Abstract
Background
Diagnosis of umbilical cord entanglement (UCE) by ultrasound (US) in monochorionic monoamniotic (MCMA) twins in the second and third trimesters is common. However, only a few cases have been reported on the diagnosis of UCE as early as the first trimester. Herein, we report a case of the earliest-ever sonographic diagnosis of UCE and demonstrate the feasibility of its diagnosis by US.
Case presentation
A 32-year-old gravida 2 para 1 woman conceived after assisted reproductive technology (ART) treatment. In transvaginal US examination at 8.5 gestational weeks, two embryos with regular heartbeats in the same amniotic sac and with only one yolk sac were demonstrated. The fetal crown-rump lengths were 20 and 21 mm, appropriate for 8.4 and 8.5 gestational weeks, respectively. HD-flow power Doppler 2D and 3D US demonstrated two tightly entangled umbilical cords of the two fetuses. Spectral Doppler US showed two different heart rates (162 and 167 beats per minute) and blood flow in opposite directions from the point of entanglement of the two umbilical cords. This was consistent with a diagnosis of a first-trimester MCMA pregnancy with UCE. Missed abortion of the two embryos was diagnosed by US examination at 10.5 weeks, and the pregnancy was terminated by dilatation and curettage without further complications.
Conclusions
UCE in the first trimester may occur as early as eight gestational weeks, and its diagnosis by ultrasound is feasible. UCE diagnosed in the first trimester may be a poor prognostic factor.
Keywords: First trimester, Umbilical cord, Entanglement, Monochorionic-monoamniotic, Twin
Background
Monochorionic monoamniotic (MCMA) twin pregnancy occurs when more than one fetus shares a single placenta and a single amniotic cavity. It results from a single fertilized egg and embryo splitting between 9 and 13 days after fertilization [1, 2]. It is a rare event, with an estimated incidence of 8 per 100,000 pregnancies, constituting approximately 1% of all twin pregnancies and approximately 5% of all monochorionic pregnancies [3].
The pregnancies of MCMA twins are associated with significantly increased complication rates compared to dichorionic or monochorionic diamniotic pregnancies. The risk of congenital anomalies in MCMA twins ranges between 15% and 25%, with a predominance of cardiac anomalies accounting for approximately one-third of all anomalies observed [1]. The high incidence of anomalies is explained by the delayed splitting of the embryo and hemodynamic fluctuations through extensive placental anastomoses [4].
MCMA twin pregnancy presents an enormous challenge following its accurate diagnosis, where the absence of an intertwin membrane results in a consistently fluctuating fetal position. This results in an almost impossible task to label each of the two specific fetuses to provide continuity in assessments, especially in the absence of discordant growth or anomalies [2]. Although only 2–4% of MCMA twin pregnancies will develop twin-to-twin transfusion syndrome, it is hard to diagnose since, in this case, the typical combination of oligohydramnios and polyhydramnios is not present [5, 6]. The rate of preterm labor is high, and over 55% of all MCMA pregnancies will deliver before 34 W [3].
The fetal loss in MCMA twins is high, reaching 30–40%. Most fetal deaths occur in the first and 2nd trimesters of pregnancy. Nevertheless, even after 24 weeks gestation, the risk of fetal death is nine times higher in MCMA twin pregnancies (9%) than in dichorionic twin pregnancies (1%) [7, 8]. In a prospective observational study of 18 MCMA pregnancies, the overall perinatal loss after excluding conjoined twins, twin reversed arterial perfusion, and twins with discordant anomalies - was 11.1% after 16 weeks and 5.9% after 20 weeks of gestation [9]. When fetal death occurs in MCMA twin pregnancies, both twins usually die. 80% of pregnancies complicated by spontaneous fetal death have a double death, whereas 20% involve a single death. In the presence of a single death, the risk of severe brain injury to the surviving twin is very high (57%) [5].
In structurally normal MCMA pregnancies, fetal deaths are often explained by acute hemodynamic imbalances caused by extensive placental anastomoses, and they might be associated with tight umbilical cord entanglement (UCE) [10]. The rate of UCE diagnosed by ultrasound during pregnancy in MCMA twins ranges between 55% and 100% [9, 11]. UCE occurs when fetuses in the same amniotic space move around each other. This might be diagnosed during the second and third trimesters. However, to date, only a few cases of UCE diagnosed in the first trimester have been reported [12–16]. Very early entanglement of the umbilical cord may expose the fetuses to the risk of blood flow compromise, potentially affecting pregnancy complications and outcomes. This highlights the importance of diagnosing UCE in the first trimester.
In addition, UCE may facilitate the early diagnosis of MCMA twins. Typically, in twin pregnancies, the determination of chorionicity is most reliable when undertaken in the first trimester. Lack of visualization of the amniotic membranes between 7 and 9 gestational weeks allows the diagnosis of monochorionic monoamniotic twins. However, amnionicity assessment can be challenging before ten gestational weeks owing to the thin, often difficult-to-visualize amniotic membrane [17]. One yolk sac in a twin pregnancy may aid in diagnosing MCMA. However, it is a relatively unreliable tool since two yolk sacs can be present in up to one-third of such pregnancies [18]. On the other hand, UCE is exclusively present in MCMA pregnancies since only in this case are both twins present in the same amniotic sac. Although the rate of UCE in the first trimester in MCMA twins is unknown, its demonstration in the first trimester may enable early diagnosis of MCMA pregnancy. Since MCMA pregnancy is associated with significantly increased complications, accurate diagnosis of chorionicity and amnionicity in the first trimester is paramount to ensure appropriate management and follow-up.
To the best of our knowledge, only six cases of first-trimester UCE have been reported in the medical literature [12–16]. In the present study, we report a representative case of UCE diagnosed at 8.5 weeks gestation to demonstrate the mode and feasibility of diagnosing this phenomenon by ultrasound (US) in the first trimester. It is the earliest case of a UCE report in the literature. In addition, we reviewed all the reported cases of diagnosis of UCE in the first trimester to evaluate the significance of first-trimester UCE on pregnancy outcome.
Case presentation
A thirty-two-year-old gravida 2 para 1 woman was admitted to our US unit for evaluation of her first-trimester twin pregnancy. In the current pregnancy, she underwent assisted reproductive technology (ART) treatment due to polycystic ovary syndrome and her partner’s low sperm count. She conceived after the transfer of two frozen-thawed blastocysts. In her seventh week of pregnancy, US examination demonstrated two intrauterine sacs, one empty and the other containing two fetal poles with positive heartbeats. This was consistent with the implantation of one embryo that vanished while the other embryo was successfully implanted and split into monozygotic twins. A transvaginal US examination at 8.5 weeks gestation revealed two embryos with regular heartbeats in the same amniotic sac; only one yolk sac was imaged (Fig. 1).
Fig. 1.
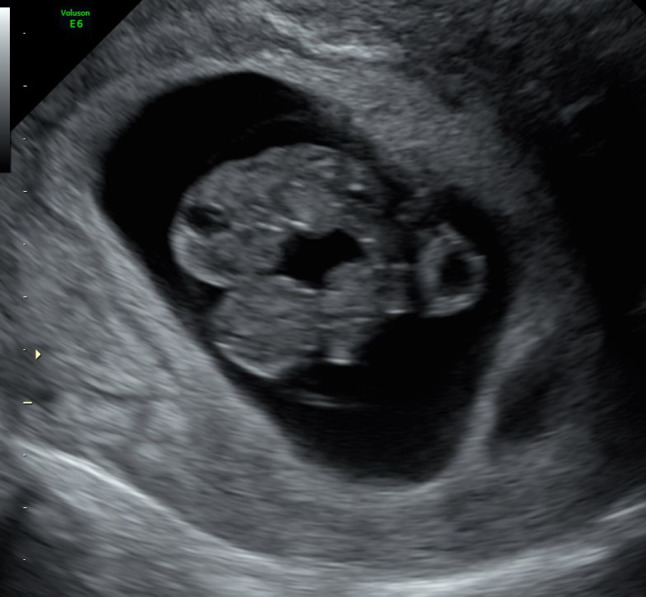
2D US at 8.5 weeks demonstrates two fetuses in the same amniotic sac with only one yolk sac
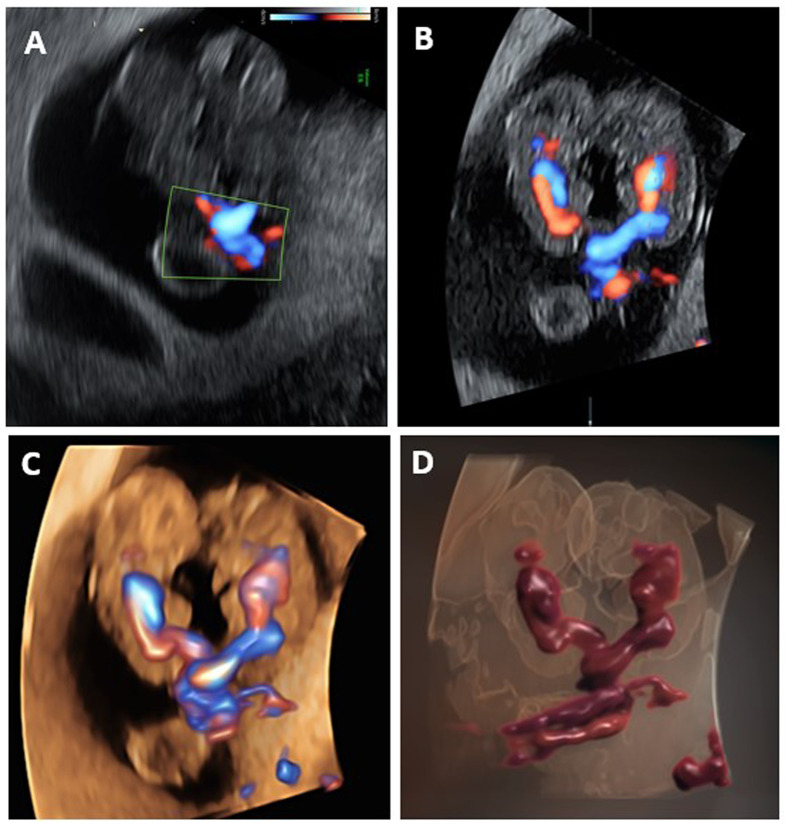
The fetal crown-rump lengths (CRL) were 20 and 21 mm, appropriate for 8.4- and 8.5-week gestation, respectively. The two fetuses’ tightly entangled umbilical cords were demonstrated by 2D, 3D multiplanar, 3D HD, and 3D HD-live power Doppler flow ultrasound modalities (Fig. 2).
Fig. 2.
Various ultrasound Doppler techniques demonstrate umbilical cord entanglement. A. 2D power Doppler shows the entangled cord point. B. 3D multiplanar power Doppler ultrasound displays the connection between the entangled point of the fetal umbilical cords and the two fetal hearts and circulations. C. 3D HD power Doppler flow highlights the bidirectional flow of the two entangled cords and the different fetal circulations. D. 3D HD-live power Doppler flow depicts the entanglement of two separate umbilical cords
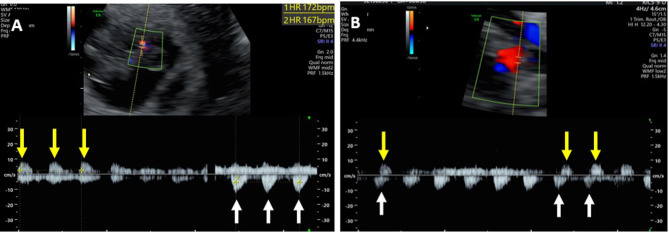
Spectral Doppler ultrasound showed two different heart rates (162 and 167 beats per minute) and simultaneous counter-arterial blood flow directions at the point where the two umbilical cords were entangled (Fig. 3).
Fig. 3.
Spectral Doppler demonstrating two different arterial heart rates with opposite blood flow directions (A) and simultaneous counter-arterial blood flow directions (B) at the point of entangled cords (white and yellow arrows)
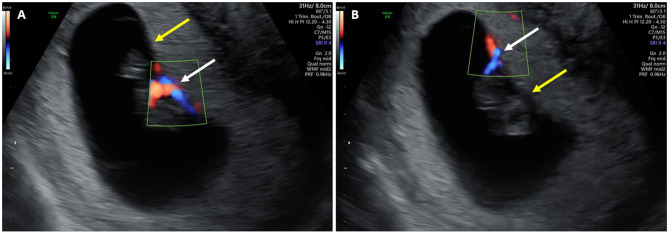
Two distinct insertion sites of the umbilical cord of each fetus to the placenta were demonstrated by 2D HD Doppler mode, 8 mm apart (Fig. 4).
Fig. 4.
Two sequential images demonstrate the distinct insertion site of the umbilical cord of each fetus to the placenta. A. the insertion site of one fetus’s umbilical cord using 2D Power Doppler mode (white arrow), while the other is shown without Doppler imaging (yellow arrow). B. The insertion site of the other fetus is displayed using Power Doppler (white arrows), with the corresponding site of the first fetus shown without Doppler (yellow arrow)
This was consistent with a diagnosis of a first-trimester MCMA pregnancy with UCE. In US examination at 10.5 weeks of pregnancy, the fetuses were shown to have entangled umbilical cords, and both were without a heartbeat. The CRLs were 31.7 and 31.8 mm, corresponding to 10.0 gestational weeks. With the diagnosis of missed abortion, the pregnancy was terminated by dilatation and curettage without further complications.
Discussion and conclusions
In the present case, to the best of our knowledge, we report the earliest-ever sonographic diagnosis of UCE. UCE probably depends on the movements of the fetuses around each other in the same amniotic sac. Fetal movements begin in utero between 7 and 8 weeks, meaning our case occurred in the very first days after the onset of fetal movements [19]. The fact that the umbilical cords were inserted into separate and distant sites of the placenta reduces the probability that the UCE was developed de novo (Fig. 4). Previously, the earliest cases of UCE were reported at ten weeks of pregnancy [13–15]. At this gestational week, the CRLs of the fetuses are approximately 30 to 40 mm, which is approximately 50 to 100% longer than the 20 mm in our case [20]. However, despite the pregnancy’s small size, we could visualize UCE clearly by transvaginal ultrasound, including two separate heartbeats demonstrable in the spectral Doppler examination (Fig. 2). Thus, our case establishes the feasibility of UCE diagnosis at a very early stage of pregnancy and its potential utility for the early diagnosis of MCMA pregnancy.
In a review of the literature on umbilical cord entanglement in the first trimester, our case is only the seventh reported case over 24 years (Table 1) [12–16]. At least 2 of the 7 cases (28%) were achieved via assisted reproductive technology (ART). The sonographic diagnosis of UCE was performed between the 8th and 13th weeks. The earliest sonographic diagnosis of UCE was at 8 W (our case). UCE was diagnosed in only 1 case (our case) of 3 (33%) US examinations performed at 8 W. In 4 of 7 cases (57%), no UCE was identified in the previous US performed one to two weeks earlier. Four of the 7 cases had missed abortion (57%), all of them of both fetuses simultaneously. One of the seven cases (our case) (15%) had early missed abortion at the 10th week of gestation, and 3 of the 7 cases (43%) had late missed abortion at 14–19 W. In three of the 7 cases (43%), the patient gave birth to two healthy newborns.
Table 1.
Summary of the seven first-trimester umbilical cord entanglement cases reported in the medical literature
| Cases | Author | Year | Conception | Previous Normal US | Week of diagnosis of uce | CRL (MM) | Two different heart rates by Doppler in the entangled cord | In utero intervention | Outcome | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-2 | Overton [12] | 1999 | * | NO | 11 | * | Yes | Medical amnioreduction at 20 weeks’ gestation with sulindac | Delivery | Two cases with the same detais. No details on delivery weeks, mode and neonatal outcome |
| 3 | Arabin [13] | 1999 | * | 8 | 10 | * | Yes | No | late missed abortion of both twins - 15W | * |
| 4 | Merién, Arabin [13, 14] | 2001 | ART | 8 | 10 | * | Yes | No | Elective CS at 35W, birthweight 2160 and 1980 | Dichorionic-Diamniotic Triplets pregnancy |
| 5 | Sherer [15] | 2002 | SPONT | NO | 10 | 30/30 | No | No | late missed abortion of both twins - 14W | * |
| 6 | Panaitescu [16] | 2021 | SPONT | 11 | 13 | * | Yes | No | late missed abortion of both twins - 19W | * |
| 7 | Present study | 2022 | ART | 7 | 8 | 20/21 | Yes | No | Early missed abortion of both twins - 10W | * |
The incidence of MCMA pregnancies is significantly increased by ART involving embryo manipulation and assisted hatching [21]. Almost 2% of pregnancies conceived with ART are monochorionic, and approximately one-third of them are MCMA pregnancies [22]. Accordingly, at least 2 of the 7 cases in our series (28%) followed ART. However, whether ART is a risk factor for early UCE is unclear.
In this small series of the present review, we found that four out of seven (57%) cases ended in missed abortions. This rate is similar to the 52% spontaneous miscarriage rate of 48 MCMA twins after a normal 1st -trimester scan and before 22 W [7]. However, in another larger series of 55 MCMA twins, the rate of fetal loss before 24 weeks was much lower (22%) [8]. The high rate of fetal loss in our review may be due to the small number of cases or the higher rate of missed abortions in the very early stage of pregnancy. However, in our series, both fetuses were lost together, and three cases were lost in the second trimester. These facts may imply that early UCE can impair fetal circulation at higher rates than in UCE, which appears at later stages of pregnancy. This knowledge may influence pregnancy management after an early diagnosis of UCE in the first trimester. If the chance of missed abortion is significantly high following the first trimester UCE diagnosis and the rate of pregnancy complications is very high - early termination of pregnancy might be considered. However, it remains to be demonstrated in larger series if this is the preferable option – especially in cases of pregnancies achieved via ART.
In MCMA pregnancies characterized by structurally normal fetuses, fetal demise is frequently attributed to acute hemodynamic perturbations precipitated by significant placental anastomoses. Fetal demise may also be associated with the presence of UCE [10]. In their review of five articles published between 2001 and 2006, Rossi et al. concluded that cord entanglement does not contribute to prenatal morbidity and mortality in monoamniotic twin pregnancies. However, the overall rate of US diagnosis of UCEs in their series was as low as 55% [11]. On the other hand, in the Dias et al. review, the rate of prenatal US diagnosis of UCE increased from 57 to 100% from 2003 to 2010, respectively [9]. Their prospective observational study diagnosed UCE at 11–16 weeks gestation in all 18 MCMA cases using B-mode and color Doppler ultrasound (9). This can be related to the improvement in the quality and technology of the US equipment. Thus, the relationship between pregnancy outcome and UCE might not rest on whether there is UCE, since it might be diagnosed today in up to 100% of MCMA pregnancies. It is possible that the real question should be when UCE occurs during the course of pregnancy. Based on the results of our literature review, we may hypothesize that the earlier the UCE, the worse the outcome. If true, this emphasizes the importance of first-trimester UCE diagnosis in MCMA twin pregnancies.
In conclusion, our study demonstrates the feasibility of first-trimester demonstration of UCE, the potential role of this demonstration as a tool for early diagnosis of MCMA twin pregnancy, and the potentially bad prognostic factor of UCE diagnosed as early as the first trimester. Larger series are necessary to establish the prognosis of first-trimester UCE and the recommended management of such cases after early diagnosis.
Acknowledgements
Not applicable.
Abbreviations
- UCE
Umbilical cord entanglement
- US
Ultrasound
- MCMA
Monochorionic monoamniotic
- ART
Assisted reproductive technology
- CRL
Crown-rump lengths
Author contributions
G.L. Data collection and manuscript writing. S.M.R. and S.S. organized the study and collected the data. E.Y.A. Project development and manuscript revision. S.M.C. Scientific and linguistic editing. S.Y. Manuscript revision and supervised the work. All authors have read and approved the manuscript.
Funding
No external funding sources were used for this study.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Mieghem T, Abbasi N, Shinar S, Keunen J, Seaward G, Windrim R, Ryan G. Monochorionic monoamniotic twin pregnancies. Am J Obstet Gynecol MFM. 2022;4(2S):100520. 10.1016/j.ajogmf.2021.100520. Epub 2021 Oct 30. PMID: 34728404. [DOI] [PubMed] [Google Scholar]
- 2.Khairudin D, Khalil A. Monochorionic monoamniotic twin pregnancies. Best Pract Res Clin Obstet Gynecol. 2022;84:96–103. 10.1016/j.bpobgyn.2022.08.004. Epub 2022 Aug 24. PMID: 36123247. [DOI] [PubMed] [Google Scholar]
- 3.Glinianaia SV, Rankin J, Khalil A, Binder J, Waring G, Sturgiss SN, Thilaganathan B, Hannon T. Prevalence, antenatal management and perinatal outcome of monochorionic monoamniotic twin pregnancy: a collaborative multicenter study in England, 2000–2013. Ultrasound Obstet Gynecol. 2019;53(2):184–92. Epub 2018 Dec 30. PMID: 29900612. [DOI] [PubMed] [Google Scholar]
- 4.Hack KE, van Gemert MJ, Lopriore E, Schaap AH, Eggink AJ, Elias SG, van den Wijngaard JP, Vandenbussche FP, Derks JB, Visser GH, Nikkels PG. Placental characteristics of monoamniotic twin pregnancies in relation to perinatal outcome. Placenta. 2009;30(1):62–5. Epub 2008 Nov 17. PMID: 19010539. [DOI] [PubMed] [Google Scholar]
- 5.Van Mieghem T, De Heus R, Lewi L, et al. Prenatal management of monoamniotic twin pregnancies. Obstet Gynecol. 2014;124:498–506. [DOI] [PubMed] [Google Scholar]
- 6.Murgano D, Khalil A, Prefumo F, Mieghem TV, Rizzo G, Heyborne KD, Melchiorre K, Peeters S, Lewi L, Familiari A, Lopriore E, Oepkes D, Murata M, Anselem O, Buca D, Liberati M, Hack K, Nappi L, Baxi LV, Scambia G, Acharya G. D’antonio F. Outcome of twin-to-twin transfusion syndrome in monochorionic monoamniotic twin pregnancy: systeMCMAic review and meta-analysis. Ultrasound Obstet Gynecol. 2020;55(3):310–317. 10.1002/uog.21889. PMID: 31595578. [DOI] [PubMed]
- 7.Madsen C, Søgaard K, Zingenberg H, Jørgensen FS, Rosbach H, Hoseth E, Pedersen LH, Petersen OB. Outcomes of monoamniotic twin pregnancies managed primarily in outpatient care-a Danish multicenter study. Acta Obstet Gynecol Scand. 2019;98:479–86. [DOI] [PubMed] [Google Scholar]
- 8.Litwinska E, Syngelaki A, Cimpoca B, Frei L, Nicolaides KH. Outcome of twin pregnancy with two live fetuses at 11–13 weeks’ gestation. Ultrasound Obstet Gynecol. 2020;55:32–8. [DOI] [PubMed] [Google Scholar]
- 9.Dias T, Mahsud-Dornan S, Bhide A, Papageorghiou AT, Thilaganathan B. Cord entanglement and perinatal outcome in monoamniotic twin pregnancies. Ultrasound Obstet Gynecol. 2010;35(2):201-4. 10.1002/uog.7501. PMID: 20069540. [DOI] [PubMed]
- 10.Heyborne KD, Porreco RP, Garite TJ, Phair K, Abril D, Obstetrix/Pediatrix Research Study Group. Improved perinatal survival of monoamniotic twins with intensive inpatient monitoring. Am J Obstet Gynecol. 2005;192(1):96–101. 10.1016/j.ajog.2004.06.037. PMID: 15672009. [DOI] [PubMed]
- 11.Rossi AC, Prefumo F. Impact of cord entanglement on perinatal outcome of monoamniotic twins: a systeMCMAic review of the literature. Ultrasound Obstet Gynecol. 2013;41:131–5. [DOI] [PubMed] [Google Scholar]
- 12.Overton TG, Denbow ML, Duncan KR, Fisk NM. First-trimester cord entanglement in monoamniotic twins. Ultrasound Obstet Gynecol. 1999;13:140–2. [DOI] [PubMed] [Google Scholar]
- 13.Arabin B, Laurini RN, van Eyck J. Early prenatal diagnosis of cord entanglement in monoamniotic multiple pregnancies. Ultrasound Obstet Gynecol. 1999;13:181–6. [DOI] [PubMed] [Google Scholar]
- 14.Merién AE, van Eyck J, Arabin B. Two cases with dichorionic diamniotic triplet pregnancy and early cord entanglement. Twin Res. 2001;4:219–22. [DOI] [PubMed] [Google Scholar]
- 15.Sherer DM, Sokolovski M, Haratz-Rubinstein N. Diagnosis of umbilical cord entanglement of monoamniotic twins by first-trimester color Doppler imaging. J Ultrasound Med. 2002;21:1307–9. [DOI] [PubMed] [Google Scholar]
- 16.Panaitescu AM, Gică N, Botezatu R, et al. Early Ultrasound Identification of Cord Entanglement in Monochorionic Monoamniotic Twin pregnancy. Diagnostics (Basel). 2021;15:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bora SA, Papageorghiou AT, Bottomley C, Kirk E, Bourne T. Reliability of transvaginal ultrasonography at 7–9 weeks’ gestation in the determination of chorionicity and amnionicity in twin pregnancies. Ultrasound Obstet Gynecol. 2008;32(5):618 – 21. 10.1002/uog.6133. PMID: 18677702. [DOI] [PubMed]
- 18.Fenton C, Reidy K, Demyanenko M, Palma-Dias R, Cole S, Umstad MP. The significance of yolk sac number in Monoamniotic Twins. Fetal Diagn Ther. 2019;46(3):193–9. 10.1159/000496204. Epub 2019 Feb 14. PMID: 30763938. [DOI] [PubMed] [Google Scholar]
- 19.Papageorghiou AT, Kennedy SH, Salomon LJ, Ohuma EO, Cheikh Ismail L, Barros FC, Lambert A, Carvalho M, Jaffer YA, Bertino E, Gravett MG, Altman DG, Purwar M, Noble JA, Pang R, Victora CG, Bhutta ZA, Villar J. International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown-rump length in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2014;44:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lüchinger AB, Hadders-Algra M, van Kan CM, de Vries JI. Fetal onset of general movements. Pediatr Res. 2008;63:191–5. [DOI] [PubMed] [Google Scholar]
- 21.Knopman JM, Krey LC, Oh C, Lee J, McCaffrey C, Noyes N. What makes them split? Identifying risk factors that lead to monozygotic twins after in vitro fertilization. Fertil Steril. 2014;102(1):82–9. 10.1016/j.fertnstert.2014.03.039. Epub 2014 Apr 29. PMID: 24794318. [DOI] [PubMed] [Google Scholar]
- 22.Alikani M, Cekleniak NA, Walters E, Cohen J. Monozygotic twinning following assisted conception: an analysis of 81 consecutive cases. Hum Reprod. 2003;18:1937–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.