Abstract
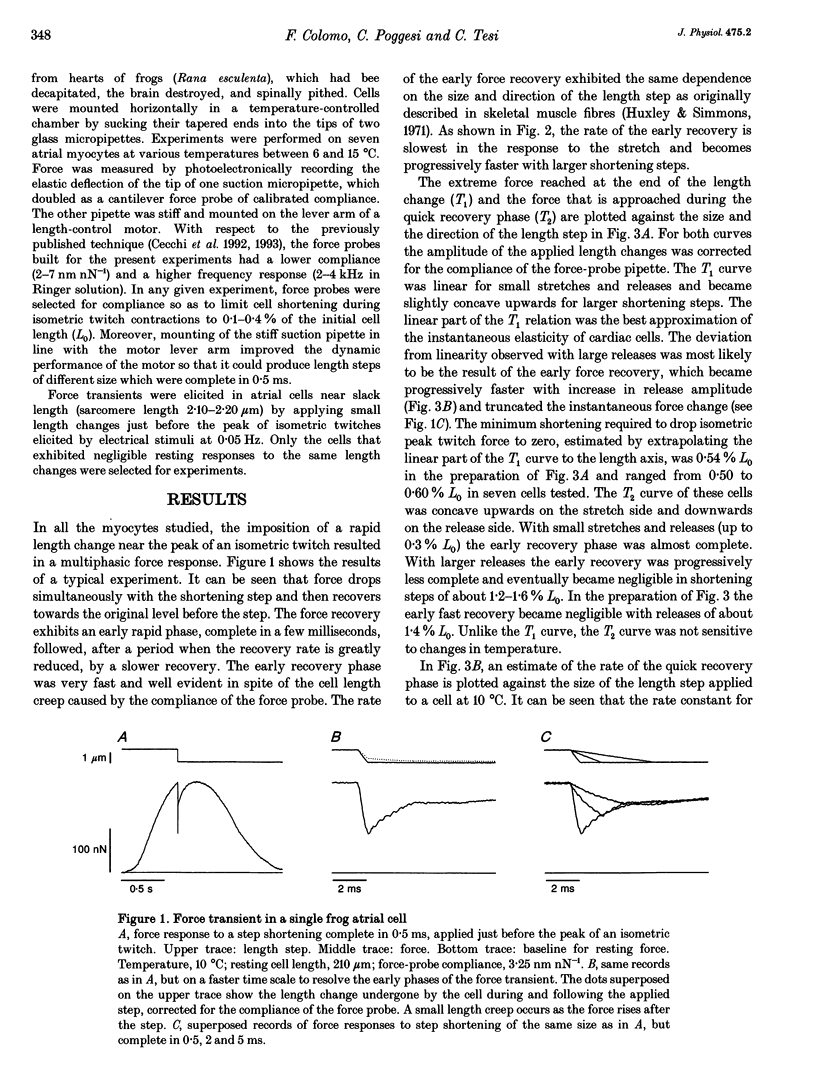
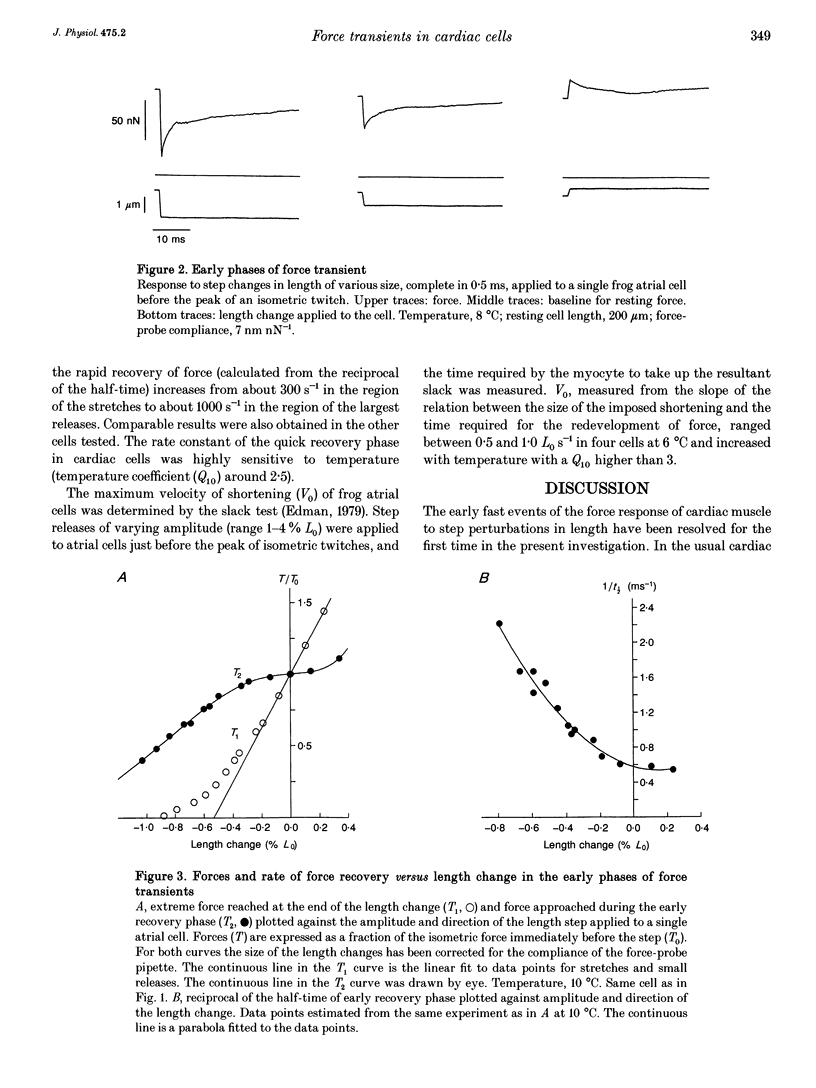
1. Force transients in response to step perturbations in length were recorded in intact atrial cells from frog heart at various temperatures (6-15 degrees C). Length changes of various sizes and in either direction, complete in 0.5 ms, were applied to single myocytes near slack length (initial sarcomere length 2.1-2.2 microns) just before the peak of an isometric twitch. The frequency response of the force transducers used was 2-4 kHz in Ringer solution. 2. An early quick force recovery phase was clearly observed after the elastic force response to the length step and before the start of much slower recovery processes. The quick recovery phase became progressively faster with larger shortening steps and was almost as fast as that originally described in intact frog skeletal muscle fibres (rate constants above 1000 s-1 in large releases at 10 degrees C). 3. The force-extension relation determined at the end of the length change (T1 curve) indicates that an instantaneous shortening of 0.5-0.6% of the initial cell length (L0) brings the force to zero. The force--extension relation determined at the end of the quick recovery phase (T2 curve) showed that the early recovery leads to an almost complete restoration of the original force with small stretches and releases (up to 0.3% L0) and that it becomes negligible in shortening steps of about 1.4% L0. 4. The results suggest that the mechanical properties of attached cross-bridges and the rate of transitions between attached cross-bridge states are approximately the same in frog atrial cells and fast skeletal muscle fibres.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman M. R., Peterson J. N., Yue D. T., Hunter W. C. Effect of isoproterenol on force transient time course and on stiffness spectra in rabbit papillary muscle in barium contracture. J Mol Cell Cardiol. 1988 May;20(5):415–426. doi: 10.1016/s0022-2828(88)80133-0. [DOI] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. Force-velocity relation in normal and nitrate-treated frog single muscle fibres during rise of tension in an isometric tetanus. J Physiol. 1978 Dec;285:257–273. doi: 10.1113/jphysiol.1978.sp012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Poggesi C., Tesi C. A force transducer and a length-ramp generator for mechanical investigations of frog-heart myocytes. Pflugers Arch. 1993 Apr;423(1-2):113–120. doi: 10.1007/BF00374968. [DOI] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Poggesi C., Tesi C. The stimulus interval-tension relation in enzymatically isolated single myocytes of the frog heart. J Physiol. 1992 Mar;448:275–291. doi: 10.1113/jphysiol.1992.sp019041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinl P., Kuhn H. J., Rüegg J. C. Tension responses to quick length changes of glycerinated skeletal muscle fibres from the frog and tortoise. J Physiol. 1974 Mar;237(2):243–258. doi: 10.1113/jphysiol.1974.sp010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Giles W. Active and passive electrical properties of single bullfrog atrial cells. J Gen Physiol. 1981 Jul;78(1):19–42. doi: 10.1085/jgp.78.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Irving M., Lombardi V., Piazzesi G., Ferenczi M. A. Myosin head movements are synchronous with the elementary force-generating process in muscle. Nature. 1992 May 14;357(6374):156–158. doi: 10.1038/357156a0. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Sagawa K., Suga H. Transient tension responses of heart muscle in Ba2+ contracture to step length changes. Am J Physiol. 1980 Mar;238(3):H340–H347. doi: 10.1152/ajpheart.1980.238.3.H340. [DOI] [PubMed] [Google Scholar]
- Sato H., Mashima H. Force-velocity relation for the tetanic contraction of frog atrial muscle. Jpn J Physiol. 1981;31(1):121–125. doi: 10.2170/jjphysiol.31.121. [DOI] [PubMed] [Google Scholar]
- Steiger G. J. Tension transients in extracted rabbit heart muscle preparations. J Mol Cell Cardiol. 1977 Aug;9(8):671–685. doi: 10.1016/s0022-2828(77)80362-3. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R., Saks V. A., Vassort G., Lauer C., Elizarova G. V. Reversible MM-creatine kinase binding to cardiac myofibrils. Am J Physiol. 1987 Sep;253(3 Pt 1):C444–C455. doi: 10.1152/ajpcell.1987.253.3.C444. [DOI] [PubMed] [Google Scholar]
- Warshaw D. M., Fay F. S. Tension transients in single isolated smooth muscle cells. Science. 1983 Mar 25;219(4591):1438–1441. doi: 10.1126/science.6828870. [DOI] [PubMed] [Google Scholar]


