Abstract
Background
CameL phase 3 study demonstrated the superiority of camrelizumab plus chemotherapy over chemotherapy alone for progression-free survival in patients with previously untreated advanced non-squamous non-small-cell lung cancer (NSCLC) without EGFR/ALK alterations. Here, we present the 5-year outcomes.
Methods
Patients were randomized (1:1) and received 4–6 cycles of camrelizumab plus carboplatin and pemetrexed (n=205) or carboplatin and pemetrexed (n=207) every 3 weeks, followed by maintenance camrelizumab plus pemetrexed or pemetrexed only. Crossover from chemotherapy group to camrelizumab monotherapy was permitted after disease progression.
Results
Median time from randomization to data cut-off was 65.2 months (range, 59.7–72.2). HR for overall survival (OS) was 0.74 (95% CI 0.58 to 0.93; one-sided p=0.0043), and was 0.62 (95% CI 0.49 to 0.79; one-sided p<0.0001) after adjustment for crossover. Five-year OS rates were 31.2% (95% CI 24.7% to 37.9%) with camrelizumab plus chemotherapy versus 19.3% (95% CI 13.9% to 25.3%) with chemotherapy alone. Among the 33 patients who completed 2 years of camrelizumab, 5-year OS rate was 84.3% (95% CI 66.4% to 93.2%), and 5-year duration of response rate was 46.5% (95% CI 24.9% to 65.6%) in the 32 responders. No new safety signals were noted.
Conclusions
Camrelizumab plus carboplatin and pemetrexed as first-line therapy continued to demonstrate long-term OS benefit over carboplatin and pemetrexed, with manageable toxicity. Patients who completed 2 years of camrelizumab had enduring response and impressive OS. Current 5-year updated analysis further supports camrelizumab plus carboplatin and pemetrexed as a standard-of-care for previously untreated advanced non-squamous NSCLC without EGFR/ALK alterations.
Trial registration number
Keywords: Immunotherapy, Lung Cancer
WHAT IS ALREADY KNOWN ON THIS TOPIC
Immune checkpoint inhibitors plus platinum-based doublet chemotherapy regimens are well-established first-line therapies for advanced non-squamous non-small-cell lung cancer (NSCLC) with no targetable driver mutations. However, long-term survival data are notably lacking.
WHAT THIS STUDY ADDS
In this 5-year update, camrelizumab (an antibody targeting programmed cell death 1) plus carboplatin and pemetrexed as first-line therapy continued to exhibit long-term benefits over chemotherapy alone, with an increase of 11.9% (31.2% vs 19.3%), 13.1% (16.1% vs 3.0%) and 21.3% (21.3% vs 0%) in overall survival (OS) rate, progression-free survival rate and duration of response (DoR) rate at 5 years, respectively. Patients who completed 2 years of camrelizumab in the combination group had enduring response (5-year DoR rate of 46.5%) and impressive OS (5-year OS rate of 84.3%). No new safety concerns were identified.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This report further supports camrelizumab plus carboplatin and pemetrexed as a standard-of-care for previously untreated advanced non-squamous NSCLC without EGFR/ALK alterations.
Background
Immune checkpoint inhibitors (ICIs) targeting programmed cell death 1 (PD-1) or programmed cell death ligand 1 (PD-L1) in combination with platinum-based doublet chemotherapy regimens are currently recommended by clinical guidelines as frontline therapy in non-squamous non-small-cell lung cancer (NSCLC) patients lacking targetable driver mutations.1,3 Numerous anti-PD-1/PD-L1 antibodies have been approved for the combination use globally or in specific countries or regions, based on the primary analyses from phase 3 clinical trials.4,12 However, it is important to underscore that, there is limited 5-year survival data to make a comprehensive evaluation of the long-term benefits of these combinations.
Camrelizumab is a humanized monoclonal antibody against PD-1 with high affinity.13 The CameL phase 3 study demonstrated a statistically significant and clinically meaningful improvement in progression-free survival (PFS) with camrelizumab plus carboplatin and pemetrexed over chemotherapy alone in patients with previously untreated advanced non-squamous NSCLC without EGFR/ALK alterations (HR, 0.60 (95% CI 0.45 to 0.79); one-sided p=0.0001).7 On the basis of results from the CameL study, the combination of camrelizumab plus carboplatin and pemetrexed has received regulatory approval as the first-line therapy for non-squamous NSCLC with no EGFR/ALK genomic alterations, regardless of PD-L1 expression level. In a descriptive analysis with extended follow-up, the camrelizumab plus chemotherapy also showed overall survival (OS) benefit over chemotherapy alone (HR, 0.72 (95% CI 0.57 to 0.92)).14 Here, we present the 5-year updated outcomes in overall study population and in those who completed 2 years of camrelizumab.
Methods
Study design and patients
The CameL study was a randomized, open-label, multicenter, phase 3 study comparing camrelizumab plus chemotherapy versus chemotherapy alone as first-line therapy for advanced non-squamous NSCLC done at 52 sites in China (ClinicalTrials.gov, number NCT03134872; online supplemental table S1). Full inclusion and exclusion criteria have been described previously.7 Briefly, patients aged 18–70 years with histologically or cytologically confirmed stage IIIB–IV non-squamous NSCLC, no prior antitumor systemic therapy for the disease, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and no untreated central nervous system metastases were enrolled.
Treatments
Patients were randomly assigned in a 1:1 ratio to receive camrelizumab (200 mg) plus carboplatin (area under curve, 5 mg/mL/min) and pemetrexed (500 mg/m2) every 3 weeks or chemotherapy alone for 4–6 cycles, followed by maintenance therapy with camrelizumab plus pemetrexed or pemetrexed alone. The randomization was stratified by sex (male vs female) and smoking history (≥20 pack-years vs <20 pack-years or never).
Patients who assigned to chemotherapy alone group were permitted to cross over to receive camrelizumab monotherapy on radiographic progression per Response Evaluation Criteria in Solid Tumors version (version 1.1). The maximum total exposure of camrelizumab was 2 years. For toxicity management, the protocol permitted interruption of up to 12 weeks for camrelizumab, as well as treatment interruptions and dose adjustments of chemotherapeutic agents.
Outcomes
The two primary endpoints of this study were PFS per blinded independent central review in overall study population and in PD-L1-positive population, which have been reported previously.7 In this report, we present the 5-year clinical outcomes, including OS, investigator-assessed PFS, objective response rate (ORR), and duration of response (DoR), as well as safety, in both overall study population and patients who completed 2 years of camrelizumab.
Statistical analysis
All randomly assigned patients who received at least one dose of the study treatment were evaluated for efficacy, and safety was assessed in the as-treated population. Time-to-event endpoints including OS, PFS, and DoR were estimated with the Kaplan-Meier method. Comparisons between groups were done using the log-rank test stratified by the randomization strata, with HRs and 95% CIs determined using the stratified Cox proportional hazards model. The rank-preserving structural failure time model was used to adjust crossover when comparing OS between groups. No specific alpha was allocated for the current analysis, and all reported p values were nominal and one-sided. Statistical analyses were performed with SAS V.9.4.
Results
Patients and treatments
Between May 12, 2017, and June 6, 2018, 412 patients were enrolled and received camrelizumab plus chemotherapy (n=205) or chemotherapy alone (n=207). The two groups had generally similar baseline characteristics (age<65 years, 78.0% and 74.4%; male, 71.2% and 72.0%; ECOG performance status of 1, 76.6% and 82.6%; all patients had metastatic lesions, with the majority of metastases in the chest (60.5% and 60.9%), bone (31.7% and 35.3%), and abdomen (21.0% and 13.5%)).
As of May 28, 2023, with a median time from randomization to cut-off date of 65.2 months (range, 59.7–72.2), nine (4.3%) patients in the camrelizumab plus chemotherapy group and one (0.5%) patient in the chemotherapy alone group remained on maintenance treatment with pemetrexed. After the allocated study treatment, 114 (55.6%) patients in the camrelizumab plus chemotherapy group and 146 (70.5%) patients in the chemotherapy alone group received at least one subsequent therapy. In the chemotherapy alone group, 95 (45.9%) patients crossed over to receive camrelizumab monotherapy on radiological progression and additional 14 (6.8%) received subsequent immunotherapy (either alone or combined with other therapies) outside the study, demonstrating an effective crossover rate of 52.7% (online supplemental figure S1).
Five-year efficacy in overall study population
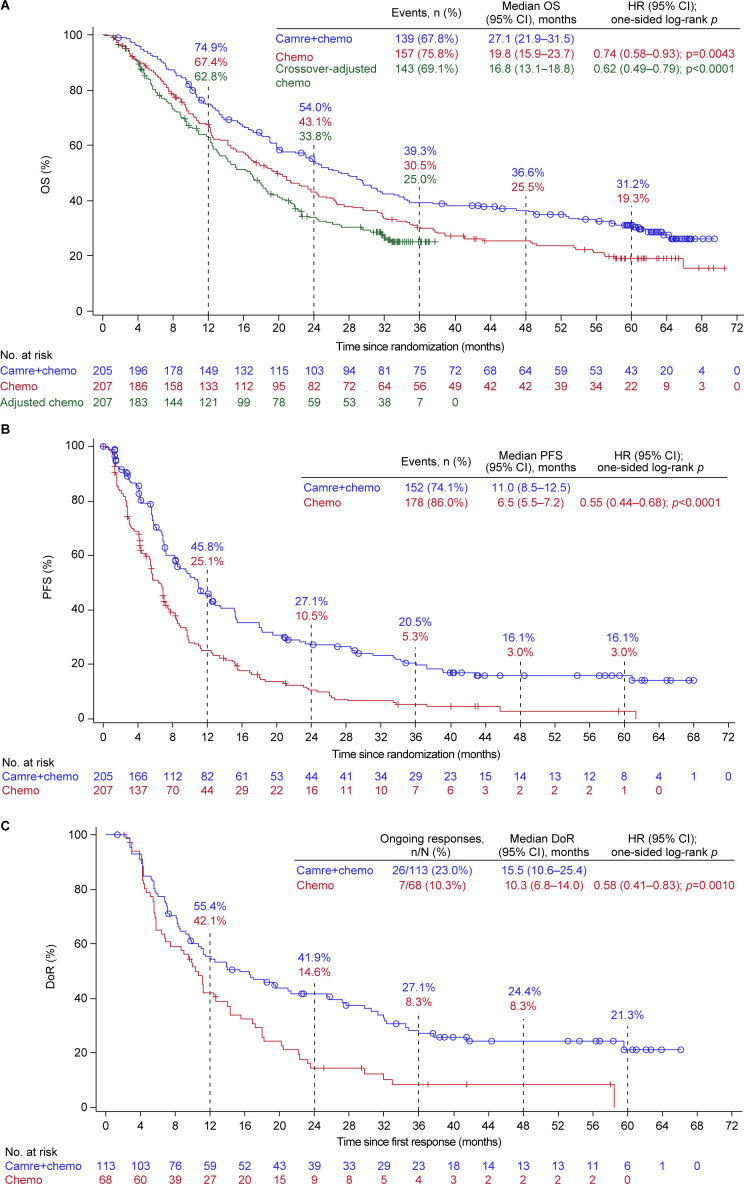
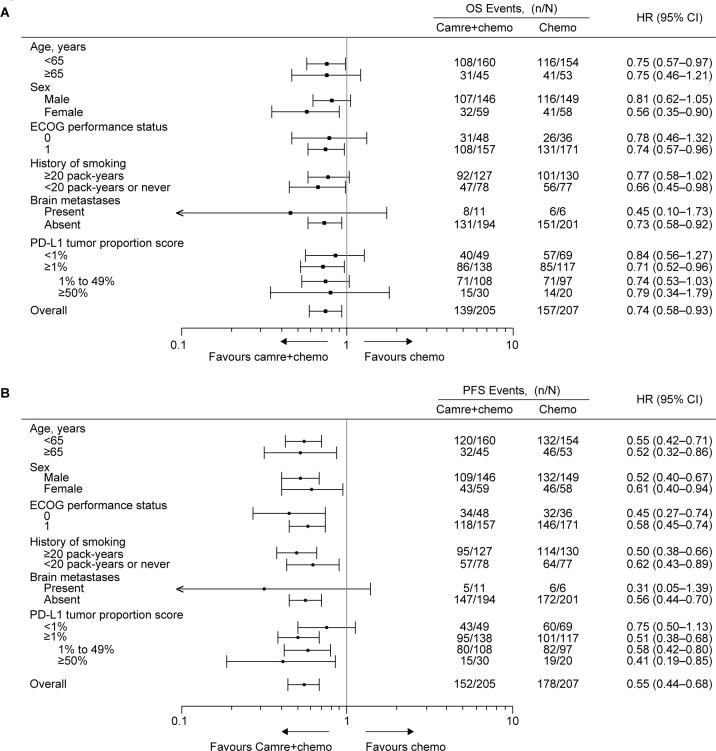
Overall, 296 (71.8%) deaths occurred, including 139 (67.8%) in the camrelizumab plus chemotherapy group and 157 (75.8%) in the chemotherapy alone group. The median OS remained longer with camrelizumab plus chemotherapy comparing chemotherapy alone (27.1 months (95% CI 21.9 to 31.5) vs 19.8 months (95% CI 15.9 to 23.7); HR, 0.74 (95% CI 0.58 to 0.93); one-sided p=0.0043; figure 1A). The 5-year OS rate was 31.2% (95% CI 24.7% to 37.9%) with camrelizumab plus chemotherapy versus 19.3% (95% CI 13.9% to 25.3%) with chemotherapy alone. Consistent with previous reports, OS benefit with camrelizumab plus chemotherapy continued to be observed regardless of age, sex, history of smoking, ECOG performance status, brain metastases status, and tumor PD-L1 expression, with an HR less than 1 (figure 2A and online supplemental figure S2).
Figure 1. Clinical outcomes with camrelizumab plus chemotherapy versus chemotherapy alone. (A) Kaplan-Meier estimates for OS. (B) Kaplan-Meier estimates for PFS. (C) Kaplan-Meier estimates for DoR. The HR was estimated from the stratified Cox proportional hazards model with treatment as the fixed effect. Comparisons between groups were analyzed using the stratified one-sided log-rank test. Stratified factors included sex (male vs female) and smoking history (≥20 pack-years vs ˂20 pack-years or never). Tumor responses were assessed by investigator per RECIST version 1.1. Camre+chemo, camrelizumab plus chemotherapy; Chemo, chemotherapy; DoR, duration of response; OS, overall survival; PFS, progression-free survival.
Figure 2. Subgroup analysis. (A) Subgroup analysis of OS. (B) Subgroup analysis of PFS. For sex and smoking history, the HR was estimated from an unstratified Cox proportional hazards model with treatment as the fixed effect. For other variables, the HR was estimated from a Cox proportional hazards model stratified by sex (male vs female) and smoking history (≥20 pack-years vs ˂20 pack-years or never), with treatment as the fixed effect. Camre+chemo, camrelizumab plus chemotherapy; Chemo, chemotherapy; ECOG, Eastern Cooperative Oncology Group; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival.
The crossover-adjusted median OS for chemotherapy alone was 16.8 months (95% CI 13.1 to 18.8), and the survival benefit was in favor of camrelizumab plus chemotherapy, with an HR of 0.62 (95% CI 0.49 to 0.79; p<0.0001; figure 1A).
As of data cut-off, 330 (80.1%) patients had disease progression or died, including 152 (74.1%) in the camrelizumab plus chemotherapy group and 178 (86.0%) in the chemotherapy alone group. Camrelizumab plus chemotherapy continued to exhibit a clinically meaningful improvement over chemotherapy alone in PFS (median, 11.0 months (95% CI 8.5 to 12.5) vs 6.5 months (95% CI 5.5 to 7.2); HR, 0.55 (95% CI 0.44 to 0.68); p<0.0001; figure 1B). The 5-year PFS rate was 16.1% (95% CI 10.9% to 22.2%) with camrelizumab plus chemotherapy versus 3.0% (95% CI 0.9% to 7.6%) with chemotherapy alone. PFS benefit with camrelizumab plus chemotherapy was consistently observed across all tested subgroups (figure 2B and online supplemental figure S3).
PD-L1 expression was not used as a stratification factor; however, when adding PD-L1 as a covariate in the Cox models, the OS and PFS benefits with camrelizumab plus chemotherapy was still superior to chemotherapy alone (OS: HR, 0.76 (95% CI 0.60 to 0.97); one-sided p=0.0137; PFS: HR, 0.58 (95% CI 0.46 to 0.74); one-sided p<0.0001).
The ORR was 55.1% (95% CI 48.0% to 62.1%) with camrelizumab plus chemotherapy versus 32.9% (95% CI 26.5% to 39.7%) with chemotherapy alone (p<0.0001). Compared with chemotherapy alone, camrelizumab plus chemotherapy demonstrated a more sustained response (median DoR, 15.5 months (95% CI 10.6 to 25.4) vs 10.3 months (95% CI 6.8 to 14.0); HR, 0.58 (95% CI 0.41 to 0.83); p=0.0010; figure 1C). The 5-year DoR rate with camrelizumab plus chemotherapy was 21.3% (95% CI 12.7% to 31.4%), whereas no patients treated with chemotherapy had a response lasting 5 years (online supplemental table S2).
Five-year safety in overall study population
During induction treatment, the majority of patients completed the 4–6 cycles of assigned treatments (65 (31.7%) patients in the camrelizumab plus chemotherapy group and 62 (30.0%) patients in the chemotherapy alone group received four cycles of treatment, 20 (9.8%) and 15 (7.2%) received five cycles of treatment, 92 (44.9%) and 81 (39.1%) received six cycles of treatment, respectively). Overall, the median number of treatment cycles in the camrelizumab plus chemotherapy group was 10 (range, 1–35) for camrelizumab, 5 (range, 1–7) for carboplatin, and 11 (range, 1–93) for pemetrexed. In the chemotherapy group, the median number of treatment cycles was 4 (range, 1–6) for carboplatin and 7 (range, 1–66) for pemetrexed.
Treatment-related adverse events (TRAEs) of grade ≥3 occurred in 146 (71.2%) and 102 (49.3%) patients in the camrelizumab plus chemotherapy and chemotherapy alone group, respectively. The most common grade ≥3 TRAEs were hematological toxicities including decreased neutrophil count, decreased white blood cell count, anemia, and decreased platelet count (table 1). Serious TRAEs were reported in 78 (38.0%) patients treated with camrelizumab plus chemotherapy and 27 (13.0%) treated with chemotherapy alone. The most frequently reported serious TRAEs were decreased platelet count, myelosuppression, and hepatic function abnormal (online supplemental table S3). Six (2.9%) deaths in the camrelizumab plus chemotherapy and three (1.4%) deaths in the chemotherapy alone groups were considered to be attributed to TRAEs.
Table 1. Treatment-related adverse events.
| Camrelizumab plus chemotherapy (n=205) | Chemotherapy alone (n=207) | |||
| All grades | Grade ≥3 | All grades | Grade ≥3 | |
| Any | 204 (99.5%) | 146 (71.2%) | 199 (96.1%) | 102 (49.3%) |
| Hematological toxicities | ||||
| Neutrophil count decreased | 148 (72.2%) | 81 (39.5%) | 133 (64.3%) | 64 (30.9%) |
| WBC decreased | 146 (71.2%) | 41 (20.0%) | 133 (64.3%) | 30 (14.5%) |
| Anemia | 139 (67.8%) | 41 (20.0%) | 125 (60.4%) | 23 (11.1%) |
| Platelet count decreased | 97 (47.3%) | 34 (16.6%) | 79 (38.2%) | 24 (11.6%) |
| Lymphocyte count decreased | 23 (11.2%) | 9 (4.4%) | 22 (10.6%) | 5 (2.4%) |
| Hemoglobin decreased | 20 (9.8%) | 3 (1.5%) | 22 (10.6%) | 2 (1.0%) |
| Non-hematological toxicities | ||||
| RCCEP | 158 (77.1%) | 4 (2.0%) | 2 (1.0%) | 0 |
| AST increased | 96 (46.8%) | 4 (2.0%) | 68 (32.9%) | 2 (1.0%) |
| ALT increased | 90 (43.9%) | 10 (4.9%) | 79 (38.2%) | 6 (2.9%) |
| Nausea | 75 (36.6%) | 2 (1.0%) | 61 (29.5%) | 2 (1.0%) |
| Asthenia | 68 (33.2%) | 7 (3.4%) | 58 (28.0%) | 3 (1.4%) |
| Decreased appetite | 66 (32.2%) | 5 (2.4%) | 56 (27.1%) | 5 (2.4%) |
| Constipation | 45 (22.0%) | 0 | 35 (16.9%) | 1 (0.5%) |
| Vomiting | 44 (21.5%) | 2 (1.0%) | 35 (16.9%) | 4 (1.9%) |
| Hepatic function abnormal | 43 (21.0%) | 4 (2.0%) | 32 (15.5%) | 1 (0.5%) |
| GGT increased | 39 (19.0%) | 6 (2.9%) | 18 (8.7%) | 1 (0.5%) |
| Blood creatinine increased | 32 (15.6%) | 1 (0.5%) | 14 (6.8%) | 1 (0.5%) |
| Rash | 29 (14.1%) | 4 (2.0%) | 11 (5.3%) | 0 |
| Pruritus | 25 (12.2%) | 1 (0.5%) | 3 (1.4%) | 0 |
| Edema peripheral | 24 (11.7%) | 0 | 17 (8.2%) | 0 |
| Myelosuppression | 24 (11.7%) | 14 (6.8%) | 10 (4.8%) | 4 (1.9%) |
| Blood bilirubin increased | 23 (11.2%) | 2 (1.0%) | 14 (6.8%) | 0 |
| Hypothyroidism | 23 (11.2%) | 1 (0.5%) | 0 | 0 |
Data are n (%). Treatment-related adverse events occurring in at least 10% of patients in either group are listed. Safety data for the chemotherapy alone group did not include data from the crossover phase.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferaseRCCEP, reactive cutaneous capillary endothelial proliferation; WBC, white blood cell
Immune-mediated AEs of all grades were reported in 182 (88.8%) patients treated with camrelizumab plus chemotherapy, and those of grade ≥3 were reported in 32 (15.6%) patients. The incidence and severity of each immune-mediated AE were exactly the same as the last report, with no additional patients developing new immune-mediated AEs. Reactive cutaneous capillary endothelial proliferation (RCCEP) was the most common immune-mediated AE; however, the majority were grade 1 or 2 in severity (154 (75.1%)), only four (2.0%) patients experienced grade 3 RCCEP, and no grade 4 or 5 RCCEP occurred. Immune-mediated AEs were characterized by an early onset, with the median time to the first occurrence of any immune-mediated AE being 1.1 months (range, 0.1–19.1). The majority of patients experienced immune-mediated AEs within the first 6 months of treatment (online supplemental figure S4).
Five-year outcomes in patients who completed 2 years of camrelizumab
Baseline characteristics of the 33 patients in the camrelizumab plus chemotherapy group who completed 2 years of camrelizumab was reported. Compared with the overall population in the camrelizumab plus chemotherapy group, the proportion of patients with PD-L1 positivity (tumor proportion score (TPS) ≥1%) (90.9% vs 67.3%) and high PD-L1 expression (TPS≥50%) (27.3% vs 14.6%) were higher in those who completed 2 years of camrelizumab. The median number of treatment cycles was 34 (range, 23–35) for camrelizumab, 6 (range, 3–6) for carboplatin, and 45 (range, 14–93) for pemetrexed.
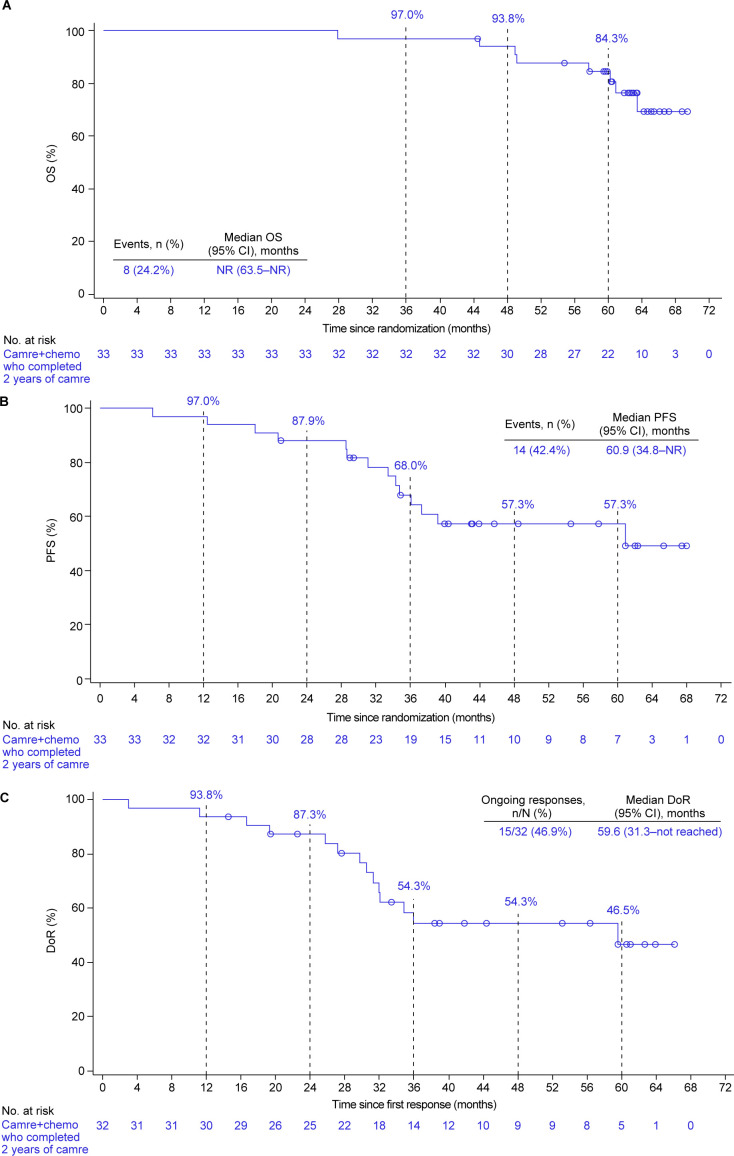
Of the 33 patients, 8 (24.2%) had died, and the median OS was not reached yet (95% CI 63.5 to not reached; figure 3A). The 5-year OS rate was 84.3% (95% CI 66.4% to 93.2%).
Figure 3. Clinical outcomes in patients who completed 2 years of camrelizumab. (A) Kaplan-Meier estimates for OS. (B) Kaplan-Meier estimates for PFS. (C) Kaplan-Meier estimates for DoR. Tumor responses were assessed by investigator per RECIST version 1.1. Camre+chemo, camrelizumab plus chemotherapy; DoR, duration of response; OS, overall survival; PFS, progression-free survival.
Totally, 14 (42.4%) patients had disease progression or died, and the median PFS was 60.9 months (95% CI 34.8 to not reached; figure 3B). The PFS rate at 5 years was 57.3% (95% CI 37.8% to 72.7%).
The ORR was 97.0% (32/33; 95% CI 84.2% to 99.9%). As of data cut-off, the response was ongoing in 15 of the 32 responders (46.9%). The median DoR was 59.6 months (95% CI 31.3 to not reached), and DoR rate at 5 years was 46.5% (95% CI 24.9% to 65.6%; figure 3C and online supplemental table S2).
21 (63.6%) patients experienced grade ≥3 TRAEs. Immune-mediated AEs occurred in all patients, with three (9.1%) patients experiencing grade ≥3 events including RCCEP, rash, and increased blood bilirubin (3.0% for each).
Discussion
In this 5-year update for the CameL study, camrelizumab plus carboplatin and pemetrexed continued to show long-term and durable clinical benefit versus chemotherapy alone for patients with previously untreated advanced non-squamous NSCLC without EGFR/ALK alterations. The HR for OS was 0.74 (95% CI 0.58 to 0.93), which was adjusted to 0.62 (95% CI 0.49 to 0.79) after accounting for crossover. The OS benefit achieved with camrelizumab plus chemotherapy over chemotherapy was maintained across all subgroups, including PD-L1 expression, with an HR less than 1.
Several studies have demonstrated the efficacy of an anti-PD-1/PD-L1 antibody plus chemotherapy in patients with non-squamous NSCLC in the first-line setting, including Keynote-189,4 15 IMpower130,5 ORIENT-11 (in China),6 16 and RATIONALE 304 (in China).8 In addition, Empower-Lung-3,17 GEMSTONE-302 (in China),12 and CHOICE-01 (in China)11 enrolled both non-squamous and squamous NSCLC. The OS curves of camrelizumab plus chemotherapy versus chemotherapy alone separated early and started to flatten at 3 years. The curve of the chemotherapy alone group also had a plateau at the tail, which was not observed in earlier studies of chemotherapy before the therapeutic landscape was changed by advent of immunotherapy.18 Actually, the tail-plateau found in the chemotherapy alone group in this study could be attributed to the effective crossover rate of 52.7% from chemotherapy to subsequent immunotherapy. Similar phenomenon was observed in the Keynote-189, IMpower130, and ORIENT-11 studies,4,615 16 in which crossover in the control arm was allowed. The HR for death with an ICI plus chemotherapy versus chemotherapy ranged from 0.48 to 0.79; the upper limits of the 95% CI were less than 1, apart from the GEMSTONE-302 study, where the upper limit was 1.01. Only the Keynote-189 study and the present study released the 5-year outcomes, whereas others at most reported the 2-year OS rate. Despite a high crossover rate, the OS improvement with camrelizumab plus chemotherapy was well maintained, with a survival gain of 8.8% (39.3% vs 30.5%) at 3 years, 11.1% (36.6% vs 25.5%) at 4 years, and 11.9% (31.2% vs 19.3%) at 5 years. The survival gain with pembrolizumab plus chemotherapy was 13.9% (31.3% vs 17.4%) at 3 years, 9.8% (23.6% vs 13.8%) at 4 years, and 8.1% (19.4% vs 11.3%) at 5 years.15 The two studies showed generally consistent long-term survival gain. Of note, camrelizumab plus chemotherapy in our study showed the longest median OS compared with other combinations. In summary, the addition of a single-agent ICI to first-line standard chemotherapy could improve the OS in patients with non-squamous NSCLC without EGFR/ALK alterations. The magnitude of improvement varied, which might be attributed to the differences in ICI agent, study population, and the efficacy of the chemotherapy control.
Of the 33 patients who completed 2 years of camrelizumab, first-line camrelizumab plus chemotherapy provided a remarkable 5-year OS rate of 84.3%. The median PFS was 60.9 months, with a 5-year PFS rate of 57.3%. The patients had high and durable response, with an ORR of 97.0%, a median DoR of 59.6 months, and a 5-year DoR rate of 46.5%. Similar to the Keynote-189 study15 and consistent with our previous report,14 our 5-year findings continued to support the feasibility of 2 years use of ICI, when combined with platinum-based doublet chemotherapy.
In our previous report, only 10.2% of patients in the camrelizumab plus chemotherapy group and 1.9% of patients in the chemotherapy alone remained on study treatment, and all were on maintenance treatment with low toxic pemetrexed.14 With additional 16 calendar months of follow-up, no new toxicity signals emerged in this updated analysis, and no additional treatment-related deaths occurred. The profile and severity of TRAEs observed in this 5-year update analysis were consistent with previous report.14 Compared with combinations of other ICIs with chemotherapy, camrelizumab plus chemotherapy showed similar safety profile, except RCCEP. However, the majority were grade 1 or 2 in severity, only 2.0% of patients experienced grade 3 RCCEP, and no grade 4 or 5 RCCEP occurred. The majority of lesions occurred shortly after the treatment and presented on the skin surface; no bleeding events from the gastrointestinal mucosa, bronchial mucosa, or abdominal organs were reported.19,22 Overall, despite with a high incidence, RCCEP lesions were generally mild or moderate, clinically controllable, and self-limiting.23
This long-term report has several limitations. First, PD-L1 expression was not used as a stratification factor. Nonetheless, the combination of camrelizumab and chemotherapy still demonstrated superiority over chemotherapy alone in terms of PFS and OS, when adding PD-L1 as a covariate in the Cox proportional hazards models. Second, the applicability of our findings is currently limited to the Chinese population. The efficacy and safety of camrelizumab in combination with chemotherapy in other populations are need to be investigated. In addition, biomarkers to monitor treatment efficacy merit extensive research.
In conclusion, camrelizumab plus carboplatin and pemetrexed maintained long-term clinical meaningful OS improvement compared with chemotherapy alone, despite a high effective crossover rate of 52.7% from chemotherapy to immunotherapy. The camrelizumab combination gave an 11.9% survival gain at 5 years, while maintaining manageable toxicity. This 5-year analysis further supports the first-line camrelizumab plus carboplatin and pemetrexed as a standard-of-care for advanced NSCLC patients without EGFR/ALK alterations in China.
supplementary material
Acknowledgements
This study was funded by Jiangsu Hengrui Pharmaceuticals, which participated in study design, data collection, and data interpretation. We are grateful to all patients and their families and all members of the collaborative group. We would also like to acknowledge PAREXEL International for statistical analyses (funded by Hengrui) and Dr Hui Dong for medical writing support (Medical Writer at Hengrui).
Footnotes
Funding: This study was funded by Jiangsu Hengrui Pharmaceuticals Co., Ltd.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and this study was approved by independent ethics committees or institutional review boards of the 52 participating institutions and conducted in accordance with the Declaration of Helsinki, Guidelines for Good Clinical Practice, and local laws and regulations of China. The name of the ethics committees or institutional review boards and the ID for ethics approval are summarized in online supplemental table S1. Participants gave informed consent to participate in the study before taking part.
Data availability free text: The clinical trial data presented in the article are not publicly available because of patient privacy and consent restrictions, but are available upon reasonable request by contacting the corresponding author. Qualified researchers should submit a proposal to the corresponding author outlining the reasons for requiring the data. The sponsor will provide the data if the proposal is approved, provided that the requestor signs a data-access agreement. Use of data must comply with the requirements of Human Genetics Resources Administration of China; and other country or region-specific regulations.
Contributor Information
Caicun Zhou, Email: caicunzhou_dr@163.com.
Gongyan Chen, Email: Gongyanchen0123@163.com.
Yunchao Huang, Email: huangych2001@aliyun.com.
Jianying Zhou, Email: drzjy@163.com.
LiZhu Lin, Email: 13501505588@139.com.
Jifeng Feng, Email: fjif@vip.sina.com.
Zhehai Wang, Email: Wzhai8778@sina.com.
Yongqian Shu, Email: shuyongqian@csco.org.cn.
Jianhua Shi, Email: shijianhualy@126.com.
Yi Hu, Email: huyi0401@aliyun.com.
QiMing Wang, Email: qimingwang1006@126.com.
Ying Cheng, Email: jl.cheng@163.com.
Fengying Wu, Email: fywu@163.com.
Jianhua Chen, Email: cjh_1000@163.com.
Xiaoyan Lin, Email: 13950482366@qq.com.
Yongsheng Wang, Email: wangys75@gmail.com.
Jianan Huang, Email: huang_jian_an@163.com.
Jiuwei Cui, Email: cuijiuwei@vip.qq.com.
Lejie Cao, Email: sycaolejie@163.com.
Yunpeng Liu, Email: cmu_trial@163.com.
Yiping Zhang, Email: zyp352@163.com.
Yueyin Pan, Email: yueyinpan1965@126.com.
Jun Zhao, Email: ohjerry@163.com.
LiPing Wang, Email: wlp@zzu.edu.cn.
Jianhua Chang, Email: changjianhua@163.com.
Qun Chen, Email: chenqun88@qq.com.
Xiubao Ren, Email: renxiubao@tjmuch.com.
Wei Zhang, Email: zhangweiliuxin@163.com.
Yun Fan, Email: fanyun@zjcc.org.cn.
Zhiyong He, Email: heyong1015@163.com.
Jian Fang, Email: fangjian5555@163.com.
Kangsheng Gu, Email: 13805692145@163.com.
XiaoRong Dong, Email: hustwhuh@126.com.
Faguang Jin, Email: jinfag@fmmu.edu.cn.
Hongjun Gao, Email: gaohj6708@hotmail.com.
Guangyu An, Email: agybjcy@163.com.
Cuimin Ding, Email: wjwdcm@sina.com.
Xiaodong Jiang, Email: jxdysy1970@163.com.
Jianping Xiong, Email: 1153581951@qq.com.
Xiangdong Zhou, Email: Xiangdongzhou@126.com.
Sheng Hu, Email: ehusmn@163.com.
Ping Lu, Email: lupingdoctor@126.com.
Anwen Liu, Email: awliu666@163.com.
Shuliang Guo, Email: GUOSL999@sina.com.
Jianjin Huang, Email: 13989889688@163.com.
Chengchu Zhu, Email: zhucc@enzemed.com.
Jian Zhao, Email: zj_hjh@163.com.
Beili Gao, Email: yshu7661@sina.com.
Yinglan Chen, Email: Doc.cyl@163.com.
Chengping Hu, Email: huchengp28@126.com.
Jian Zhang, Email: 13991802890@163.com.
Hongmei Zhang, Email: zhm@fmmu.edu.cn.
Hui Zhao, Email: zhaohuichenxi@126.com.
Zhigao Wang, Email: zhigao.wang.zw1@hengrui.com.
Xinjing Ma, Email: xinjing.ma@hengrui.com.
Wei Shi, Email: wei.shi@hengrui.com.
Data availability statement
Data are available upon reasonable request.
References
- 1.NCCN guidelines: non-small cell lung cancer, version 1.2024. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 n.d. Available.
- 2.Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer. Chin J Oncol. 2023;45:539–74. doi: 10.3760/cma.j.cn112152-20230510-00200. [DOI] [PubMed] [Google Scholar]
- 3.Hendriks LE, Kerr KM, Menis J, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:358–76. doi: 10.1016/j.annonc.2022.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078–92. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 5.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–37. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Zhou H, Zhang L. Response to Letter to the Editor: Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: A Randomized, Double-Blind, Phase 3 Study (ORIENT-11) J Thorac Oncol. 2020;15:e191–2. doi: 10.1016/j.jtho.2020.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9:305–14. doi: 10.1016/S2213-2600(20)30365-9. [DOI] [PubMed] [Google Scholar]
- 8.Lu S, Wang J, Yu Y, et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J Thorac Oncol. 2021;16:1512–22. doi: 10.1016/j.jtho.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Gogishvili M, Melkadze T, Makharadze T, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med. 2022;28:2374–80. doi: 10.1038/s41591-022-01977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou C, Wang Z, Sun M, et al. Interim survival analysis of the randomized phase III GEMSTONE-302 trial: sugemalimab or placebo plus chemotherapy as first-line treatment for metastatic NSCLC. Nat Cancer . 2023;4:860–71. doi: 10.1038/s43018-023-00578-z. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Wu L, Li B, et al. Toripalimab Plus Chemotherapy for Patients With Treatment-Naive Advanced Non-Small-Cell Lung Cancer: A Multicenter Randomized Phase III Trial (CHOICE-01) J Clin Oncol. 2023;41:651–63. doi: 10.1200/JCO.22.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou C, Wang Z, Sun Y, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 2022;23:220–33. doi: 10.1016/S1470-2045(21)00650-1. [DOI] [PubMed] [Google Scholar]
- 13.Markham A, Keam SJ. Camrelizumab: First Global Approval. Drugs (Abingdon Engl) 2019;79:1355–61. doi: 10.1007/s40265-019-01167-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhou C, Chen G, Huang Y, et al. Camrelizumab Plus Carboplatin and Pemetrexed as First-Line Treatment for Advanced Nonsquamous NSCLC: Extended Follow-Up of CameL Phase 3 Trial. J Thorac Oncol. 2023;18:628–39. doi: 10.1016/j.jtho.2022.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Garassino MC, Gadgeel S, Speranza G, et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol. 2023;41:1992–8. doi: 10.1200/JCO.22.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Wang Z, Fang J, et al. Final overall survival data of sintilimab plus pemetrexed and platinum as First-Line treatment for locally advanced or metastatic nonsquamous NSCLC in the Phase 3 ORIENT-11 study. Lung Cancer (Auckl) 2022;171:56–60. doi: 10.1016/j.lungcan.2022.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Makharadze T, Gogishvili M, Melkadze T, et al. Cemiplimab Plus Chemotherapy Versus Chemotherapy Alone in Advanced NSCLC: 2-Year Follow-Up From the Phase 3 EMPOWER-Lung 3 Part 2 Trial. J Thorac Oncol. 2023;18:755–68. doi: 10.1016/j.jtho.2023.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. 2020;13:47. doi: 10.1186/s13045-020-00886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–80. doi: 10.1016/S1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 21.Lickliter JD, Gan HK, Voskoboynik M, et al. A First-in-Human Dose Finding Study of Camrelizumab in Patients with Advanced or Metastatic Cancer in Australia. Drug Des Devel Ther. 2020;14:1177–89. doi: 10.2147/DDDT.S243787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21:832–42. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 23.Qu W, Wang F, Qin S, et al. Reactive cutaneous capillary endothelial proliferation following camrelizumab monotherapy or combination therapy for multi-cancers: a large-scale pooled analysis of 10 studies in China. Ther Adv Med Oncol . 2024;16:17588359241242607. doi: 10.1177/17588359241242607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.