Abstract
Background
Tribe Oenantheae consists mainly of aquatic species within the Apioideae. The unique morphology and habitat distinguish this group from other Apioideae groups. However, the genomic information of these group species has not been widely developed, and the molecular mechanisms of adaptive evolution remain unclear.
Results
We provide comparative analyses on 30 chloroplast genomes of this tribe representing five genera to explore the molecular variation response to plant adaptations. The Oenantheae chloroplast genomes presented typical quadripartite structures, with sizes ranging from 153,024 bp to 155,006 bp. Gene content and order were highly conserved with no significant expansion or contraction observed. Seven regions (rps16 intron–trnK, rpoB–trnC, trnE–trnT–psbD, petA–psbJ, ndhF–rpl32–trnL, ycf1a–rps15, and ycf1a gene) were identified as remarkable candidate DNA markers for future studies on species identification, biogeography, and phylogeny of tribe Oenantheae. Our study elucidated the relationships among the genera of tribe Oenantheae and subdivided the genera of Sium and Oenanthe. However, relationships among the Oenanthe I clade remain to be further clarified. Eight positively selected genes (accD, rbcL, rps8, ycf1a, ycf1b, ycf2, ndhF, and ndhK) were persuasively detected under site models tests, and these genes might have played roles in Oenantheae species adaptation to the aquatic environments.
Conclusions
Our results provide sufficient molecular markers for the subsequent molecular studies of the tribe Oenantheae, and promote the understanding of the adaptation of the Oenantheae species to aquatic environments.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05863-2.
Keywords: Oenantheae, Adaptation, Phylogeny, Positive selection, Candidate DNA markers
Background
Oenantheae Dumort is a special tribe of Apioideae (Apiaceae) that is morphologically and ecologically well-defined. Species of this tribe are generally aquatic with clumped fibrous or tuberous-thickened roots (usually rooted at the base of the stem), and pericarp of the fruits with varying degrees of corky-thickened. Molecular phylogenetic studies in recent years have shown that Oenantheae is currently recognized as a natural and stable major clade of Apioideae, located in the middle of the phylogenetic tree [1–7]. Pollen of Oenantheae species vary in shape, covering almost every type from primitive to evolutionary [8, 9]. The integration of multiple traits (morphological, phylogenetic, ecological, and palynological) has shown that Oenantheae is the key group in studying the evolutionary history of Apioideae. The tribe currently contains about 24 genera and 120 species: including 5 genera (Berula W. D. J. Koch, Cicuta L., Cryptotaenia DC., Oenanthe L., and Sium L.) distributed in both Eurasia and North America; 12 genera (Atrema DC., Ptilimnium Raf., Tiedemannia DC., Harperella Rose, Limnosciadium Mathias & Constance, Cynosciadium DC., Lilaeopsis Greene, Neogoezia Hemsl., Trepocarpus Nutt. ex DC., Daucosma Engelm. & A.Gray, Oxypolis Raf., and Perideridia Rchb.) [10–12] endemic to North America; 3 genera [Caropsis (Rouy & E.G.Camus) Rauschert、Lereschia Boiss., and Trocdaris Raf.] [5, 12] and one hybrid genus (× Beruladium A.C.Leslie) [13] endemic to Europe; one genus (Helosciadium W.D.J.Koch) [14] distributed in both Eurasia and Africa; one monotypic genus (Apodicarpum Makino) [3] endemic to Asia; and one species (Peucedanum sandwicense Hillebr.) [12] endemic to Hawaii. There are 5 genera and around 17 species distributed in China [15].
Previous studies have shown that Oenantheae was recognized as a monophyletic tribe of Apioideae, but the relationships among the genera within this tribe have not been completely resolved. Most of the previous studies on the phylogeny of Oenantheae were carried out based on small molecular markers, including nrDNA ITS [5, 10–12, 16–19], rps16 intron [20], trnQ–trnK [18], psbI–5’trnK(UUU) [11], and rps16–5’trnK(UUU) [12, 14]. The intergeneric relationships of most genera within Oenantheae have been clarified, except for the genera within the Sium alliance. Recently, a biogeographical study on Oenantheae was implemented under comprehensive sampling using ITS and rps16–trnK markers [12]. The phylogenetic reconstruction results showed that Oenantheae was well divided into four lineages and two individual species, including the North American (NA) endemic clade [10, 19], the Cicuta & Oenanthe group [21], the Sium alliance [14], the Perideridia clade [22], Caropsis verticillatoinundata (Thore) Rauschert, and Trocdaris verticillatum (L.) Raf [12]. The relationships among genera within the North American (NA) endemic clade and the Cicuta & Oenanthe group were highly supported, but that of the Sium alliance (which was formerly known as Old World endemics clade in Spalik et al., 2009) were still controversial. The relationships among genera within the Sium alliance were poorly supported. Mostly, the insufficient informative sites of small molecular markers were suggested to be the main reason for the depressed resolution of phylogenies [2, 6].
Chloroplast genomes have been widely used for the reconstruction of plant phylogenies in recent years [6, 23–28]. Compared with small molecular markers, chloroplast genomes have sufficient information sites to meet the needs of phylogenetic reconstruction. The unique mode of inheritance ultimately reflected the “true” phylogenetic relationships of a given lineage. Nearly 2.2% of the published chloroplast genomes (NCBI, September 2023) were generated from Apiales species [27], most of which were from Apiaceae species. Apiaceae chloroplast genomes generally shared a typical quadripartite structure including a larger single copy region (LSC), a small single copy region (SSC), and two inverted repeat regions (IRA and IRB). However, the characteristics of genome structure varied among different species/genera of Apiaceae [6, 29, 30]. Comparative genomic studies concerning the characteristics of genes or functional groups of genes are of great importance in understanding plastome evolution and phylogenetic inference [27, 29, 31–33]. The chloroplast genome data were successfully used for phylogenetic reconstruction of Apiaceae in many studies [6, 24, 29, 30, 34–37]. However, the chloroplast genome of Oenantheae has not been paid much attention, and most of the chloroplast genome studies on this tribe involved individual species [38–41] or specific genus (for genus Sium) [42]. Regrettably, there was a lack of plastid phylogenomic analysis conducted for this tribe, and the genome differences of intergeneric plastids are still unknown within Oenantheae.
In this study, we filled this gap with a comparative analysis of the chloroplast genomes of 30 taxa (covering five genera) within Oenantheae. We conducted comprehensive analyses (1) to reveal the chloroplast genome characteristics and evolution of Oenantheae; (2) to identify suitable hotspot regions to use as candidate DNA barcodes for genera identification within the tribe; (3) to investigate the efficacy of chloroplast genome in resolving the relationships within the tribe. This is the first comprehensive chloroplast genome analysis of the tribe Oenantheae, which will provide genetic resources and complete the phylogeny analysis of this tribe. This study is crucial for reconstructing the phylogeny and evolution of the global Oenantheae.
Materials and methods
Taxon sampling, DNA extraction, genome sequencing, assembly, and annotation
Seventeen chloroplast genomes were newly sequenced, covering 4 genera and eleven species within Oenantheae (Fig. 1). The collections and voucher information for these newly sequenced species are provided in Table S1. Fresh and well-developed leaves were collected and dried in silica gel. Total genomic DNA was extracted from the leaf tissues using the Plant Genomic DNA Kit following the manufacturer’s protocol (Tiangen Biotech, Beijing, China), and the DNA quality and purity were further determined with the agarose gel electrophoresis. The high-quality DNA was used for library construction and sequencing. Paired-end sequencing libraries were generated on the Illumina Novaseq 6000 platform at Novogene (Beijing, China), with 2 × 150 bp paired-end reads and an insert size of 300 bp. DNA libraries were prepared using Rapid Plus DNA Lib Prep Kit for Illumina (RK20208). We sequenced 3G raw data for each sample.
Fig. 1.
Morphological diversity of Oenantheae species. A, Oenanthe javanica; B, O. hookeri; C, O. thomsonii; D, Sium ventricosum; E, S. serra; F, S. suave; G, Cicuta virosa; H, Cryptotaenia canadensis; I, Cryptotaenia japonica
Qualities of raw reads were checked by FastQC v0.11.9 [43]. The chloroplast genomes were assembled using NOVOPlasty v4.3.3 [44]. The Rubisco-bis-phosphate oxygenase (RUBP) sequences from multiple reference genomes (Table 1) were extracted to serve as seed files for different genera as appropriate. The assembled sequences were checked and annotated under Geneious Prime 2023.2.1 (created by the Biomatters development team, Ltd.), with gaps or degenerate bases corrected by Sanger sequencing. The chloroplast genome maps were drawn using OGDRAW [45]. All newly generated chloroplast genomes have been uploaded to the National Center for Biotechnology Information (NCBI) and are accompanied by detailed accession numbers, as shown in Table 1.
Table 1.
Summary of major characteristics of the Oenantheae chloroplast genomes, including sequence size (bp), number of genes, GC content, GenBank accession number, and Vouchers/References
| Taxon | Size (bp) | GC content | Number of Genes | GenBank accession No. | Vouchers/References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | LSC | IRs | SSC | Total | LSC | IR | SSC | Total | CDS | rRNA | tRNA | pseudogene | |||
| Cicuta virosa | 154,438 | 84,193 | 26,355 | 17,535 | 37.50% | 35.60% | 42.70% | 30.80% | 133 | 86 | 8 | 37 | 2 | PQ283862 | LHM1252 |
| Cicuta virosa | 154,569 | 84,177 | 26,407 | 17,578 | 37.50% | 35.60% | 42.70% | 31.00% | 133 | 86 | 8 | 37 | 2 | NC_037711 | NCBI direct submission |
| Cicuta virosa | 154,341 | 84,066 | 26,377 | 17,521 | 37.50% | 35.60% | 42.70% | 30.90% | 133 | 86 | 8 | 37 | 2 | PQ283863 | WJ2368 |
| Oenanthe hookeri | 154,597 | 84,414 | 26,456 | 17,271 | 37.50% | 35.60% | 42.70% | 31.10% | 133 | 86 | 8 | 37 | 2 | PQ283867 | WJ2334 |
| Oenanthe javanica | 154,246 | 84,096 | 26,456 | 17,238 | 37.60% | 35.80% | 42.70% | 31.30% | 133 | 86 | 8 | 37 | 2 | NC_049874 | Zhang et al., 2020b [41] |
| Oenanthe javanica | 154,378 | 84,118 | 26,491 | 17,278 | 37.60% | 35.80% | 42.70% | 31.20% | 133 | 86 | 8 | 37 | 2 | PQ283868 | WJ2305_02 |
| Oenanthe javanica | 154,048 | 84,063 | 26,244 | 17,497 | 37.60% | 35.80% | 42.80% | 31.30% | 133 | 86 | 8 | 37 | 2 | PQ283869 | WJ2320 |
| Oenanthe javanica | 154,223 | 84,073 | 26,456 | 17,238 | 37.60% | 35.80% | 42.70% | 31.20% | 133 | 86 | 8 | 37 | 2 | PQ283870 | WJ2372_AH01 |
| Oenanthe linearis | 154,486 | 84,286 | 26,458 | 17,284 | 37.50% | 35.60% | 42.70% | 31.10% | 133 | 86 | 8 | 37 | 2 | PQ283871 | ZJW034 |
| Oenanthe linearissubsp.rivulari | 154,628 | 84,348 | 26,451 | 17,378 | 37.50% | 35.60% | 42.70% | 31.00% | 133 | 86 | 8 | 37 | 2 | PQ283872 | NZD2301 |
| Oenanthe linearissubsp.rivulari | 154,660 | 84,426 | 26,451 | 17,332 | 37.50% | 35.60% | 42.70% | 31.10% | 133 | 86 | 8 | 37 | 2 | PQ283873 | WJ2319 |
| Oenanthe thomsonii | 154,640 | 84,373 | 26,479 | 17,309 | 37.50% | 35.60% | 42.70% | 31.10% | 133 | 86 | 8 | 37 | 2 | PQ283875 | WJ2321 |
| Oenanthe virgata | 154,464 | 84,410 | 26,445 | 17,163 | 37.50% | 35.60% | 42.70% | 31.20% | 133 | 86 | 8 | 37 | 2 | KX832335 | Spooner et al., 2017 [39] |
| Oenanthe pimpinelloides | 154,438 | 84,415 | 26,421 | 17,181 | 37.60% | 35.70% | 42.70% | 31.30% | 133 | 86 | 8 | 37 | 2 | PQ283874 | wj20230607_01 |
| Cryptotaenia canadensis | 154,101 | 84,048 | 26,435 | 17,183 | 37.50% | 35.50% | 42.70% | 30.90% | 133 | 86 | 8 | 37 | 2 | PQ283864 | WJ220908 |
| Cryptotaenia japonica | 153,385 | 83,616 | 26,419 | 16,931 | 37.50% | 35.60% | 42.70% | 30.90% | 133 | 86 | 8 | 37 | 2 | PQ283865 | WJ2375 |
| Cryptotaenia japonica | 153,228 | 83,451 | 26,417 | 16,943 | 37.50% | 35.60% | 42.70% | 30.90% | 133 | 86 | 8 | 37 | 2 | PQ283866 | WJ2384 |
| Cryptotaenia japonica | 153,259 | 83,477 | 26,419 | 16,944 | 37.50% | 35.60% | 42.70% | 30.90% | 133 | 86 | 8 | 37 | 2 | NC_046737 | Luo and Yu, 2019 [40] |
| Sium crispulifolium | 154,099 | 84,707 | 26,150 | 17,092 | 37.40% | 35.40% | 42.70% | 31.00% | 133 | 86 | 8 | 37 | 2 | OP234518 | Zhou et al., 2023 [42] |
| Sium medium | 154,598 | 84,116 | 26,472 | 17,538 | 37.40% | 35.50% | 42.70% | 30.70% | 133 | 86 | 8 | 37 | 2 | NC_072108 | Zhou et al., 2023 [42] |
| Sium ninsi | 155,006 | 85,036 | 26,208 | 17,554 | 37.40% | 35.50% | 42.70% | 30.60% | 133 | 86 | 8 | 37 | 2 | NC_072107 | Zhou et al., 2023 [42] |
| Sium serra | 154,690 | 84,491 | 26,223 | 17,753 | 37.40% | 35.60% | 42.70% | 30.60% | 133 | 86 | 8 | 37 | 2 | PQ283876 | WJ2374 |
| Sium suave | 154,676 | 84,155 | 26,469 | 17,583 | 37.40% | 35.50% | 42.70% | 30.70% | 133 | 86 | 8 | 37 | 2 | NC_071929 | Zhou et al., 2023 [42] |
| Sium suave | 154,642 | 84,111 | 26,466 | 17,599 | 37.40% | 35.50% | 42.70% | 30.70% | 133 | 86 | 8 | 37 | 2 | OP234519 | Zhou et al., 2023 [42] |
| Sium suave | 154,646 | 84,128 | 26,466 | 17,586 | 37.40% | 35.50% | 42.70% | 30.70% | 133 | 86 | 8 | 37 | 2 | PQ283877 | WBC2301 |
| Sium tenue | 154,929 | 84,952 | 26,204 | 17,569 | 37.40% | 35.50% | 42.70% | 30.70% | 133 | 86 | 8 | 37 | 2 | NC_072106 | Zhou et al., 2023 [42] |
| Sium ventricosum | 153,024 | 84,294 | 24,970 | 18,790 | 37.40% | 35.50% | 43.10% | 30.80% | 133 | 86 | 8 | 37 | 2 | NC_070095 | NCBI direct submission |
| Sium ventricosum | 153,029 | 84,300 | 24,970 | 18,789 | 37.40% | 35.50% | 43.10% | 30.80% | 133 | 86 | 8 | 37 | 2 | OP234514 | Zhou et al., 2023 [42] |
| Sium ventricosum | 153,031 | 84,273 | 25,027 | 18,704 | 37.40% | 35.50% | 43.10% | 30.80% | 133 | 86 | 8 | 37 | 2 | PQ283878 | WJ2339 |
| Tiedemannia filiformis subsp. greenmannii | 154,737 | 84,581 | 26,510 | 17,136 | 37.30% | 35.40% | 42.60% | 30.40% | 133 | 86 | 8 | 37 | 2 | HM596071 | Downie and Jansen, 2015 [38] |
Chloroplast genomes comparative analyses
A total of 30 chloroplast genomes from five genera of Oenantheae have been adopted to implement comparative analyses. Detailed accession numbers are listed in Table 1. We investigated the chloroplast genome structure of Oenantheae taxa, including gene number, length, and GC content of the whole genomes. Boundaries of the large single copy (LSC), small single copy (SSC), and inverted repeat (IR) regions among Oenantheae chloroplast genomes were compared in Geneious Prime 2023.2.1 to detect the IR expansion/contraction. To identify potential rearrangement and inversion events in chloroplast genomes, we utilized the Mauve Alignment [46] implemented in Geneious Prime.
To identify mutation hotspot regions among species within Oenantheae, the whole chloroplast genomes aligned matrix generated from MAFFT v7 [47] plug-in Geneious Prime 2023.2.1 was used to calculate nucleotide diversity (Pi). The sliding window analysis was conducted using DnaSP v6.10 [48], with a step size of 200 bp and a window length of 600 bp. We performed additional nucleotide diversity calculations for specific regions, including fragments previously utilized in the phylogenetic analysis of Oenantheae, the protein-coding genes (PCGs), their coding sequences (CDSs), and introns.
Positively selected analyses
To understand the process of evolution of chloroplast protein-coding genes, we calculated the non-synonymous (Ka) and synonymous mutations (Ks) of the CDSs. The ratios between non-synonymous and synonymous mutations (ω = Ka/Ks) can reveal the direction and strength of nature selection acting on the protein. Values of ω < 1, = 1, > 1 indicate negative purifying selection, neutral evolution, and positive selection, respectively [49]. A total of 80 CDSs were extracted from 30 chloroplast genomes of Oenantheae taxa, and the protein-coding sequence alignments were generated using Translation Align under the MAFFT program with stop codons removed accordingly. The petN and psbN genes were excluded due to their identical nucleotide sequences among taxa. Thus, only 78 CDSs were included in the later analysis. The DnaSP v6.10 was subsequently utilized to calculate the Ka and Ks.
We tested positive selection on site (codon) based models. Five (petN, psbL, psbN, rps7, and ycf3) were excluded from the testing due to having identical amino acid sequences. A total of 75 CDSs were employed to test positive selection. Firstly, 75 aligned CDSs were manually concatenated into a supermatrix in MEGA v7 [50] and subsequently used to infer an unrooted ML tree under RAxML v8.2.12 [51]. The unrooted ML tree and each CDS alignment served as input files in EasyCodeML analysis [52]. Site models in CodeML [53–55] executed in EasyCodeML were utilized to detect positively selected sites of PCGs in Oenantheae chloroplast genomes. These models treat the ω ratio for any site (codon) in the gene as a random variable from a statistical distribution, thus allowing ω to vary among codons [53, 54, 56]. Two pairs of particularly effective site models were adopted in our study: M1a (Nearly Neutral) vs. M2a (Positive Selection) and M7 (beta) vs. M8 (beta&ω). For these two tests, M1a and M7 are null models that do not allow for any sites with ω > 1, and the M2a and M8 are alternative models that allow some sites with ω > 1. These tests were widely used for positive selection [32, 57–62]. The presence of some sites at which ω > 1 was used to define positive selection [56]. Likelihood ratio tests (LRTs) [54] were performed to compare the null models against the alternative models. The P-values were calculated to measure the significance level of positive selection. If the LRT favors M2a or M8 (P < 0.05), we will identify sites in the genes that are under positive selection [56]. The Bayesian Empirical Bayes (BEB) method [57] was adopted and performed for the identification. A gene with LRT P < 0.05 and positively selected sites was considered a positively selected gene.
Phylogenetic reconstruction
A total of 32 taxa were sampled for phylogenetic analysis, including five genera from Oenantheae and two species from Scandiceae (Apioideae) as outgroups. The outgroups [Anthriscus sylvestris (L.) Hoffm., and Torilis scabra (Thunb.) DC.] were selected according to Wen et al., 2021, the NCBI accession numbers were MT561042 and MT561029, respectively. The 30 Oenantheae taxa contain 11 taxa from each genus Sium and Oenanthe, four taxa from Cryptotaenia species, three taxa from Cicuta, and one taxon from Tiedemannia. The five genera cover three lineages of Oenantheae (except for the Perideridia clade): including the sium alliance (Sium and Cryptotaenia), Cicuta & Oenanthe group, and Nouth American endemics clade (Tiedemannia). Eight datasets were adopted for reconstructing the phylogenetic tree. Five intergenic regions, including three small fragments previously used [1), psbI–trnK, 2), rps16–trnK, and 3), trnQ–trnK] [11, 12, 14, 18], 4), the noncoding region of the total genome, and 5), one dataset concatenated nine introns extracted from the corresponding PCGs (excluded introns of the rpl2 and ndhB genes due to the very small Pi). Two protein-coding sequences (CDSs) matrixes: 6), one dataset concatenated eight CDSs with high nucleotide diversity value, and 7), another concatenated 78 CDS from the whole genome. 8), The entire genome matrix was also used for analyses.
Two methods were employed to conduct phylogenetic analysis: maximum likelihood (ML) and Bayesian inference (BI). The ML analysis was performed by RAxML v8.2.4 [51], under the GTRGAMMA model as suggested in the manual. Rapid bootstrap analysis was implemented using 1000 bootstrap replicates to search for the best-scoring ML tree. MrBayes v3.2.7a [63] was employed to conduct the BI analysis under the best-fit model GTR + I + G recommended by jModelTest v2.1.4 [64]. Two independent Markov chain Monte Carlo (MCMC) runs were performed, each with three heated chains and one cold chain for 10,000,000 generations. The average standard deviation of split frequencies should approach zero, otherwise generations will be added to continue the analysis. Each run started with a random tree, sampling trees every 1000 generations, with the initial 25% discarded as burn-in. The bootstrap support (BS) and posterior probability (PP) were used to measure the supports of the phylogenetic tree implemented under ML and BI methods, respectively. The final tree was viewed in FigTree v1.4 [65].
Results and discussion
Gene characteristic diversity in Oenantheae chloroplast genomes
Among the seventeen newly sequenced plastid genomes, seven species were first reported. The size of Oenantheae chloroplast genomes ranged from 153,024 bp [Sium ventricosum (H.Boissieu) Li S.Wang & M.F.Watson, NC_070095] to 155,006 bp (Sium ninsi L.) (Table 1). Chloroplast genomes of Oenantheae species shared a typical quadripartite structure, which was consistent with that of most other Apioideae species [6, 29, 30, 36, 42], possessing two copies of IR regions (IRA and IRB, 24,970–26,510 bp) separated by the LSC region (83,451–85,036 bp) and SSC region (16,931–18,790 bp). The total GC content ranged from 37.30 to 37.60% roughly comparable to that in some other chloroplast genomes of Apioideae distal clades species [6, 66–70]. The IR region held the highest GC content (42.6–43.1%) when compared to LSC (35.4–35.8%) and SSC (30.4–31.3%) (Table 1, Fig. S1). The North American endemics Tiedemannia filiformis subsp. Greenmannii (Mathias & Constance) Feist & S.R.Downie showed the lowest GC content among the 30 Oenantheae chloroplast genomes in all regions (the total genome, LSC, SSC, IR regions) (Table 1). For the remaining species, GC contents of the total genome, LSC, and SSC were highest in the Oenanthe species, and lowest in the Sium species. The GC contents of IR regions were basically the same among species except S. ventricosum, which possessed a unique extra high GC content of 43.1%. We hypothesized that it might be related to the high-altitude habitat of this species. We reviewed the chloroplast genomes of Apioideae species, the GC content of IR regions of some high-altitude plants (e.g. Pleurospermum s. l.) did have high GC values (42.7–45.4%) [24], while others (e.g. Tongoloa H. Wolff) did not (40.5–42.4%) [37]. Therefore, the higher GC content may be related to the more complex evolutionary history of this species, which needs to be further studied.
Each of the 30 chloroplast genomes contained 133 genes, consisting of 86 PCGs, eight rRNA genes, 37 tRNA genes, and two pseudogenes. A total of 19 genes have been detected duplicated, including seven protein-coding genes (rpl2, rpl23, ycf2, ndhB, rps7, rps12, and ycf1), four rRNA genes (rrn4.5, rrn5, rrn16, and rrn23), seven tRNA genes (trnA-UGC, trnI-GAU, trnI-CAU, trnL-CAA, trnN-GUU, trnR-ACG, and trnV-GAC), and the pseudogene ycf15. Fifteen genes were owning some introns: 11 genes (atpF, ndhA, rpoC1, petB, petD, rpl16, rps16, and two copies of ndhB and rpl2) contained one intron, four genes (clpP, ycf3, and two copies of rps12) contained two introns. No significant expansion or contraction has been detected in Oenantheae chloroplast genomes (Table S2), so did the rearrangement and inversion events (Fig. S2). Each of the LSC/IRA junctions (JLA) and SSC/IRA junctions (JSA) in the Oenantheae chloroplast genomes were at the same positions. The JLA was located between the rpl2 and trnH-GUG genes, and the JSA was at the ycf1a gene (the copy of the ycf1 gene was largely located in the IRA region). The junction of LSC/IRB (JLB) was located at the rps19 gene, except for Cicuta virosa L. (no. LHM1252, intergenic region of rps19 and rpl2), which was consistent with that mentioned in Wen et al., 2021. The junction of SSC/IRB (JSB) was located at the ycf1b gene (the copy of the ycf1 gene was largely located in the IRB region) or the overlap region of ycf1b and ndhF.
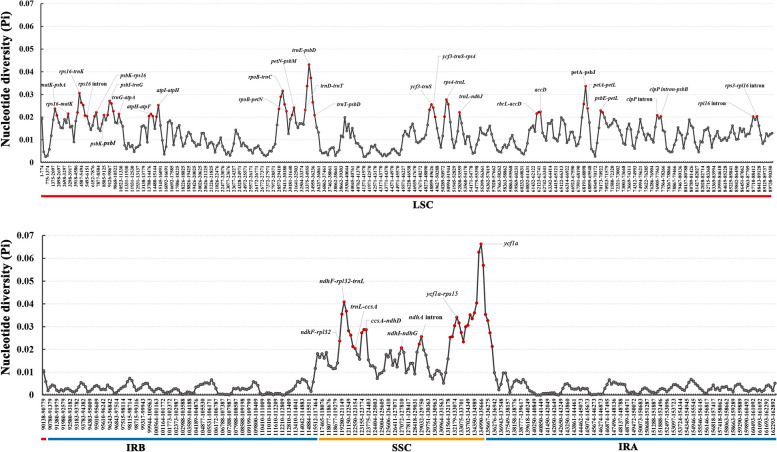
Mutation hotspot regions among species of Oenantheae
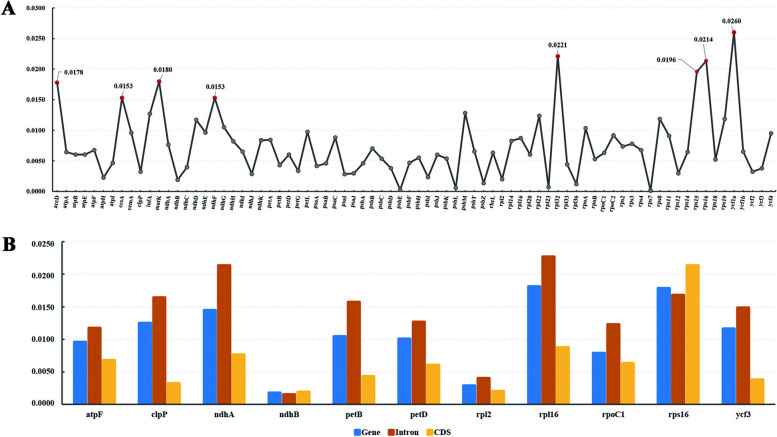
Gene sequences with high nucleotide diversity (Pi) were suggested to be mutation hotspot regions. The sliding window analyses across the 30 Oenantheae chloroplast genomes showed that the Pi ranged from 0.0000 to 0.0663 (Fig. 2, Table S3). Overall, 188 sequences were owning Pi > 0.015. Among them 89 sequences with Pi > 0.02 (highlighted with red dots in Figs. 2), 22 sequences with Pi > 0.03, and six sequences with Pi > 0.04 (Table S3). The results showed that the sequences with Pi > 0.02 were all located in LSC and SSC, and sequences in IR regions generally had significantly lower Pi (Fig. 2). The sequences with high Pi were identified. Sequences with Pi > 0.02 were located at 30 regions, including matK–psbA, rps16–matK, rps16 intron–trnK, rps16 intron, rps16 intron–psbK, psbK–psbI, psbI–trnS–trnG, trnG–atpA, atpH–atpF intron, atpI–atpH, rpoB–trnC–petN, petN–psbM, trnD–trnY–trnE–trnT–psbD, ycf3–trnS–rps4, rps4–trnT–trnL, trnL–trnF–ndhJ, rbcL–accD, accD, petA–psbJ–psbL, psbE–petL, clpP intron, clpP intron–psbB, rpl16 intron, rps3–rpl16 intron, ndhF–rpl32–trnL–ccsA, ccsA–ndhD, ndhI–ndhG, ndhA intron, ycf1a–rps15–ndhH, ycf1a gene. Among these regions, 14 were formerly used or identified to reconstruct the phylogeny of Apiaceae groups [2, 42, 71–77]. Our study provides more alternative molecular markers for the phylogenetic studies of Apiaceae groups (especially for Apioideae major clades). Sequences with 0.03 < Pi < 0.04 were identified at seven regions, including ycf1a gene, trnE–trnT–psbD, ndhF–rpl32–trnL, rpoB–trnC, ycf1a–rps15, petA–psbJ, and rps16 intron–trnK regions. Among these regions, the ycf1a gene, trnE–trnT–psbD, ndhF–rpl32–trnL regions also contained the sequences with Pi above 0.04 (Table S3, Fig. 2). Sequences with Pi > 0.02 were mostly distributed in intergenic regions or introns of genes, with only three CDSs of the PCGs (rpl32, rps16, and ycf1a) owning Pi > 0.02 (Table S3, S4). In addition to the three, there were a few PCGs (accD, ccsA, matK, ndhF, and rps15) with Pi greater than 0.015 in their CDS regions. The remaining PCGs possessed generally low Pi (Table S4, Fig. 3A).
Fig. 2.
The nucleotide diversity (Pi) of 30 Oenantheae chloroplast genomes. Regions with high Pi (above 0.02) were marked out
Fig. 3.
The nucleotide diversity (Pi) of sequences. A, Pi of 78 protein-coding sequences (CDSs), sequences with Pi above 0.015 were marked out. B, Pi of 11 protein-coding genes, their CDSs, and introns
We calculated the Pi of genes, introns, and coding sequences (CDS) of the 11 genes that had introns (except the rps12 gene). Compared with the genes and coding regions, the introns each possessed a high Pi, except for the case of rps16 and ndhB. The Pi of these two genes are displayed as CDS > Gene > Intron (Fig. 3B). Overall, introns of five genes (including rpl16, ndhA, rps16, clpP, and petB) possessed Pi > 0.015, among them rpl16 (0.0227) and ndhA (0.0213) with Pi above 0.02 (Table S5). The Pi of the 11 CDSs were very low, except for the rps16 CDS (0.0215). The rps16 intron has been used to infer the phylogeny and evolution of Apiaceae, the subfamily, and their major clades for a long time [2, 71–75, 77]. Our study found that both the CDS and intron of the rps16 gene possessed Pi > 0.015. The Pi of other three small fragments previously used (psbI–trnK, rps16–trnK, and trnQ–trnK) in phylogenetic analyses of the Oenantheae group [11, 12, 14, 18] were also calculated with values above 0.02 (Table S6). Based on previous studies, all these fragments (rps16 intron, psbI–trnK, rps16–trnK, and trnQ–trnK) provided a certain resolution in the phylogenetic analysis of Oenantheae, but could not completely resolve all relationships within the tribe. Thus, we proposed that sequence regions with Pi greater than 0.03 (ycf1a gene, trnE–trnT–psbD, ndhF–rpl32–trnL, rpoB–trnC, ycf1a–rps15, petA–psbJ, and rps16 intron–trnK regions) can be considered as candidate barcodes for the phylogenetic analyses. We extracted the seven regions with Pi > 0.03 and aligned. All sequences are free of alignment ambiguities. A comparison of the number of variable sites, parsimony-informative positions, and the percent of parsimony-informative across these regions and the four previously used fragments have revealed that regions with Pi > 0.03 were identified as candidate DNA markers (Table S6).
Phylogenetic relationships among Oenantheae species inferred from multiple datasets
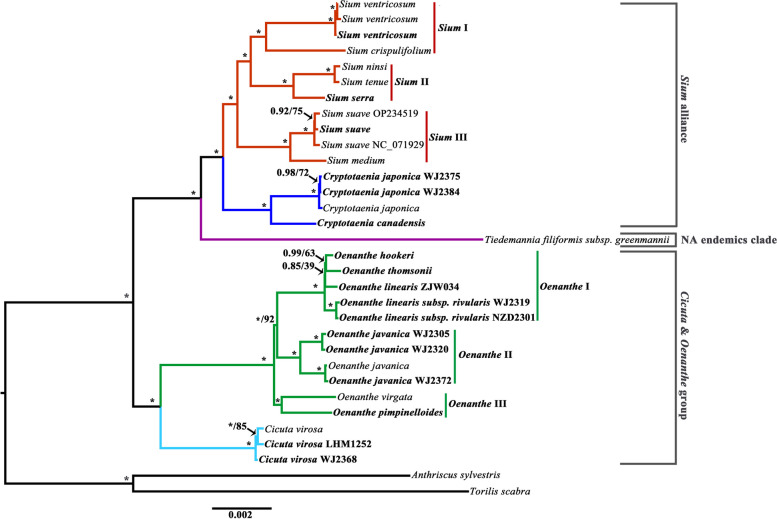
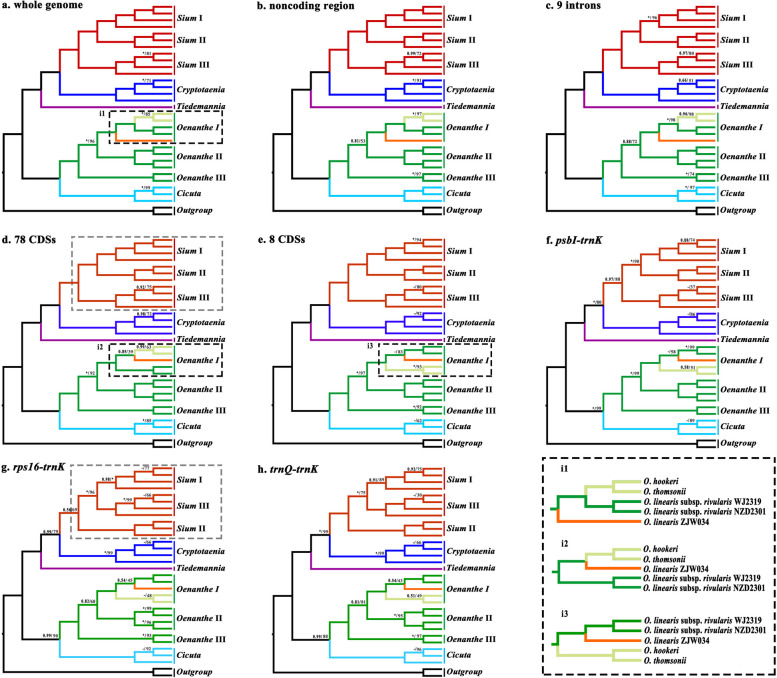
The robust phylogenetic trees were reconstructed from eight concatenated matrices, which exhibited clear relationships among genera within the tribe Oenantheae (Figs. 4 and 5). Our phylogenetic results strongly supported (BS = 100, PP = 1.00) that Oenantheae was divided into three clades: the Cicuta & Oenanthe group, the Sium alliance, and the NA endemics clade. The latter two clades formed the closest sister group (Figs. 4 and 5). This was basically consistent with previous studies [11, 12, 14]. The Sium alliance clade includes two genera, the Sium and the Cryptotaenia. Within the Cryptotaenia, the populations of Cryptotaenia japonica Hassk. gathered a branch, and subsequently formed a sister group to the Cryptotaenia canadensis (L.) DC. The Sium genus here was highly supported to be split into three branches: Sium I, Sium II, and Sium III. Two high-altitude species [Sium crispulifolium (H.Boissieu) Jing Zhou and S. ventricosum] clustered together to form the Sium I clade. Three species [S. ninsi, Sium tenue (Kom.) Kom., and Sium serra (Franch. & Sav.) Kitag.] gathered Sium II. The Sium III consist of Sium suave Walter and Sium medium Fisch. & C.A.Mey. Among these three branches, Sium I and Sium II were supported to be the closest sister (Figs. 4 and 5a–f), gathering the southern Palearctic clade. This topology structure was consistent with the previous studies that combined nrDNA ITS and cpDNA fragments (rps16–trnK) with improved support [12, 14]. Although an alternative result [((Sium I, Sium III), Sium II)] was proved in the phylogenetic analyses inferred from rps16–trnK and trnQ–trnK, this topology was moderately supported at the key nodes (Fig. 5g–h).
Fig. 4.
Phylogenetic relationships inferred from 32 species based on 78 shared CDSs. Support values marked above the branches follow the order Bayesian inference (PP, posterior probability)/maximum likelihood (BS, bootstrap support), * represent the best support (100%). Species names in bold indicate that these species are newly sequenced
Fig. 5.
Comparison of eight phylogenetic trees. Phylogenetic tree each inferred from a, the whole genome matrix; b, the noncoding region concatenated matrix; c, 9 introns concatenated matrix; d, 78 CDSs concatenated matrix; e, eight CDSs concatenated matrix; f, psbI–trnK; g, rps16–trnK; h, trnQ–trnK. Support values marked above the branches follow the order Bayesian inference (PP, posterior probability)/maximum likelihood (BS, bootstrap support), * represent the best support (100%), - represent PP values < 50%. In the black dotted box at the bottom right are the three topologies of the Oenanthe I clade
For the Cicuta & Oenanthe group, the taxa of Cicuta and Oenanthe each formed a nature branch. The genus Oenanthe was strongly supported to be divided into three branches. The relationships among these branches were incontrovertible with Oenanthe I and Oenanthe II forming the closest sister group with high supports inferred from the whole genome, 78 CDSs, 8 CDSs, and psbI–trnK (Figs. 4 and 5). Although the results inferred from the remaining four datasets have only moderate support, the topology is consistent. Two tuberous geophyte species [Oenanthe pimpinelloides L. and Oenanthe virgata Poir.] were gathered together to form Oenanthe III. The Oenanthe II consisted of populations of Oenanthe javanica (Blume) DC. and divided into two branches. The populations of high-altitude and low-altitude clustered separately (Table S1, Fig. 4). The Oenanthe I contained four taxa with narrow leaf blades, two alpine taxa (Oenanthe hookeri C.B.Clarke and Oenanthe thomsonii C.B.Clarke), Oenanthe linearis Wall. ex DC., and Oenanthe linearis subsp. rivularis (Dunn) C.Y.Wu & F.T.Pu. The interspecific relationships within Oenanthe I were ambiguous due to the extremely short differentiated branches of the Oenanthe I clade and the depress supports within the clade. Three topologies have been inferred from the eight datasets. Two populations of O. linearis subsp. rivularis formed a stable branch within Oenanthe I. The first topology (i1) reconstructed based on the whole genome, non-coding region, and 9 introns was very stable with the alpine branch well supported to form the closest sister group with O. linearis subsp. rivularis (Fig. 5a–c). The phylogenetic tree inferred from 78 CDSs has a unique topology (i2) of the Oenanthe I: the alpine branch and O. linearis were the closest sister groups with very lower supports (BS = 39, PP = 0.85). The remaining datasets shared the third topology (i3), the O. linearis and its subspecies clustered together, but the supports were not high (Fig. 5e–h). The interspecific relationships of the genus have been unresolved for a long time, and phylogenetic relationships based on small fragments (nrDNA ITS, cpDNA rps16–trnK) have often received poor support in previous studies [1, 11, 12, 14]. Our study provides a basis for resolving the phylogenetic relationships within the genus, to better solve the problem within Oenanthe I, we recommend obtaining rich nuclear genes for comprehensive analyses. Populations of the S. suave, C. japonica, and C. virosa were divided with very short branch lengths, with moderate node supports in the four large datasets (whole genome, noncoding region, 9 introns, and 78 CDSs) and very low supports in the four small datasets (Fig. 5).
Our results provided stable phylogenetic relationships among species of Oenantheae except the Oenanthe I clade. Molecular data with sufficient non-coding sequences appear to be effective in resolving interspecific relationships within the Oenanthe I clade (Fig. 5a–c). However, some additional data may need to be adopted into the study to clarify the relationships between species within the Oenanthe I clade. At present, many molecular data have been proposed to resolve generic-level phylogenies, such as single-copy nuclear genes (SCG), low-copies nuclear genes, and SNPs extracted from transcriptome or genome [78–82]. Despite the discordance between nuclear-based and plastid-based phylogenetic trees, sufficiently stable topologies can support a more comprehensive interpretation of taxa evolution [6, 83–85]. The previous phylogenetic analyses of the tribe Oenantheae rarely involved taxa distributed in China [11, 12, 14]. Our research contributes in part to the replenishment of Oenantheae’s global resources.
Positively selected gene responses to the adaptive evolution of Oenantheae
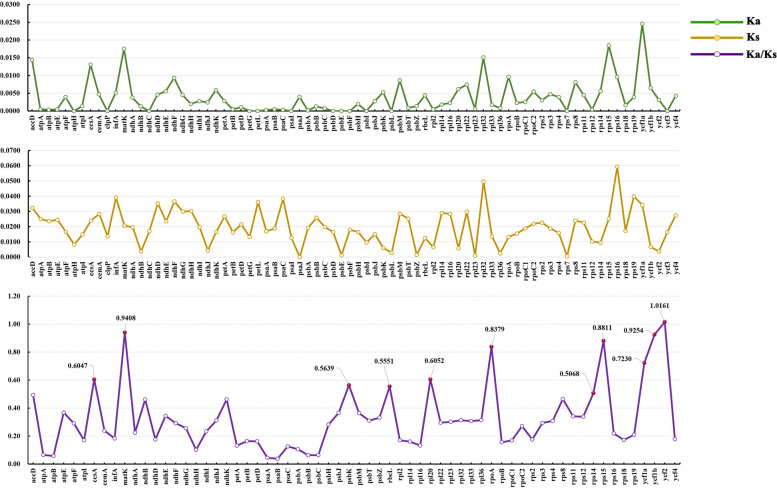
We calculated the Ka, Ks, and their ratio Ka/Ks (ω) to evaluate the selection pressure of the 78 PCGs (Table S4). The results showed that the Ka values ranged from 0 to 0.0246 and the Ks values ranged from 0 to 0.0594. Thirteen PCGs (atpH, clpP, ndhC, petG, petL, psaI, psbD, psbE, psbF, psbI, psbL, rps7, and ycf3) have Ka = 0, which indicated these PCGs without any non-synonymous mutations. Only one PCG (psaJ) with Ks = 0, which suggested this gene has non-synonymous mutations but no synonymous mutations. The Ka/Ks (ω) value of psaJ was not calculated because Ks = 0 and the ω value tended to infinity. The ω values for the remaining PCGs ranged from 0 to 1.0161 (Table S4). A total of 12 PCGs were identified with ω values above 0.5 (ccsA, matK, psbK, psaJ, rbcL, rpl20, rpoA, rps14, rps15, ycf1a, ycf1b, and ycf2) (Fig. 6). Among these genes, psaJ and ycf2 possessed ω values greater than 1. The results suggested ten PCGs might be under relaxed selection with 0.5 < ω < 1 and two genes have undergone strong positive selection.
Fig. 6.
The average Ka, Ks, and Ka/Ks values curves of the protein-coding sequences (CDS). CDSs with Ka/Ks > 0.5 were marked out with red circles in the Ka/Ks curve
To detect sites under positive selection in each PCG, we performed tests by comparing null models (M1a and M7) against alternative models (M2a and M8). We have calculated several parameters to estimate the positive selection, including the LRT P-values of the compared models (M1a vs. M2a, M7 vs. M8), the detailed log-likelihood values and free parameters of M7 and M8, and the positively selected sites calculated under BEB method (Table S7). The calculation of LRT P-values showed that eight PCGs (accD, rbcL, rps8, ycf1a, ycf1b, ycf2, ndhF, and ndhK) were significant (P < 0.05) for both M1a-M2a and M7-M8, two PCGs (ndhH and rps11) were not significant (P > 0.05) for the M1a-M2a comparison but significant under the M7-M8. Based on BEB analysis, sites had probability > 50% for the positive-selection class with ω > 1 were detected in 44 PCGs (Table S7). Among these genes, 14 PCGs (rpoC2, rpl20, psbT, psaJ, and the ten PCGs significant under M7-M8 mentioned above) have some positively selected sites with a probability > 95% (Table 2). The LRT of the four PCGs (rpoC2, rpl20, psbT, and psaJ) were not significant (P > 0.05) for both comparisons although their positively selected sites probability greater than 95%. Sites that had probabilities > 99% were presented in seven PCGs (rbcL, rps8, ycf1a, ycf1b, ycf2, ndhF, and rps11).
Table 2.
Positive selected sites identified in the chloroplast genomes of Oenantheae
| Gene name | LRT P-value | Positively selected sites (BEB, *: PP > 95%; **: PP > 99%) | sites number | |
|---|---|---|---|---|
| M7 (beta) vs. M8 (beta&ω) | M1a (nearly neutral) vs. M2a (positive selection) | |||
| accD | 0.000004515 | 0.000000015 | 100 H 0.983*,207 K 0.954* | 2 |
| rbcL | 0.000000000 | 0.000000000 | 28 D 0.974*,91 A 1.000**,95 N 0.997**,97 Y 0.998**,225 I 0.997**,309 I 0.998**,328 S 1.000**,354 I 0.956*,355 A 0.972*,443 E 0.998** | 10 |
| rps8 | 0.000000000 | 0.000000000 | 57 D 1.000** | 1 |
| ycf1a | 0.000000000 | 0.000000000 | 469 R 0.960*, 795 V 0.982*,1014 K 0.971*,1085 I 0.969*,1092 K 1.000**,1093 I 0.965*,1094 L 0.986*,1111 Y 0.962* | 8 |
| ycf2 | 0.000000000 | 0.000000000 | 103 F 1.000**,503 Q 0.996**,746 L 0.996**,833 R 0.955*,915 P 0.950*,1025 Q 0.951* | 6 |
| ycf1b | 0.000000888 | 0.000000131 | 200 S 0.976*,205 K 0.994**,206 Y 0.980*,215 R 0.975*,217 S 0.975*,244 N 0.971*,270 K 1.000** | 7 |
| ndhF | 0.000004851 | 0.000065086 | 465 Q 0.988*,655 N 0.952*,663 C 1.000** | 3 |
| ndhH | 0.003524280 | 0.082768146 | 198 I 0.960* | 1 |
| ndhK | 0.011201551 | 0.022716790 | 181 F 0.964*,218 K 0.957* | 2 |
| rps11 | 0.012214628 | 0.054106065 | 14 G 0.993** | 1 |
| rpoC2 | 0.082830660 | 0.347241769 | 239 I 0.969* | 1 |
| rpl20 | 0.096456032 | 0.117651549 | 12 R 0.964* | 1 |
| psbT | 0.225892513 | 0.225960517 | 33 K 0.972* | 1 |
| psaJ | 0.422979316 | 0.423014425 | 10 V 0.977*,14 L 0.977*,36 A 0.977* | 3 |
Our Ka and Ks calculation results have suggested ten PCGs (ccsA, matK, psbK, rbcL, rpl20, rpoA, rps14, rps15, ycf1a, and ycf1b) under relaxed selection and two (psaJ and ycf2) undergone strong positive selection. Among these PCGs, four (rbcL, ycf1a, ycf1b, and ycf2) have detected significant positive selection in some sites. The LRTs of these PCGs were significant at a 1% level under both M7-M8 and M1a-M2a tests. Also, the BEB calculation provided strong evidence for sites under positive selection as the posterior probabilities for ω > 1 were high (Table 2). Together, these results suggested strong evidence for sites under positive selection in these four PCGs (rbcL, ycf1a, ycf1b, and ycf2). The BEB calculation also provided some evidence for sites under positive selection in PCGs rpl20 and psaJ as the posterior probabilities for ω > 1 were high (probability > 0.95). However, the LRTs of these two PCGs were not significant (P > 0.05) for both M7-M8 and M1a-M2a comparisons (Table 2). These situations also existed in the rpoC2 and psbT genes. These results suggested some weak evidence for sites under positive selection in the four PCGs (rpl20, psaJ, rpoC2, and psbT). For the remaining six PCGs (ccsA, matK, psbK, rpoA, rps14, and rps15), the LRT P-values were above 0.05 for both two comparisons, and the BEB calculation only provided weak evidence for sites under positive selection as the posterior probability for ω > 1 was low (< 95%) and the posterior distribution for ω was diffuse at every site (Table S7). All these suggested that these six PCGs might be under weak positive selection. Site models have detected extra four PCGs (accD, rps8, ndhF, and ndhK) possessing significant positively selected sites. Both the LRT and BEB calculations have provided strong evidence. The tests for sites under positive selection in the ndhH and rps11 genes were equivocal with the M1a-M2a and M7-M8 comparison giving conflicting results. We tend to accept the test of M1a-M2a because this test is noted to be more stringent when the evidence for positive selection exists but is not very strong [56, 86]. These results together suggested some evidence for sites under positive selection in the ndhH and rps11 genes, although this evidence was not very strong.
Tribe Oenantheae has a high proportion of bog and water species relative to terrestrial members. The occupation of the aquatic environments indicates that Oenantheae species have adapted to conditions of damp, marshy, or truly aquatic habitats. Compared with terrestrial species, emergent aquatic plants occupy stressful ecological habitats characterized by low light, and reduced carbon and oxygen availability [87]. Survival in aquatic habitats has resulted in adaptations specific to low oxygen concentrations. Plants of the tribe Oenantheae exhibit various adaptations to these habitats, such as clusters of fibrous or tuberous roots and corky-thickened fruits [7, 11]. Here possible evidence of positive selection in chloroplast coding genes was detected to reveal the adaptation of Oenantheae species to aquatic environments at the molecular level. Based on our results, a total of eight genes (accD, rbcL, rps8, ycf1a, ycf1b, ycf2, ndhF, and ndhK) are considered positive selection genes. Gene accD encodes the beta carboxyl transferase subunit of ACCase and has been verified to affect several biological processes such as storage compound metabolism, leaf and seed development [88–91]. The two significantly positively selected sites we detected in the accD gene varied mainly between the Cicuta & Oenanthe group and the other species. We identified these genes as possibly associated with the unique traits Cicuta & Oenanthe group owned particularly in leaf and seed development. We have found statistically that the leaf and fruit morphology of Oenanthe and Cicuta are different from other groups. The leaf blades of these two genera species always have one or more pinnates. The fruits of them are overall ovoid with thick and corky fruit ribs, with vittae solitary in each furrow and 2 on commissure [7, 18, 92–95]. The chloroplast ribosomal proteins act as important constituents of protein synthesis machinery and participate in various processes of plant growth, and development as well as reaction to unfavorable conditions [96–99]. Adaptive evolution of the rps8 gene may be helpful for the normal growth and development of Oenantheae species in aquatic environments. The remaining six positive selection genes are functionally associated with photosynthesis. The rbcL gene encodes the Rubisco large subunit, which catalyzes the assimilation of atmospheric CO2 during photosynthesis [100–102]. Positive selection of this gene has been detected in some plants and suggested adaptation to low CO2 concentration, shade-tolerant, and aquatic environments [32, 60, 103, 104], but not prevalent in the Apiaceae. We detected ten positive selection sites in the rbcL gene, the increased amino acid replacement may reflect the continuous fine-tuning of Rubisco under varying species’ ecological conditions. The ndh genes (ndhF and ndhK) encode NADH-dehydrogenase subunits of the Ndh1-complex bound to the thylakoid membrane, which were fundamental to the electron transport chain for the generation of ATP, and photosynthesis of plants [105–108]. Positive selection of these two ndh genes may reflect the adaptation to aquatic environments stress, such as low light and reduced oxygen availability. Positive selections of these two genes in Apiaceae have been detected commonly [24, 36, 109]. Essential genes ycf1 and ycf2 in plant chloroplast genomes encode proteins that are indispensable for cell survival and photosynthesis [110–112]. Overall, positive selection of the eight chloroplasts protein-coding genes (accD, rbcL, rps8, ycf1a, ycf1b, ycf2, ndhF, and ndhK) may be responses to aquatic environment adaptation of Oenantheae species. There may be partial positive selection in six genes that with positive selection sites (posterior probability above 95%), including rpl20, psaJ, rpoC2, psbT, ndhH, and rps11, but it is not statistically significant. Nevertheless, we suggest that these genes still require attention because they may be potential positive selection genes.
Conclusion
In conclusion, we generated the complete chloroplast genomes of 17 Oenantheae species. A deep comparative analysis of 30 Oenantheae chloroplast genomes has been conducted to create an accurate map of the genomic structure of hydrophytic umbellifers. A total of 30 regions (matK–psbA, rps16–matK, rps16 intron–trnK, rps16 intron, rps16 intron–psbK, psbK–psbI, psbI–trnS–trnG, trnG–atpA, atpH–atpF intron, atpI–atpH, rpoB–trnC–petN, petN–psbM, trnD–trnY–trnE–trnT–psbD, ycf3–trnS–rps4, rps4–trnT–trnL, trnL–trnF–ndhJ, rbcL–accD, accD, petA–psbJ–psbL, psbE–petL, clpP intron, clpP intron–psbB, rpl16 intron, rps3–rpl16 intron, ndhF–rpl32–trnL–ccsA, ccsA–ndhD, ndhI–ndhG, ndhA intron, ycf1a–rps15–ndhH, and ycf1a gene) have been identified with high nucleotide diversity (Pi > 0.02), which suggested to be mutation hotspot regions among Oenantheae species. Among these, the ycf1a gene, trnE–trnT–psbD, ndhF–rpl32–trnL, rpoB–trnC, ycf1a–rps15, petA–psbJ, and rps16 intron–trnK regions were considered as candidate barcodes for the phylogenetic analyses of Oenantheae due to well aligned and rich informative sites. Eight genes (accD, rbcL, rps8, ycf1a, ycf1b, ycf2, ndhF, and ndhK) with strong signatures of positive selection were convincingly detected. Two (accD and rps8) are related to plant growth and development, and the remaining are mainly related to the photosynthesis system. Such a comparative genomic analysis of the Oenantheae chloroplast genomes offers us an unprecedented opportunity to reveal footprints of the adaptive evolution of chloroplast genes that make Oenantheae species adapt to aquatic environments. Our phylogenetic analyses also recovered reliable relationships among species of Oenantheae except within the Oenanthe I clade. Phylogeny and evolution of the genus Oenanthe may be further clarified by incorporating more nuclear genes in further studies. In addition, expanding species sampling of North American endemic species will contribute to a deeper understanding of the molecular adaptive evolution of Oenantheae. Our research only serves as an introduction, and we urge other researchers to join us in the future.
Supplementary Information
Acknowledgements
We are grateful to the China National Botanical Garden in Beijing and the Yaoluoping Nature Reserve in Anhui Province for allowing us to collect plant material. We would like to thank Zhu Xin-Xin from Xinyang Normal University for providing information on the distribution of some materials.
Author contributions
Conceptualization, WJ and SCF; validation, WBC, LHM, ZW, YJX; interpretation of data, ZJW and MXD; analysis, WJ and YJX; writing—original draft preparation, WJ; writing—review and editing, SCF and WJ; funding acquisition, WJ. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant no. 32200191), the Foundation of Jiangsu Key Laboratory for the Research and Utilization of Plant Resources (JSPKLB202214).
Data availability
The datasets generated and analyzed during the current study are available in the NCBI (https://www.ncbi.nlm.nih.gov) repository. The raw reads have been submitted to GenBank at NCBI BioProject PRJNA1154308, with SRA numbers SRR30524793–SRR30524809. The assembly and annotated chloroplast genomes were deposited in NCBI with accession numbers PQ283862–PQ283878. Voucher specimens were identified by Jun Wen and deposited in NAS (Herbarium, Institute of Botany, Chinese Academy of Sciences, Jiangsu Province) with deposition numbers (NAS00640404– NAS00640420, Fig. S3).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Banasiak Ł, Piwczyński M, Uliński T, Downie SR, Watson MF, Shakya B, et al. Dispersal patterns in space and time: a case study of Apiaceae subfamily Apioideae. J Biogeogr. 2013;40:1324–35. [Google Scholar]
- 2.Zhou J, Gong X, Downie SR, Peng H. Towards a more robust molecular phylogeny of Chinese Apiaceae subfamily Apioideae: additional evidence from nrDNA ITS and cpDNA intron (rpl16 and rps16) sequences. Mol Phylogenet Evol. 2009;53:56–68. [DOI] [PubMed] [Google Scholar]
- 3.Clarkson JJ, Zuntini AR, Maurin O, Downie SR, Plunkett GM, Nicolas AN, et al. A higher-level nuclear phylogenomic study of the carrot family (Apiaceae). Am J Bot. 2021;108:1–18. [DOI] [PubMed] [Google Scholar]
- 4.Downie SR, Katz-Downie DS, Watson MF. A phylogeny of the flowering plant family Apiaceae based on chloroplast DNA rpl16 and rpoC1 intron sequences: towards a suprageneric classification of subfamily Apioideae. Am J Bot. 2000;87:273–92. [PubMed] [Google Scholar]
- 5.Downie SR, Spalik K, Katz-Downie DS, Reduron JP. Major clades within Apiaceae subfamily Apioideae as inferred by phylogenetic analysis of nrDNA ITS sequences. Plant Div Evol. 2010;128:111–36. [Google Scholar]
- 6.Wen J, Xie DF, Price M, Ren T, Deng YQ, Gui LJ, et al. Backbone phylogeny and evolution of Apioideae (Apiaceae): new insights from phylogenomic analyses of plastome data. Mol Phylogenet Evol. 2021;161: 107183. [DOI] [PubMed] [Google Scholar]
- 7.Wen J, Yu Y, Xie DF, Peng C, Liu Q, Zhou SD, et al. A transcriptome-based study on the phylogeny and evolution for taxonomic controversial subfamily Apioideae (Apiaceae). Ann Bot-London. 2020;125:937–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu P, Sheh ML. Pollen photographs and flora of umbelliferae in China. Shanghai: Shanghai Scientific and Technical Publishers; 2001.
- 9.Xi YZ, Sun XJ. Pollen morphology of Umbelliferae in China and its evolution. In: The Institute of Botany Academia Sinica, editor. Botanical Research, No. 1. Beijing: Science Press; 1983. p. 57–83.
- 10.Hardway TM, Spalik K, Watson MF, Katz-Downie DS, Downie SR. Circumscription of Apiaceae tribe Oenantheae. S Afr J Bot. 2004;70:393–406. [Google Scholar]
- 11.Downie SR, Katz-Downie DS, Sun FJ, Lee CS. Phylogeny and biogeography of Apiaceae tribe Oenantheae inferred from nuclear rDNA ITS and cpDNA psbI–5’trnK(uuu) sequences, with emphasis on the north American endemics clade. Botany. 2008;86:1039–64. [Google Scholar]
- 12.Spalik K, Banasiak Ł, Feist MAE, Downie SR. Recurrent short-distance dispersal explains wide distributions of hydrophytic umbellifers (Apiaceae tribe Oenantheae). J Biogeogr. 2014;41:1559–71. [Google Scholar]
- 13.Desjardins SD, Leslie AC, Stace CA, Schwarzacher T, Bailey JB. Intergeneric hybridisation between Berula erecta and helosciadium nodiflorum (Apiaceae). Taxon. 2015;64:784–94. [Google Scholar]
- 14.Spalik K, Downie SR, Watson MF. Generic delimitations within the Sium alliance (Apiaceae tribe Oenantheae) inferred from cpDNA rps16-5’trnK(UUU) and nrDNA ITS sequences. Taxon. 2009;58:735–48. [Google Scholar]
- 15.Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. http://www.plantsoftheworldonline.org/. Retrieved 10 Sept 2024.
- 16.Spalik K, Downie SR. The evolutionary history of Sium Sensu Lato (Apiaceae): dispersal, vicariance, and domestication as inferred from ITS rDNA phylogeny. Am J Bot. 2006;93:747–61. [DOI] [PubMed] [Google Scholar]
- 17.Spalik K, Downie SR. Intercontinental disjunctions in Cryptotaenia (Apiaceae, Oenantheae): an appraisal using molecular data. J Biogeogr. 2007;34:2039–54. [Google Scholar]
- 18.Feist MAE, Downie SR, Magee AR, Liu MR. Revised generic delimitations for Oxypolis and Ptilimnium (Apiaceae) based on leaf morphology, comparative fruit anatomy, and phylogenetic analysis of nuclear rDNA ITS and cpDNA trnQ-trnK intergenic spacer sequence data. Taxon. 2012;61:402–18. [Google Scholar]
- 19.Feist MAE, Downie SR. A phylogenetic study of Oxypolis and Ptilimnium based on nuclear rDNA ITS sequences. Syst Bot. 2008;33:447–58. [Google Scholar]
- 20.Bone TS, Downie SR, Affolter JM, Spalik K. A phylogenetic and biogeographic study of the genus Lilaeopsis (Apiaceae tribe Oenantheae). Syst Bot. 2011;36:789–805. [Google Scholar]
- 21.Lee CS, Downie SR. Phylogenetic relationships within Cicuta (Apiaceae tribe Oenantheae) inferred from nuclear rDNA ITS and cpDNA sequence data. Can J Bot. 2006;84:453–68. [Google Scholar]
- 22.Downie SR, Sun FJ, Katz-Downie DS, Colletti GJ. A phylogenetic study of Perideridia (Apiaceae) based on nuclear ribosomal DNA ITS sequences. Syst Bot. 2004;29:737–51. [Google Scholar]
- 23.Zhou BF, Yuan S, Crowl AA, Liang YY, Shi Y, Chen XY, et al. Phylogenomic analyses highlight innovation and introgression in the continental radiations of Fagaceae across the Northern Hemisphere. Nat Commun. 2022;13:1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng C, Guo XL, Zhou SD, He XJ. Backbone phylogeny and adaptive evolution of Pleurospermum s. l.: new insights from phylogenomic analyses of complete plastome data. Front Plant Sci. 2023;14:1148303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma XG, Ren YB, Sun H. Introgression and incomplete lineage sorting blurred phylogenetic relationships across the genomes of sclerophyllous oaks from southwest China. Cladistics. 2024;40:357–73. [DOI] [PubMed] [Google Scholar]
- 26.Li QQ, Khasbagan, Zhang ZP, Wen J, Yu Y. Plastid phylogenomics of the tribe potentilleae (Rosaceae). Mol Phylogenet Evol. 2024;190:107961. [DOI] [PubMed]
- 27.Wang J, Kan S, Liao X, Zhou J, Tembrock LR, Daniell H, et al. Plant organellar genomes: much done, much more to do. Trends Plant Sci. 2024;29:754–69. [DOI] [PubMed] [Google Scholar]
- 28.Zeng ZH, Zhong L, Sun HY, Wu ZK, Wang X, Wang H, et al. Parallel evolution of morphological and genomic selfing syndromes accompany the breakdown of heterostyly. New Phytol. 2024;242:302–16. [DOI] [PubMed] [Google Scholar]
- 29.Liu LJ, Liu CK, Cai J, Deng JJ, He XJ, Zhou SD. The complete plastomes of thirteen Libanotis (Apiaceae, Apioideae) plants: comparative and phylogenetic analyses provide insights into the plastome evolution and taxonomy of Libanotis. BMC Plant Biol. 2024;24:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song BN, Liu CK, Zhao AQ, Tian RM, Xie DF, Xiao YL, et al. Phylogeny and diversification of genus Sanicula L. (Apiaceae): novel insights from plastid phylogenomic analyses. BMC Plant Biol. 2024;24:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Sun Y, Landis JB, Lv Z, Shen J, Zhang H, et al. Plastome phylogenomic study of Gentianeae (Gentianaceae): widespread gene tree discordance and its association with evolutionary rate heterogeneity of plastid genes. BMC Plant Biol. 2020;20:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li QQ, Zhang ZP, Aogan, Wen J. Comparative chloroplast genomes of Argentina species: genome evolution and phylogenomic implications. Front Plant Sci. 2024;15:1349358. [DOI] [PMC free article] [PubMed]
- 33.Wen J, Wu BC, Li HM, Zhou W, Song CF. Plastome structure and phylogenetic relationships of genus Hydrocotyle (Apiales): provide insights into the plastome evolution of Hydrocotyle. BMC Plant Biol. 2024;24:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu CK, Deng JJ, Song BN, Qin HH, Zhou SD, He XJ. Plastid phylogenomics provide evidence to accept a new genus Pseudopeucedanum (Apiaceae) separated from Peucedanum s.l. Bot J Linn Soc. 2023;205:243–52. [Google Scholar]
- 35.Tian R, Aou X, Song B, Li Z, He X, Zhou S. Plastid phylogenomic analyses reveal a cryptic species of Ligusticopsis (Apiaceae, Angiosperms). Int J Mol Sci. 2023;24:7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin HH, Cai J, Liu CK, Zhou RX, Price M, Zhou SD, et al. The plastid genome of twenty-two species from Ferula, Talassia, and Soranthus: comparative analysis, phylogenetic implications, and adaptive evolution. BMC Plant Biol. 2023;23:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gui LJ, Xie DF, Peng C, Ren T, Yu LY, Zhou SD, He XJ. Chloroplast genomes and ribosomal DNA provide insights into divergence and morphological evolution of alpine Tongoloa. J Syst Evol. 2023. 10.1111/jse.13028. [Google Scholar]
- 38.Downie SR, Jansen RK. A comparative analysis of whole plastid genomes from the Apiales: expansion and contraction of the inverted repeat, mitochondrial to plastid transfer of DNA, and identification of highly divergent noncoding regions. Syst Bot. 2015;40:336–51. [Google Scholar]
- 39.Spooner DM, Ruess H, Iorizzo M, Senalik D, Simon P. Entire plastid phylogeny of the carrot genus (Daucus, Apiaceae): concordance with nuclear data and mitochondrial and nuclear DNA insertions to the plastid. Am J Bot. 2017;104:296–312. [DOI] [PubMed] [Google Scholar]
- 40.Luo L, Yu Y. The complete chloroplast genome of Cryptotaenia japonica. Mitochondrial DNA B. 2019;4:1650–1. [Google Scholar]
- 41.Zhang Z, Dong H, Yuan M, Yu Y. The complete chloroplast genome of Oenanthe javanica. Mitochondrial DNA B. 2020;5:3151–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, Niu JM, Wang XY, Yue JR, Zhou SL, Liu ZW. Plastome evolution in the genus Sium (Apiaceae, Oenantheae) inferred from phylogenomic and comparative analyses. BMC Plant Biol. 2023;23:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrews S, Lindenbaum P, Howard B, Ewels P. FastQC: a quality control tool for high throughput sequence data. Cambridge (UK): The Babraham Institute; 2011. [Google Scholar]
- 44.Dierckxsens N, Mardulyn P, Smits G. NOVOPlasty: denovo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017;45:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lohse M, Drechsel O, Kahlau S, Bock R. Organellargenomedraw–a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013;41:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darling ACE, Mau B, Blattner FR, Perna NT, Mauve. Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14:1394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rozas J, Ferrer-Mata A, Sanchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34:3299–302. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Nielsen R. Codon-substitution models for detecting molecular adaptation at individual sites along specific lineages. Mol Biol Evol. 2002;19:908–17. [DOI] [PubMed] [Google Scholar]
- 50.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao FL, Chen CJ, Arab DA, Du ZG, He YH, Ho SYW. EasyCodeML: a visual tool for analysis of selection using CodeML. Ecol Evol. 2019;9:3891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen R, Yang Z. Likelihood models for detecting positively selected amino acid sites and applications to the HIV-1 envelope gene. Genetics. 1998;148:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Z, Nielsen R, Goldman N, Pedersen AMK. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics. 2000;155:431–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–91. [DOI] [PubMed] [Google Scholar]
- 56.Álvarez-Carretero S, Kapli P, Yang Z. Beginner’s guide on the Use of PAML to detect positive selection. Mol Biol Evol. 2023;40:msad041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Z, Wong WS, Nielson R. Bayes empirical Bayes inference of amino acid sites under positive selection. Mol Biol Evol. 2005;22:1107–18. [DOI] [PubMed] [Google Scholar]
- 58.Hou ZC, Xu GY, Su Z, Yang N. Purifying selection and positive selection on the myxovirus resistance gene in mammals and chickens. Gene. 2007;396:188–95. [DOI] [PubMed] [Google Scholar]
- 59.Figueiró HV, Li G, Trindade FJ, Assis J, Pais F, Fernandes G, et al. Genome-wide signatures of complex introgression and adaptive evolution in the big cats. Sci Adv. 2017;3:e1700299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao LZ, Liu YL, Zhang D, Li W, Gao J, Liu Y, et al. Evolution of Oryza chloroplast genomes promoted adaptation to diverse ecological habitats. Commun Biol. 2019;2:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ren T, Li ZX, Xie DF, Gui LJ, Peng C, Wen J, et al. Plastomes of eight Ligusticum species: characterization, genome evolution, and phylogenetic relationships. BMC Plant Biol. 2020;20:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao L, Zhou W, He J, Li DZ, Li HT. Positive selection and relaxed purifying selection contribute to rapid evolution of male-biased genes in a dioecious flowering plant. Elife. 2024;12:RP89941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ronquist F, Teslenko M, van der Mark P, Ayres D, Darling A, Ohna SH, et al. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Sys Biol. 2012;61:539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rambaut A. FigTree v1.4. University of Edinburgh, Edinburgh, UK. 2012. http://tree.bio.ed.ac.uk/software/gtree/.
- 66.Kang L, Xie D, Xiao Q, Peng C, Yu Y, He X. Sequencing and analyses on chloroplast genomes of tetrataenium candicans and two allies give new insights on structural variants, DNA barcoding and phylogeny in Apiaceae subfamily Apioideae. PeerJ. 2019;7:e8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang M, Wang X, Sun J, Wang Y, Ge Y, Dong W, et al. Phylogenomic and evolutionary dynamics of inverted repeats across Angelica Plastomes. BMC Plant Biol. 2021;21:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li ZX, Guo XL, Price M, Zhou SD, He XJ. Phylogenetic position of Ligusticopsis (Apiaceae, Apioideae): evidence from molecular data and carpological characters. Aob Plants. 2022;14:plac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samigullin T, Logacheva M, Terentieva E, Degtjareva G, Pimenov M, Valiejo-Roman C. Plastid phylogenomic analysis of tordylieae tribe (Apiaceae, Apioideae). Plants. 2022;11:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weng L, Jiang Y, Wang Y, Zhang X, Zhou P, Wu M, et al. Chloroplast genome characteristics and phylogeny of the sinodielsia clade (apiaceae: apioideae). BMC Plant Biol. 2023;23:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Downie SR, Watson MF, Spalik K, Katz-Downie DS. Molecular systematics of Old World Apioideae (Apiaceae): relationships among some members of tribe Peucedaneae Sensu Lato, the placement of several island-endemic species, and resolution within the apioid superclade. Can J Bot. 2000;78:506–28. [Google Scholar]
- 72.Calviño CI, Teruel FE, Downie SR. The role of the Southern Hemisphere in the evolutionary history of Apiaceae, a mostly north temperate plant family. J Biogeogr. 2016;43:398–409. [Google Scholar]
- 73.Calviño CI, Tilney PM, van Wyk BE, Downie SR. A molecular phylogenetic study of southern African Apiaceae. Am J Bot. 2006;93:1828–47. [DOI] [PubMed] [Google Scholar]
- 74.Liao C, Downie SR, Li Q, Yu Y, He X, Zhou B. New insights into the phylogeny of Angelica and its allies (Apiaceae) with emphasis on east Asian species, inferred from nrDNA, cpDNA, and morphological evidence. Syst Bot. 2013;38:266–81. [Google Scholar]
- 75.Fernández M, Ezcurra C, Calviño CI. Chloroplast and ITS phylogenies to understand the evolutionary history of southern South American Azorella, Laretia and Mulinum (Azorelloideae, Apiaceae). Mol Phylogenet Evol. 2017;108:1–21. [DOI] [PubMed] [Google Scholar]
- 76.Liu CK, Lei JQ, Jiang QP, Zhou SD, He XJ. The complete plastomes of seven Peucedanum plants: comparative and phylogenetic analyses for the Peucedanum Genus. BMC Plant Biol. 2022;22:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panahi M, Banasiak Ł, Piwczyński M, Puchałka R, Oskolski AA, Spalik K. Phylogenetic relationships among Dorema, Ferula and Leutea (Apiaceae: Scandiceae: Ferulinae) inferred from nrDNA ITS and cpDNA noncoding sequences. Taxon. 2015;64:770–83. [Google Scholar]
- 78.Jin ZT, Ma DK, Liu GN, Hodel RGJ, Jiang Y, Ge BJ, et al. Advancing Pyrus phylogeny: deep genome skimming-based inference coupled with paralogy analysis yields a robust phylogenetic backbone and an updated infrageneric classification of the pear genus (Maleae, Rosaceae). Taxon. 2024;73:784–99. [Google Scholar]
- 79.Messeder JVS, Carlo TA, Zhang G, Tovar JD, Arana C, Huang J, et al. A highly resolved nuclear phylogeny uncovers strong phylogenetic conservatism and correlated evolution of fruit color and size in Solanum L. New Phytol. 2024;243:765–80. [DOI] [PubMed] [Google Scholar]
- 80.Hu XZ, Guo C, Qin SY, Li DZ, Guo Z-H. Deep genome skimming reveals the hybrid origin of Pseudosasa Gracilis (Poaceae: Bambusoideae). Plant Diversty. 2024;46:344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu JX, Guo C, Ma PF, Zhou MY, Luo YH, Zhu GF, et al. The origin and morphological character evolution of the paleotropical woody bamboos. J Integr Plant Biol. 2024. 10.1111/jipb.13751. [DOI] [PubMed] [Google Scholar]
- 82.Wei ZR, Jiao D, Wehenkel CA, Wei XX, Wang XQ. Phylotranscriptomic and ecological analyses reveal the evolution and morphological adaptation of Abies. J Integr Plant Biol. 2024. 10.1111/jipb.13760. [DOI] [PubMed]
- 83.Liu BB, Ren C, Kwak M, Hodel RGJ, Xu C, He J, et al. Phylogenomic conflict analyses in the apple genus Malus s.l. reveal widespread hybridization and allopolyploidy driving diversification, with insights into the complex biogeographic history in the Northern Hemisphere. J Integr Plant Biol. 2022;64:1020–43. [DOI] [PubMed] [Google Scholar]
- 84.Jin ZT, Hodel RGJ, Ma DK, Wang H, Liu GN, Ren C, et al. Nightmare or delight: taxonomic circumscription meets reticulate evolution in the phylogenomic era. Mol Phylogenet Evol. 2023;189: 107914. [DOI] [PubMed] [Google Scholar]
- 85.Zhang G, Ma H. Nuclear phylogenomics of angiosperms and insights into their relationships and evolution. J Integr Plant Biol. 2024;66:546–78. [DOI] [PubMed] [Google Scholar]
- 86.Zhang J, Nielsen R, Yang Z. Evaluation of an improved branch site likelihood method for detecting positive selection at the molecular level. Mol Biol Evol. 2005;22:2472–9. [DOI] [PubMed] [Google Scholar]
- 87.Chen LY, Lu B, Morales-Briones DF, Moody ML, Liu F, Hu GW, et al. Phylogenomic analyses of Alismatales Shed Light into adaptations to aquatic environments. Mol Biol Evol. 2022;39:msac079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madoka Y, Tomizawa KI, Mizoi J, Nishida I, Nagano Y, Sasaki Y. Chloroplast transformation with modified accD operon increases acetyl-CoA carboxylase and causes extension of leaf longevity and increase in seed yield in tobacco. Plant Cell Physiol. 2002;43:1518–25. [DOI] [PubMed] [Google Scholar]
- 89.Rawsthorne S. Carbon flux and fatty acid synthesis in plants. Prog Lipid Res. 2002;41:182–96. [DOI] [PubMed] [Google Scholar]
- 90.Kode V, Mudd EA, Iamtham S, Day A. The tobacco plastid accD gene is essential and is required for leaf development. Plant J. 2005;44:237–44. [DOI] [PubMed] [Google Scholar]
- 91.Caroca R, Howell KA, Malinova I, Burgos A, Tiller N, Pellizzer T, et al. Knockdown of the plastid-encoded acetyl-CoA carboxylase gene uncovers functions in metabolism and development. Plant Physiol. 2021;185:1091–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pu FD, Watson MF. Oenanthe Linnaeus. In: Wu ZY, Raven PH, editors. Flora of China V.14. Beijing and St. Louis: Science Press and Missouri Botanical Garden Press; 2005. p. 130–2.
- 93.Pu FD, Watson MF. Sium Linnaeus. In: Wu ZY, Raven PH, editors. Flora of China V.14. Beijing and St. Louis: Science Press and Missouri Botanical Garden; 2005. p. 115–6.
- 94.Pan ZH, Watson MF. Cryptotaenia de Candolle. In: Wu ZY, Raven PH, editors. Flora of China V.14. Beijing and St. Louis: Science Press and Missouri Botanical Garden; 2005. p. 80.
- 95.Sheh ML, Watson MF. Cicuta Linnaeus. In: Wu ZY, Raven PH, editors. Flora of China V.14. Beijing and St. Louis: Science Press and Missouri Botanical Garden Press; 2005. p. 77.
- 96.Fleischmann TT, Scharff LB, Alkatib S, Hasdorf S, Schottler MA, Bock R. Nonessential plastid-encoded ribosomal proteins in tobacco: a developmental role for plastid translation and implications for reductive genome evolution. Plant Cell. 2011;23:3137–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schippers JHM, Mueller-Roeber B. Ribosomal composition and control of leaf development. Plant Sci. 2010;179:307–15. [Google Scholar]
- 98.Tiller N, Weingartner M, Thiele W, Maximova E, Schottler MA, Bock R. The plastid-specific ribosomal proteins of Arabidopsis thaliana can be divided into non-essential proteins and genuine ribosomal proteins. Plant J. 2012;69:302–16. [DOI] [PubMed] [Google Scholar]
- 99.Robles P, Quesada V. Unveiling the functions of plastid ribosomal proteins in plant development and abiotic stress tolerance. Plant Physiol Bioch. 2022;189:35–45. [DOI] [PubMed] [Google Scholar]
- 100.Wicke S, Schneeweiss GM, depamphilis CW, Müller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 2011;76:273–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wilson RH, Hayer-Hartl M. Complex chaperone dependence of Rubisco biogenesis. Biochemistry. 2018;57:3210–6. [DOI] [PubMed] [Google Scholar]
- 102.Whitney SM, Sharwood RE. Rubisco engineering by plastid transformation and protocols for assessing expression. Methods Mol Biol. 2021;2317:195–214. [DOI] [PubMed] [Google Scholar]
- 103.Kapralov MV, Filatov DA. Widespread positive selection in the photosynthetic rubisco enzyme. BMC Evol Biol. 2007;7: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iida S, Miyagi A, Aoki S, Ito M, Kadono Y, Kosuge K. Molecular adaptation of rbcL in the heterophyllous aquatic plant Potamogeton. PLoS ONE. 2009;4:e4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rumeau D, Peltier G, Cournac L. Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ. 2007;30:1041–51. [DOI] [PubMed] [Google Scholar]
- 106.Yamori W, Sakata N, Suzuki Y, Shikanai T, Makino A. Cyclic electron flow around photosystem I via chloroplast NAD(P)H dehydrogenase (NDH) complex performs a significant physiological role during photosynthesis and plant growth at low temperature in rice. Plant J. 2011;68:966–76. [DOI] [PubMed] [Google Scholar]
- 107.Suorsa M, Sirpiö S, Aro EM. Towards characterization of the chloroplast NAD(P)H dehydrogenase complex. Mol Plant. 2009;2:1127–40. [DOI] [PubMed] [Google Scholar]
- 108.Martin M, Sabater B. Plastid ndh genes in plant evolution. Plant Physiol Bioch. 2010;48:636–45. [DOI] [PubMed] [Google Scholar]
- 109.Xie DF, Xie C, Ren T, Song BN, Zhou SD, He XJ. Plastid phylogenomic insights into relationships, divergence, and evolution of Apiales. Planta. 2022;256:117. [DOI] [PubMed] [Google Scholar]
- 110.Drescher A, Ruf S, Calsa TJr, Carrer H, Bock R. The two largest chloroplast genome-encoded open reading frames of higher plants are essential genes. Plant J. 2000;22:97–104. [DOI] [PubMed] [Google Scholar]
- 111.Kikuchi S, Asakura Y, Imai M, Nakahira Y, Kotani Y, Hashiguchi Y, et al. A Ycf2- FtsHi heteromeric AAA-ATPase complex is required for chloroplast protein import. Plant Cell. 2018;30:2677–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakai M. YCF1: a green TIC: response to the De Vries et al commentary. Plant Cell. 2015;27:1834–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available in the NCBI (https://www.ncbi.nlm.nih.gov) repository. The raw reads have been submitted to GenBank at NCBI BioProject PRJNA1154308, with SRA numbers SRR30524793–SRR30524809. The assembly and annotated chloroplast genomes were deposited in NCBI with accession numbers PQ283862–PQ283878. Voucher specimens were identified by Jun Wen and deposited in NAS (Herbarium, Institute of Botany, Chinese Academy of Sciences, Jiangsu Province) with deposition numbers (NAS00640404– NAS00640420, Fig. S3).