Abstract
Blending multiple polymers together to form the so-called “high-entropy polymers (HEPs)” can generate the effects of molecular dispersion in addition to suppressing polymer phase separation. We embedded a semiconducting polymer (conjugated polymers, CPs) in an optically inert matrix composed of n polymer species and found that a molecule-level dispersion is attained in HEPs defined as n ≥ 5. In the regime of dilute CP concentrations, the photonic properties vary widely in the n = 1 matrices owing to diverse solubility parameters, but the distribution narrows with n, and the CP starts to exhibit behaviors of molecule-level dispersion at n ≥ 5, where the matrix polymers compete with each other to exert direct influences on the embedded CP. Specifically, for MEH-PPV, increasing n reduces the fluorescence redshift and spectral width from diminishing aggregation. For the rigid PFO molecules, increasing n creates a dilution effect facilitating formation of the low-energy planar β-phase. For the flexible regioregular P3HT-rr, HEPs offer well-dispersed amorphous chains highly susceptible to chain environments, thus influencing ηR’s in the quasi-fixed amorphous–crystalline energy transfer landscape. The HEP effects continue for greater CP concentrations, consistent with the matrix dispersing behaviors in the dilute regime. This work demonstrates a molecular-level dispersion by HEPs, offering a method of molecular tailoring for polymer research and applications via simple mixing.
Keywords: high-entropy polymers, polymer blends, photoluminescence, photonic materials, conjugated polymers, molecular dispersion
Over the past two decades, high-entropy alloys have transformed the design and production of metallic alloys, leading to numerous breakthroughs and applications.1−3 This concept has recently been applied to polymeric materials, where blending multiple polymer species to form high-entropy polymers (HEPs) does significantly suppress polymer phase separation, a persistent challenge for developing the binary or tertiary polymer blends.4 The high-entropy polymers (HEPs) are defined as multicomponent polymer blends with five or more distinct polymer species inspired by the concept of high-entropy alloys. Like high-entropy alloys, generally no single component should dominate the compositions of the HEPs. Since mixing different species together is a simple and cost-effective method for creating new materials, the emergence of HEPs offers an attractive method for developing polymer materials. This study explores the photonic behaviors of light-emitting conjugated polymers (CPs) embedded in HEP thin films. It focuses on how CP molecules interact with the environment to influence their photonic performances, in an attempt to investigate the molecular state on finer lenght scales.
Semiconducting CPs, finding wide applications relating to display, lighting, photodetectors, and microelectronics,5−7 are highly susceptible to the states of molecular conformations and aggregation that may substantially impact the energy transfer pathways to dictate the photonic behaviors. For example, segmental stresses arising from nonequilibrated molecular packaging strongly influence the emission wavelength (λmax) and the self-trapping propensity; the latter can sabotage the emissive quantum efficiencies (ηR).8−16 Therefore, photonic behaviors are very sensitive to the dispersion state of CP when it is embedded in a matrix. Hence, it would be interesting to study the photoluminescence (PL) of the model CPs of varied backbone stiffnesses and intermolecular properties when embedded in polymer blends of various numbers of components (n’s) to scrutinize molecular dispersion as affected by HEPs.
For this purpose, we choose three conjugated polymers of various backbone stiffnesses and photonic properties: the P3HT-rr (regioregular poly(3-hexylthiophene-2,5-diyl)), MEH-PPV (poly(2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene)), and PFO (poly(9,9-di-n-octylfluorenyl-2,7-diyl)), in the order of increasing backbone rigidity. The CP is then embedded in optically inert polymer matrices composed of varied species from a polymer set of diverse glass transition temperatures (Tg), segmental hydrophobicity, and solubility parameters: polystyrene (PS), poly(methyl methacrylate) (PMMA), cis-polyisoprene (PIP), polyvinylpyrrolidone (PVP), poly(vinyl acetate) (PVAc), poly(bisphenol A carbonate) (PC), ethyl cellulose (EC), and poly(2,6-dimethyl-1,4-phenylene oxide) (PPO). Among the CPs, MEH-PPV luminesces in a fashion sensitive to segmental aggregation as the latter can cause redshifted λmax and reduced ηR despite a long side group in place designed to mitigate intermolecular π–π attractions.17−20 The PFO, a derivative of the blue-emitting polyfluorene family that generally has high ηR’s,21−23 emits different λmax’s according to the intermonomer torsion angle ϕ: random ϕ (the α-phase), 140° < ϕ< 160° (the γ-phase), and 160° < ϕ < 180° (the β-phase), with the β-phase emitting the reddest capable to dominate the emission via Forster energy transfer once it exceeds 7% in coexistent states.24−28 The β-phase, normally favored due to its relatively greater ηR, is energetically the lowest, formed preferentially when the polymer chains are plasticized or isolated in diluted states.24,29−35 The P3HT-rr is regioregular, capable of interdigitated edge-on crystalline ordering via on-plane π stacking.36,37 The π-stacked aggregates lead to extended conjugations that can serve as the “red chains” to funnel photoexcited energies from the “blue chains” residing in the amorphous regions.38−42
As shown in the following, HEP blending not only suppresses phase separation of the component polymers but also finely disperses the CP molecules in the matrix, allowing the photonic properties to be tailored by the matrix species, which, at the same time, signifies further the molecular dispersion of the component polymers.
Results and Discussion
The photonic behaviors of HEPs were examined mainly by blending 1 wt % of a CP (MEH-PPV, PFO, or P3HT-rr) in the optically inert polymer blends to examine the molecular interactions. The CP concentration was ultimately increased to 50 wt % for MEH-PPV to further explore the stability of molecular dispersion. Clearly, as n increases, phase separation of the polymers is increasingly suppressed. Although minor demixing of a length scale ∼300–500 nm continues (Figure S1, Supporting Information A), segmental dispersion down to molecular scales is attained in HEPs for both the embedded CP and the matrix polymers, as shown by the photonic behaviors. It implies a molecular dispersion length scale estimated of ∼20 nm via HEPs, much smaller than that implied by the morphology demixing, rendering the photonic behaviors not closely relevant to the morphologies (Figure S2, Supporting Information A).
PL of Diluted Conjugated Polymers in the Blends
The three CPs of diverse backbone and interchain properties (MEH-PPV, PFO, and P3HT-rr) exhibit distinct dispersion effects unique to each characteristic photonic behavior. The individual behaviors are explored and discussed in the following.
MEH-PPV
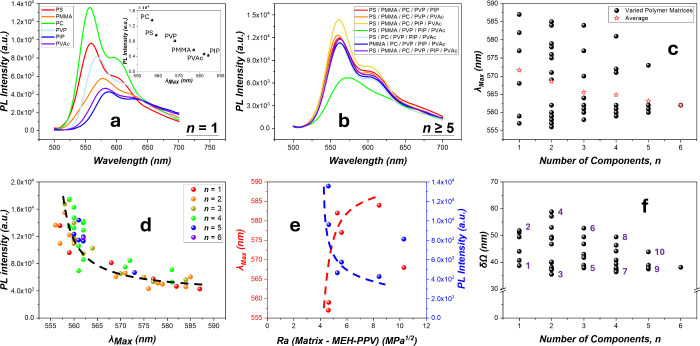
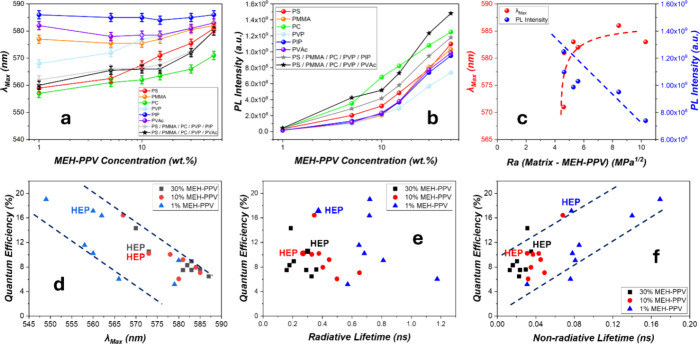
As n increases, the embedded CP molecules exhibit significantly reduced redshifts, during which the matrix polymers may influence directly the PL behaviors. At n = 1, the PL spectra exhibit distinct emission characteristics with varied degrees of molecular aggregation depending on the matrix polymer species (Figure 1a). In PC or PS, the quasi-single molecules' PL behavior of λmax at ∼557 and 559 nm was observed (single-molecule MEH-PPV emission at λmax = 555 nm),43 indicating minimal aggregation in the blends. In contrast, in PIP, PVAc, or PMMA, considerable redshifts were observed (λmax = 587, 582, and 577 nm), which implicate substantial molecular aggregation. The redshifts cause pronounced PL reductions, reflecting aggregation-caused quenching (ACQ) for MEH-PPV optical properties (Figure 1a, inset).
Figure 1.
PL spectra of 1 wt % MEH-PPV in the various matrices: (a,b) PL spectra in the n = 1 (a) and n ≥ 5 (b) polymer matrices. The PL spectra of other n’s are shown in Figure S3, Supporting Information B. (c) λmax vs n (the polymer compositions of each film can be seen in Figure 2). (d) PL intensity vsλmax for various n’s. (e) λmax and PL intensity vs Ra. (f) Breadths δΩ of the 0–0 peaks vs n’s. (1: PC, 2: PVAc, 3: PC/PIP, 4: PVP/PIP, 5: PC/PIP/PVAc, 6: PMMA/PIP/PVAc, 7: PC/PVP/PIP/PVAc, 8: PS/PMMA/PVP/PIP and PS/PMMA/PIP/PVAc, 9: PS/PMMA/PC/PIP/PVAc, and 10: PS/PMMA/PVP/PIP/PVAc).
The compatibility between the matrix polymer and CP significantly influences the π aggregation. With PVP being the only exception, λmax increases rapidly with Ra (the Hansen solubility limit, Table S1, Supporting Information C), as shown in Figure 1e, revealing stronger propensity of intersegmental aggregation for poorer matrix-CP affinities. The observation is consistent with the notion that molecular aggregates arise when the matrix molecules start to pervade and mix with the CP molecules near the end of the solvent evaporation during spin coating. The anomalous deviation by PVP (showing smaller λmax and larger PL intensity than expected on Ra) is tentatively attributed to sizeable separations between the hydrophilic short PVP chains (MPVP = 40 kg/mol) and the hydrophobic CP segments during cosolvent-mediated convergence at the final stage of film formation.
For films of n > 1, the PL spectra vary remarkably with the matrix polymer composition and n (Figure 1b and Figure S3, Supporting Information B), with λmax distribution shrinking with n (Figure 1c). The narrowing of the λmax distribution is drastic when n increases from 4 to ≥5. It indicates that not only decreased MEH-PPV aggregation but also a convergence into similar emissive structures has occurred as the matrix evolves into the HEP regime.
Moreover, the PL spectral breadth δΩ that reflects the distribution of conjugation lengths exhibits a steep narrowing of its distribution for n > 4, after a brief increase at n = 2 (Figure 1f). The breadth δΩ is the full width at half-maximum (fwhm) of the 0–0 emission band (Figure S5, Supporting Information D). The fact that δΩ at n = 6 is near the minimum for all surveyed samples reveals the uniform emissive states of MEH-PPV in the HEP regime, ruling out the possibility of PL superposition from various phases. Furthermore, the loose correlation between δΩ and λmax (Figure S4c, Supporting Information B), as well as the highest ηR’s of 17.13% in HEP matrices among all samples (Table S3, Supporting Information E), is attributed to the reduced molecular aggregation.
Like the n = 1 samples, the PL intensities correlate with λmax in an exponential decay fashion (Figure 1d) for films of various n's, revealing the pronounced ACQ effect on MEH-PPV optical behaviors. On the other hand, the quantum efficiency ηR does not exhibit any good correlation with radiative lifetimes τR's (Figure S4b, Supporting Information B); rather, it correlates loosely in a negative fashion with the nonradiative lifetimes τNR’s (Figure S 4d, Supporting Information B), indicating that nonradiative events strongly impede the emission processes.9,44
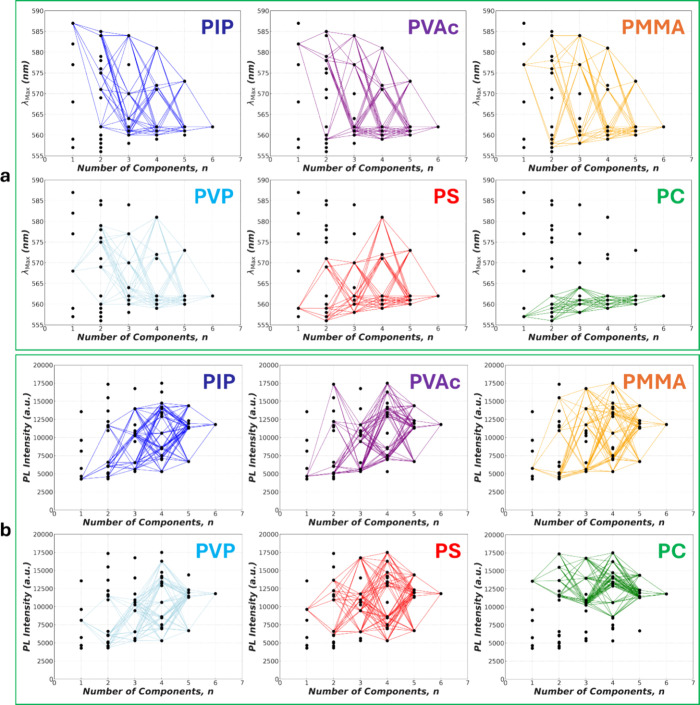
We further surveyed λmax across all n's for each matrix species (Figure 2a). We found that the polymer blends containing PC always exhibit the lowest λmax’s, indicating that PC facilitates the reduction of MEH-PPV aggregates even when the PC fraction dwindles in the HEP regime. This implies effective dispersion of the matrix species itself in addition to CP dispersion in HEPs. Conversely, the films containing PIP always manifest the highest λmax's unless they also contain PC, indicating PIP behaving as a CP aggregation promoter while PC can override the effect for all n’s. Along the same line, those exhibiting intermediate λmax’s at n = 1 also demonstrate intermediate λmax’s in their blends unless they are blended with PC or PIP.
Figure 2.
Detailed tracing of the λmax’s (a) and the PL intensities of the 0–0 transition (b) of the 1.0 wt % MEH-PPV for each polymer component in the various matrices of varying n.
Similarly, we found that all films that contain PC always deliver among the highest PL intensities, while those containing PIP always deliver the lowest unless blended with PC (Figure 2b). The polymers of intermediate PL intensities at n = 1 also manifest the intermediate PL intensities in the blended films unless they are blended with either PIP or PC. This reiterates molecular-scale dispersion of each matrix species in the HEP regime, allowing specific segmental interactions to influence the photonic behaviors of MEH-PPV.
PFO
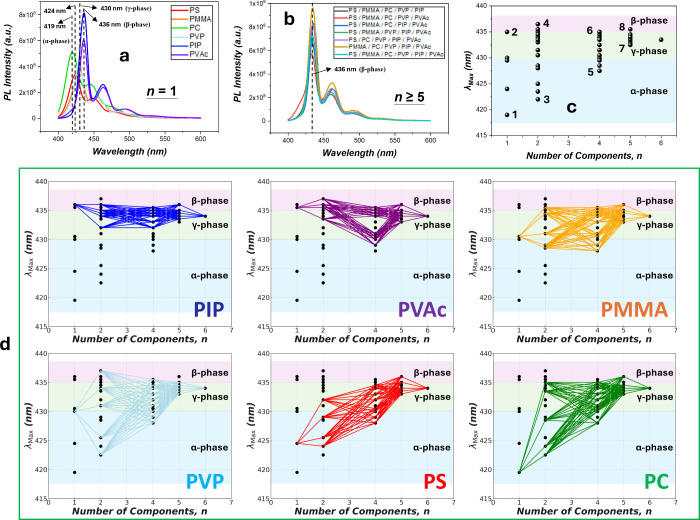
The PFO molecules, in contrast, interact differently with the HEP matrices to affect the photonic behaviors owing to their more rigid backbones and shorter conjugation lengths. As shown in Figure 3a, at n = 1, the PFO exhibits λmax = 417 nm of the 0–0 band in the PC matrix, revealing a metastable amorphous α-phase. The emission redshifts to λmax= 424 nm in PS, indicating an incipient degree of order of the largely amorphous PFO chains. The chain order is further enhanced in PMMA and PVP, with the PFO adopting the γ-phase emitting λmax = 430 nm. Finally, the chain order grows in PIP and PVAc, exhibiting λmax = 436 nm to signify formation of the planar β-phase in these matrices.
Figure 3.
(a) PL spectra of 1 wt % PFO in the various n = 1 matrices, showing distinct phase behaviors of the amorphous α-phase, γ-phase, and planar β-phase. (b) PL spectra of 1 wt % PFO in HEPs (comprising n = 5 and 6), exhibiting the formation of the β-phase. (c) λmax’s of 1 wt % PFO in the various polymer matrices. 1: PC, 2: PIP, 3: PC/PVP, 4: PVP/PVAc, 5: PS/PMMA/PC/PVP, 6: PMMA/PVP/PIP/PVAc, 7: PS/PMMA/PC/PVP/PVAc, and 8: PS/PMMA/PC/PIP/PVAc. (d) Tracing on λmax’s vs n for each polymer component.
The emission phases at n = 1 appear to reflect the extent of PFO segmental relaxation during interactions with the matrix polymer during spin coating. In that, we believe that the low Tg matrices, such as PIP (Tg= −67 °C) and PVAc (Tg= 42 °C), provide the environments for PFO chains to relax to the low-energy planar β-phase (λmax = 436 nm), an effect akin to that by high-boiling-point solvent residuals (such as isodurene and cyclopentanone) in PFO films.45 Conversely and consistently, the metastable α-phase (λmax = 417 nm) prevails in the highest Tg matrix of PC (Tg= 147 °C). In matrices of intermediate Tg’s between 100 and 120 °C (PS, PMMA, and PVP), λmax emerges between 424 and 430 nm, a spectral range between the emissions of the α- and β-phases.
As n increases beyond n = 1, the PL spectrum evolves toward the β-phase (Figure 3b,c and Figure S8, Supporting Information F), with λmax's narrowing their distribution that finally converge to well above 430 nm at large n’s, testifying the prevalence of the planar phase (Figure 3c). The dominance of the β-phase in the HEP matrices (Figure 3b) indicates an HEP environment promoting chain relaxation to the low-energy planar order, for which we assert that the molecular-level dispersion conferred by HEPs renders the CP to interact with the low-Tg matrix polymers, producing an effect similar to that at n = 1 even with much less fractions of the matrix polymer at higher n’s. The detailed tracings in Figure 3d consistently illustrate the persistence of such CP-matrix polymer interactions that influence PFO chain relaxation up to the HEP regime. Since the β-phase is commonly associated with respectable quantum yields ηR's (Figure 3a),24−35 the HEP strategy may help enhance PFO photonic performance, although other molecular interactions may also simultaneously influence ηR.
P3HT-rr
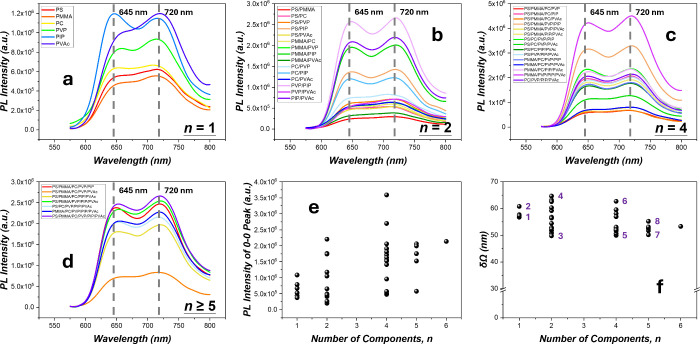
P3HT-rr, owing to its soft backbones and strong intersegmental ordering, presents a window for investigating how polymer crystallinity influences photonic behaviors in HEP environments. In contrast to the behaviors of MEH-PPV and PFO, the PL spectra in the various n = 1 matrices reveal a conspicuously constant spectral shape with little peak shifts (Figure 4a). It suggests the existence of a stable emission structure. Moreover, P3HT-rr molecules in solid matrices form a morphology composed of isolated amorphous chain segments interspersed within aggregated phases of varied orders. Since ordered chains offer longer conjugation lengths,41 they may serve as the “red chains” to funnel photoexcited energies, including that absorbed by the amorphous “blue chains”, into photon emissions. This stable energy transfer (Forster resonance energy transfer, FRET) effectively defines a constant emission structure that could have an effect on the PL spectrum. Notably, the light-emitting crystalline structures in this context are highly “preserved” to secure a quasi-constant emission spectral shape in the various matrices.
Figure 4.
PL of the 1.0 wt % P3HT-rr in the various polymer blends: (a–d) PL spectra in n = 1 (a), n = 2 (b), n = 4 (c), and n ≥ 5 (d) matrices; (e) PL intensity in the various matrices vs n. The PL intensity across all n's for every specific matrix species can be seen in Figure S9, Supporting Information G. (f) Breadth of the 0–0 peak δΩ in the different matrices vs n. 1: PIP, 2: PVP, 3: PVP/PIP, 4: PS/PVAc, 5: PS/PMMA/PVP/PIP, 6: PS/PMMA/PC/PVAc, 7: PS/PMMA/PC/PVP/PIP, and 8: PS/PMMA/PC/PVP/PVAc.
The PL intensities, however, vary enormously in the different matrix polymers at n = 1 (Figure 4a). The intensity variation seems to arise from changes in the amorphous environments that affect the ηR. We observed that the “softer” chain environments generally confer more efficient emissions, in that the rubbery PIP (Tg= −67 °C) gives the most efficient emissions followed by the low-Tg polymer of PVAc (Tg = 30–45 °C). Conversely, the glassy polymers of PS and PMMA (PS: Tg = 100 °C, PMMA: Tg = 105 °C) afford the chain environments giving the lowest PL emissions. Consistently, lying in between are PVP and PC matrices, glassy but of lower molecular weights (PVP: Tg = 120 °C, 40 kg/mol, PC: Tg = 147 °C, 45 kg/mol), following the trend that “plasticizing” environments result in higher PL emissions for the amorphous P3HT-rr chains.
Furthermore, the generally low ηR’s of P3HT-rr (∼2% for pristine P3HT-rr films) indicate that the excited states of P3HT-rr predominantly relax nonradiatively with rampant self-trapping.9,16 Since the strain energies causing self-trapping are mitigated by increased segmental flexibility,9,16 the softer chain environments would allow the amorphous chains to exhibit more effective energy transfer to the emissive crystalline aggregates. In addition, enhanced energy transfer may have contributed to the higher 0–0 band (645 nm) relative to the 0–1 band (720 nm) in PIP (Figure 4a).41,46−48 For P3HT, varied distributions of the J- and H-aggregates as proposed in the literature may also play a role in the emission behaviors, nevertheless.47
For samples of n > 1, the same spectral shape persists, with emission peaks at 645 and 720 nm corresponding, respectively, to the 0–0 and 0–1 bands, identical to those of the n = 1 samples (Figure 4b–d). We also found that the molecular factors influencing PL efficiency at n = 1 continue to operate for n > 1, in that the matrices containing rubbery PIP chains exhibit the highest PL intensities among the blends of the same n for all n's (Figure 4b–d and Figure S9, Supporting Information G). In addition, the PL intensities generally increase with n, except for an anomaly at n = 4, and reach a relatively high value at n = 6 (Figure 4e). The increasing trend of PL with n, recalling that ηR of P3HT-rr increases with molecular dilution (from ηR ∼ 2% in the pristine state to ηR ∼ 21% as diluted at 0.1 wt % in PS),16 implies a dilution effect derived from effective molecular dispersion as n increases. The moderate anomalous downturn beyond n = 4, on the other hand, seems to indicate the existence of a threshold fraction of PIP for effective ηR enhancement, below which (∼25 wt %) the enhancement may start to decline. Remarkably, that a small fraction of the softening polymer at n = 4 (∼25 wt % PIP) can generate more than 3.5-fold PL enhancements relative to that in the n = 1 matrix (100% PIP) indicates the prominent molecular dispersion in HEPs.
In addition, a trend of narrowing δΩ distribution with n was observed (Figure 4f), which was akin to that of MEH-PPV (Figure 1f). The narrowing underscores the uniformity of emissive states as dictated by the crystalline aggregates. It also indicates the relatively minor impact due to changes of the polymer matrix to the emissive crystalline morphology and consequently the δΩ, in contrast to that in MEH-PPV where the emissive noncrystalline aggregates are highly sensitive to matrix interactions and hence give rise to broader δΩ distribution (22 nm for MEH-PPV vs 15 nm for P3HT-rr at n = 2).
PL of Concentrated Conjugated Polymers in the Blends
The photonic behaviors of greater CP concentrations (c’s) up to c = 50 wt % were further explored for MEH-PPV, where two n = 5 high-entropy polymers (HEP-1 and HEP-2; HEP-1: equal-parted PS, PMMA, PC, PVP, and PIP; HEP-2: equal-parted PS, PMMA, PC, PVP, and PVAc) were prepared.
The MEH-PPV PL spectra in these systems exhibit an approximately constant spectral shape vs c, featuring a prominent 0–0 band and a lesser 0–1 band (Figure S10, Supporting Information H). The λmax’s, however, vary in different n = 1 matrices and undergo redshifts as c increases (Figure 5a). In the PIP matrix, λmax stays at ∼590 nm in the full c range from 1 to 50 wt %. Since the pristine MEH-PPV also emits at 590 nm,9 it seems to indicate that MEH-PPV always forms certain characteristic molecular aggregates in PIP independent of c to dominate the emissions. In contrast, MEH-PPV disperses to various degrees in other polymers, e.g., in the PC matrix, the emissions are located at λmax = 557 nm for 1 wt %, very close to MEH-PPV single-molecule emissions (∼555 nm), then increase slowly to 571 nm at 50 wt %, signifying the progressive CP aggregation as c increases. With λmax being the reddest in PIP, always staying at ∼590 nm, while others undergo redshifting with c, the distribution of λmax thus narrows as c increases (Figure 5a). In the concentrated CP regime, λmax correlates well with the Hansen solubility distance Ra (Figure 5c), similar to that in the 1 wt % diluted systems (Figure 1e), signifying lesser aggregation for smaller Ra’s, and vice versa, for CP mixing with the matrix polymer.
Figure 5.
(a,b) λmax (a) and PL intensity (b) vs MEH-PPV concentration in the various n = 1 matrices as well as the two n = 5 HEP matrices (HEP-1: equal-parted PS, PMMA, PC, PVP, and PIP; HEP-2: equal-parted PS, PMMA, PC, PVP, and PVAc). (c) Correlations between the emission properties (PL intensity and λmax) and Ra for the 50 wt % MEH-PPV in the n = 1 matrices. (d–f) ηR vs λmax (d), radiative lifetime τR (e), and nonradiative lifetime τNR (f) for MEH-PPV of various concentrations (1, 10, and 30 wt %) in the n = 1 and n = 7 matrices, with n = 7 data marked.
In the high-entropy polymers (HEP-1 and HEP-2), λmax’s persistently exhibit the lowest among films of the same CP concentration in the entire explored c range. In the c range from 1 to 15 wt %, dubbed the HEP regime where no polymer species overwhelm the others, the λmax’s remain almost constant with c, ranging in a small span of ∼562–567 nm (Figure 5a). As c increases, the λmax’s then increase slowly to 580 nm at c = 50 wt %. The emission peaks λmax’s of the HEP-1 and HEP-2 are nearly indistinguishable, indicating that swapping PIP with PVAc produces insignificant changes in terms of CP aggregation up to c = 50 wt %, testifying the robust suppression of CP phase separation in the HEP systems. The CP dispersion persistent into the high c’s agrees excellently with the observed molecular dispersion of the component polymers in the HEP matrices and by which the component polymer can directly influence the photonic behavior of the embedded CP molecules.
The PL intensity of any of these concentrated samples increases with c (Figure 5b), indicating that the rates of aggregation-caused quenching (ACQ) vs c for MEH-PPV are slower than that on increasing emissive species. Nevertheless, at n = 1, the PL intensity is among the lowest for CP in PIP and at the same time the highest in PC for the whole explored c range, consistent with the correlation of λmax with Ra (Figure 5c), like that in the diluted systems (c = 1 wt % vs n, Figure 2b), which clearly demonstrates the strong effect of polymer miscibility on PL intensity under the influence of ACQ. In the high-entropy polymers, the CP emits among the highest, believably owing to the more dispersed states of the CP molecules. In the concentrated films of 30 and 50 wt %, the HEP-2 emits moderately stronger than HEP-1, a behavior to be explored further but is tentatively attributed to lower residual stresses in the rubber-containing HEP-1 films.13−16
Quantum Efficiencies, Luminescence Lifetimes, and Some General Discussions
We further analyzed quantum efficiencies ηR’s and luminescence lifetimes τ’s of MEH-PPV in the c range up to 30 wt % at n = 1 and n = 7, examining the HEP effects. We found that ηR decreases continuously with λmax from ∼20 to ∼5% as the latter increases from ∼550 to 587 nm (Figure 5d), which can be interpreted as due to aggregation-caused quenching (ACQ). Furthermore, we found that greater c's are generally associated with shorter radiative lifetimes τR (Figures S6 and S7 and Tables S2–S5, Supporting Information E), and ηR does not correlate well with τR's (Figure 5e), although generally shorter τR's are believably correspond to greater propensities for radiative decay. Instead, ηR seems to increase with decreasing nonradiative lifetime τNR (Figure 5f), suggesting that the nonradiative pathways are competing strongly with the radiative processes in the CPs explored here.
In light of this observation, we also noticed that ηR’s of MEH-PPV in HEP matrices are among the highest, and there is a positive correlation between ηR and τΝR across all films, including HEPs. This suggests that better molecular dispersion via HEPs suppresses nonradiative pathways. Specifically, molecular dispersion attained by HEP mixing facilitates β-phase formation for PFO and allows segmental plasticization for amorphous P3HT-rr chains, both producing the effects of improved ηR.
Molecular dispersion via HEPs, in essence, is derived from diminishing encounter probabilities between like-polymers during processing at large n’s. This behavior is independent of the choice of component polymers and thus can apply to other multiple polymer systems in general. Although good dispersion conceptually would lead to the average over that of the individual components, if a strong dependence arises for a specific photonic perofrmance on molecular isolation, molecular dispersion via HEPs would engender the best or among the best performance results. In addition, when prominent interactions prevail conferred by some specific component polymers, the robust interactions would sway the averaged results. The phase behaviors of PFO and P3HT-rr as affected by PIP plasticization belong to the latter, while λmax and ηR of MEH-PPV the former. The averaging effect would also result in decreased spreads of photonic behaviors in general, unless phase separation or new phases cause an increase in the spread at small n’s before the final converging at large n’s when molecular dispersion via HEPs dominates.
Note that this work adopted a simplified “equal-parted approach” for analyzing the HEP blending, which only serves as a stepping stone for further in-depth explorations. For example, as illustrated by the “tracing” of each photonic attribute vs n for specific component polymers (Figures 2 and 3b and Figure S9, Supporting Information G), we can identify the favorable or unfavorable matrix polymers and then go on to adjust the compositions or modify the choices of component polymers for optimized performances.
Obviously, owing to the capability of precision tailoring via molecular tuning, HEPs are expected to be useful in many applications where polymers are already being used, in addition to the areas that call for new functions, potentially, e.g., optoelectronic devices, barrier materials, biomedical materials, stimulus-responsive materials, polymer compatibilizers, and recycling without classification, among others. However, further research and development endeavors are required to expand these possibilities. Capable of molecular-scale dispersion even for dissimilar polymers, simple blending via HEPs offers a method of molecular tailoring for polymer research and applications.
Conclusions
By studying the photonic behaviors of conjugated polymers (CPs) embedded in matrices of varied numbers of polymer species (n’s), we demonstrate that molecule-level dispersion of polymers can be attained in the high-entropy polymer (HEP) regime defined as n ≥ 5. For a dilute concentration of CP (c = 1 wt %), the photonic properties vary widely in the n = 1 matrices owing to the diverse solubility parameters, but the distribution narrows with n, and the CPs start to exhibit behaviors of molecule-level dispersion at n ≥ 5, where the matrix polymers compete with each other to exert direct influences on the embedded CP. Specifically, via HEPs, the MEH-PPV exhibits quasi-single-molecule emissions, the PFO demonstrates β-phase fluorescence upon molecular dilution, and the P3HT-rr renders well-dispersed amorphous chains highly susceptible to segmental plasticization. For higher CP concentrations, molecular-level dispersion also arises showing effects of molecular dispersion for c’s up to ∼15–20 wt %, consistent with the matrix dispersion behaviors operative in the dilute regime. With CP molecules still finely dispersed in HEPs, the photonic performances are enhanced up to c = 50 wt %. Based on the simple principle of diminishing encounter probabilities between like-polymers during processing, the HEP strategy offers a method of molecular tailoring via simple mixing.
Experimental Section
Chemicals and Materials
Eight optically inert polymers were selected for constructing the polymer matrices: polystyrene (PS, Mw = 123,000 g/mol), poly(methyl methacrylate) (PMMA, Mw = 350,000 g/mol), poly(bisphenol A carbonate) (PC, Mw = 45,000 g/mol), polyvinylpyrrolidone (PVP, Mw = 40,000 g/mol), cis-polyisoprene, (PIP, Mw = 35,000 g/mol), poly(vinyl acetate) (PVAc, Mw = 100,000 g/mol), poly(2,6-dimethyl-1,4-phenylene oxide) (PPO, Mw = 244,000 g/mol), and ethyl cellulose (EC, 48% ethoxyl), all purchased from Sigma-Aldrich except that PS was bought from Pressure Chemical Co. For the three conjugated polymers (CPs), PFO (poly(9,9-di-n-octylfluorenyl-2,7-diyl), Mw = 74,766 g/mol, PDI = 3.68) was purchased from Ossila Ltd., MEH-PPV (poly(2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene), Mn = 150,000–250,000 g/mol) was bought from Sigma-Aldrich, and P3HT-rr (poly(3-hexylthiophene-2,5-diyl), regioregular, Mw = 58,000 g/mol) was obtained from Rieke Metals. Chloroform (ACS grade) used as a cosolvent for all these polymers was purchased from Sigma-Aldrich.
Preparation of Solutions and Films
Each polymer was dissolved separately in chloroform before mixing and casting into thin films. The hygroscopic polymers (PMMA, PPO, and PVP) were dried at 85 °C under vacuum for 24 h to remove moisture before solution preparation. The solution of each matrix polymer was prepared by stirring at 25–30 °C for a day in ambient conditions, while the CP solutions were prepared by stirring at 35 °C for a day under wrapped aluminum foil in a nitrogen-maintained glovebox controlled under 3 ppm for both oxygen and moisture. For the n = 1 samples, the solution of the matrix polymer was added to the CP solution at an amount according to the desired concentration and stirred for half a day before spin coating into films. For the n > 1 samples, the matrix polymer solutions were first mixed in equal parts before adding the CP solution. Spin coating was carried out at 4000 rpm for 20 s, producing films of 35–45 nm thickness on a glass slide. The film thickness was determined using a scanning probe microscope (Icon, Bruker, the Instrument Center at NTHU). Multiple duplicated samples were prepared and tested to ensure data reproducibility. No new polymer species or chain cross-linking was produced via any chemical reactions from the sample preparation.
Characterization of the Ultrathin Films
The topography and phase images of the samples were examined also using the scanning probe microscope (Icon, Bruker, the Instrument Center at NTHU). The AFM data were further processed via a fast Fourier transform for quantitative analyses. The photoluminescence (PL) spectra were obtained by using a Horiba FluoroLog-3 (NanoLog-3) PL spectrometer, excited at 380, 480, and 550 nm, respectively, for PFO, MEH-PPV, and P3HT-rr. The quantum efficiencies (ηR’s) were obtained also by using the PL spectrometer with the equipped integrating sphere accessory, the errors of determination being around ∼3–5%. The fluorescence radiative lifetimes (τR) were measured using a time-correlated single-photon counting setup (TCSPC, the Instrument Center at NTHU), excited at 405 nm and detected at 560 and 437 nm, respectively, for MEH-PPV and PFO, using the PicoHarp software for data fitting. The nonradiative lifetime was then calculated from τR and ηR using the conventional equation.
Acknowledgments
This work was financially supported by the High Entropy Materials Center from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. We also acknowledge financial support from grants from the National Science and Technology Council in Taiwan (NSTC): NSTC 112-2221-E-007-005 and NSTC 113-2221-E-007-002. In addition, we thank the Instrument Center supported by NSTC of Taiwan at the National Tsing Hua University, for allowing the use of the TCSPC and AFM.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.4c10585.
Film morphology vs PL spectra, photonic properties of the 1.0 wt % MEH-PPV in the various polymer blends, solution properties of the polymer components, determination of δΩ and fwhm of the 0–0 band, luminescence lifetimes and quantum efficiencies, photonic properties of the 1.0 wt % PFO in the various polymer blends, photonic properties of the 1.0 wt % P3HT-rr in the various polymer blends, and photonic properties of concentrated CP in the polymer matrices (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Yeh J.-W.; Chen S.-K.; Lin S.-J.; Gan J.-Y.; Chin T.-S.; Shun T.-T.; Tsau C.-H.; Chang S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. 10.1002/adem.200300567. [DOI] [Google Scholar]
- Yeh J.-W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. 10.1007/s11837-013-0761-6. [DOI] [Google Scholar]
- Murty B. S.; Yeh J.-W.; Ranganathan S.; Bhattacharjee P. P.. High Entropy Alloys, 2nd ed., Elsevier, 2019. [Google Scholar]
- Huang Y.-J.; Yeh J.-W.; Yang A. C.-M. High-entropy polymers”: A new route of polymer mixing with suppressed phase separation. Materialia 2021, 15, 100978 10.1016/j.mtla.2020.100978. [DOI] [Google Scholar]
- Hojati-Talemi P.; Bächler C.; Fabretto M.; Murphy P.; Evans D. Ultrathin Polymer Films for Transparent Electrode Applications Prepared by Controlled Nucleation. ACS Appl. Mater. Interfaces 2013, 5, 11654–11660. 10.1021/am403135p. [DOI] [PubMed] [Google Scholar]
- Li H.; Wu Y.; Wang X.; Kong Q.; Fu H. A self-assembled ultrathin crystalline polymer film for high performance phototransistors. Chem. Commun. 2014, 50, 11000–11003. 10.1039/C4CC04547E. [DOI] [PubMed] [Google Scholar]
- Qiu L.-Z.; Wei S.-Y.; Xu H.-S.; Zhang Z.-X.; Guo Z.-Y.; Chen X.-G.; Liu S.-Y.; Wu D.; Luo L.-B. Ultrathin Polymer Nanofibrils for Solar-Blind Deep Ultraviolet Light Photodetectors Application. Nano Lett. 2020, 20, 644–651. 10.1021/acs.nanolett.9b04410. [DOI] [PubMed] [Google Scholar]
- Nguyen T.-Q.; Kwong R. C.; Thompson M. E.; Schwartz B. J. Improving the performance of conjugated polymer-based devices by control of interchain interactions and polymer film morphology. Appl. Phys. Lett. 2000, 76, 2454–2456. 10.1063/1.126374. [DOI] [Google Scholar]
- Lu H.; Weng Z.-M.; Chen C.-C.; Liao Y.-T.; Chang Y.-M.; Yang A. C.-M. Quantum Efficiency Increasing of a Pristine Polymer by Curbing Picosecond Self-Trapping via Segmental Stretching. Macromolecules 2021, 54, 11248–11255. 10.1021/acs.macromol.1c01487. [DOI] [Google Scholar]
- Liu Y.-H.; Huang C.-C.; Cheng C.-C.; Yang A. C.-M. Supramolecular confinement and photoluminescence enhancements of a conjugated polymer by hydrogen bonding in solid films. Mater. Chem. Phys. 2022, 277, 125505 10.1016/j.matchemphys.2021.125505. [DOI] [Google Scholar]
- Nijegorodov N. I.; Downey W. S. The Influence of Planarity and Rigidity on the Absorption and Fluorescence Parameters and Intersystem Crossing Rate Constant in Aromatic Molecules. J. Phys. Chem. 1994, 98, 5639–5643. 10.1021/j100073a011. [DOI] [Google Scholar]
- Lee P.; Li W.-C.; Chen B.-J.; Yang C.-W.; Chang C.-C.; Botiz I.; Reiter G.; Lin T.-L.; Tang J.; Yang A. C.-M. Massive Enhancement of Photoluminescence through Nanofilm Dewetting. ACS Nano 2013, 7, 6658–6666. 10.1021/nn4009752. [DOI] [PubMed] [Google Scholar]
- Chen P.-T.; Yang Y.-W.; Reiter G.; Yang A. C.-M. Large quantum efficiency enhancements of pristine conjugated polymer MEH-PPV by interlayer polymer diffusion. Polymer 2020, 204, 122753 10.1016/j.polymer.2020.122753. [DOI] [Google Scholar]
- Tung K.-P.; Chen C.-C.; Lee P.; Liu Y.-W.; Hong T.-M.; Hwang K. C.; Hsu J. H.; White J. D.; Yang A. C.-M. Large Enhancements in Optoelectronic Efficiencies of Nano-plastically Stressed Conjugated Polymer Strands. ACS Nano 2011, 5, 7296–7302. 10.1021/nn202117e. [DOI] [PubMed] [Google Scholar]
- Zorn M.; Bae W. K.; Kwak J.; Lee H.; Lee C.; Zentel R.; Char K. Quantum Dot–Block Copolymer Hybrids with Improved Properties and Their Application to Quantum Dot Light-Emitting Devices. ACS Nano 2009, 3, 1063–1068. 10.1021/nn800790s. [DOI] [PubMed] [Google Scholar]
- Lu H.; Chang C.-H.; Wu B.-R.; Wu N.-C.; Liang J.-Z.; Dai C.-A.; Yang A. C.-M. Reaching Nearly 100% Quantum Efficiencies in Thin Solid Films of Semiconducting Polymers via Molecular Confinements under Large Segmental Stresses. ACS Nano 2022, 16, 8273–8282. 10.1021/acsnano.2c02083. [DOI] [PubMed] [Google Scholar]
- Burroughes J. H.; Bradley D. D. C.; Brown A. R.; Marks R. N.; Mackay K.; Friend R. H.; Burns P. L.; Holmes A. B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. 10.1038/347539a0. [DOI] [Google Scholar]
- Braun D.; Heeger A. J. Visible light emission from semiconducting polymer diodes. Appl. Phys. Lett. 1991, 58, 1982–1984. 10.1063/1.105039. [DOI] [Google Scholar]
- Moses D. High quantum efficiency luminescence from a conducting polymer in solution: A novel polymer laser dye. Appl. Phys. Lett. 1992, 60, 3215–3216. 10.1063/1.106743. [DOI] [Google Scholar]
- Schwartz B. J. What makes a chromophore?. Nat. Mater. 2008, 7, 427–428. 10.1038/nmat2191. [DOI] [PubMed] [Google Scholar]
- Kuik M.; Wetzelaer G.-J. A. H.; Laddé J. G.; Nicolai H. T.; Wildeman J.; Sweelssen J.; Blom P. W. M. The Effect of Ketone Defects on the Charge Transport and Charge Recombination in Polyfluorenes. Adv. Funct. Mater. 2011, 21, 4502–4509. 10.1002/adfm.201100374. [DOI] [Google Scholar]
- Bright D. W.; Dias F. B.; Galbrecht F.; Scherf U.; Monkman A. P. The Influence of Alkyl-Chain Length on Beta-Phase Formation in Polyfluorenes. Adv. Funct. Mater. 2009, 19, 67–73. 10.1002/adfm.200800313. [DOI] [Google Scholar]
- Yang X. H.; Jaiser F.; Neher D.; Lawson P. V.; Brédas J.-L.; Zojer E.; Güntner R.; Scanduicci de Freitas P.; Forster M.; Scherf U. Suppression of the Keto-Emission in Polyfluorene Light-Emitting Diodes: Experiments and Models. Adv. Funct. Mater. 2004, 14, 1097–1104. 10.1002/adfm.200305012. [DOI] [Google Scholar]
- Wilhelm P.; Blank D.; Lupton J. M.; Vogelsang J. Control of Intrachain Morphology in the Formation of Polyfluorene Aggregates on the Single-Molecule Level. ChemPhysChem 2020, 21, 961–965. 10.1002/cphc.202000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei D.; Guo Y.; Lu D. Study of the Chain Condensation Process from a Dilute to a Concentrated Solution and the Transformation of the Chain Conformation from a Solution to a Film for the Conjugated Polymer PFO. ACS Omega 2022, 7, 8498–8505. 10.1021/acsomega.1c06144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng T.-W.; Yan H.; Nakamura T.; Omagari S.; Kim J.-S.; Vacha M. Real-Time Monitoring of Formation and Dynamics of Intra- and Interchain Phases in Single Molecules of Polyfluorene. ACS Nano 2020, 14, 16096–16104. 10.1021/acsnano.0c08038. [DOI] [PubMed] [Google Scholar]
- Huang L.; Huang X.; Sun G.; Gu C.; Lu D.; Ma Y. Study of β phase and Chains Aggregation Degrees in Poly(9,9-dioctylfluorene) (PFO) Solution. J. Phys. Chem. C 2012, 116, 7993–7999. 10.1021/jp301102t. [DOI] [Google Scholar]
- Chen C.-Y.; Chang C.-S.; Huang S.-W.; Chen J.-H.; Chen H.-L.; Su C.-I.; Chen S.-A. Phase-Separation-Induced Gelation of Poly(9,9-dioctylfluorene)/Methylcyclohexane Solution. Macromolecules 2010, 43, 4346–4354. 10.1021/ma100335c. [DOI] [Google Scholar]
- Deng Y.; Yuan W.; Jia Z.; Liu G. H- and J-Aggregation of Fluorene-Based Chromophores. J. Phys. Chem. B 2014, 118, 14536–14545. 10.1021/jp510520m. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Zhang X.; Wang Y.; Lu W.; Wang R.; Fan L.; Xu Y.; Lou H.; Zhang X. Controllable β-phase formation in poly(9,9-dioctylfluorene) by dip-coating for blue polymer light-emitting diodes. Thin Solid Films 2022, 746, 139118 10.1016/j.tsf.2022.139118. [DOI] [Google Scholar]
- Eggimann H. J.; Le Roux F.; Herz L. M. How β-Phase Content Moderates Chain Conjugation and Energy Transfer in Polyfluorene Films. J. Phys. Chem. Lett. 2019, 10, 1729–1736. 10.1021/acs.jpclett.9b00483. [DOI] [PubMed] [Google Scholar]
- Hamilton I.; Chander N.; Cheetham N. J.; Suh M.; Dyson M.; Wang X.; Stavrinou P. N.; Cass M.; Bradley D. D. C.; Kim J.-S. Controlling Molecular Conformation for Highly Efficient and Stable Deep-Blue Copolymer Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 11070–11082. 10.1021/acsami.8b00243. [DOI] [PubMed] [Google Scholar]
- Wu C.; Bull B.; Szymanski C.; Christensen K.; McNeill J. Multicolor Conjugated Polymer Dots for Biological Fluorescence Imaging. ACS Nano 2008, 2, 2415–2423. 10.1021/nn800590n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Chi L.; Hai G.; Fang Y.; Li X.; Xia R.; Huang W.; Gu E. An easy approach to control β-phase formation in PFO films for optimized emission properties. Molecules 2017, 22, 315. 10.3390/molecules22020315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perevedentsev A.; Chander N.; Kim J.-S.; Bradley D. D. C. Spectroscopic properties of poly(9,9-dioctylfluorene) thin films possessing varied fractions of β-phase chain segments: enhanced photoluminescence efficiency via conformation structuring. J. Polym. Sci., Part B: Polym. Phys. 2016, 54, 1995–2006. 10.1002/polb.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T.; Brazard J.; Ono R. J.; Hanson B.; Traub M. C.; Wu Z.-Q.; Li Z.; Bolinger J. C.; Ganesan V.; Bielawski C. W.; et al. Regioregularity and Single Polythiophene Chain Conformation. J. Phys. Chem. Lett. 2011, 2, 1400–1404. 10.1021/jz200546x. [DOI] [Google Scholar]
- Sirringhaus H.; Brown P. J.; Friend R. H.; Nielsen M. M.; Bechgaard K.; Langeveld-Voss B. M. W.; Spiering A. J. H.; Janssen R. A. J.; Meijer E. W.; Herwig P.; de Leeuw D. M. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 1999, 401, 685–688. 10.1038/44359. [DOI] [Google Scholar]
- Brown P. J.; Thomas D. S.; Köhler A.; Wilson J. S.; Kim J.-S.; Ramsdale C. M.; Sirringhaus H.; Friend R. H. Effect of interchain interactions on the absorption and emission of poly(3-hexylthiophene). Phys. Rev. B 2003, 67, 064203 10.1103/PhysRevB.67.064203. [DOI] [Google Scholar]
- Spano F. C.; Silva C. H- and J-Aggregate Behavior in Polymeric Semiconductors. Annu. Rev. Phys. Chem. 2014, 65, 477–500. 10.1146/annurev-physchem-040513-103639. [DOI] [PubMed] [Google Scholar]
- Niles E. T.; Roehling J. D.; Yamagata H.; Wise A. J.; Spano F. C.; Moulé A. J.; Grey J. K. J-Aggregate Behavior in Poly-3-hexylthiophene Nanofibers. J. Phys. Chem. Lett. 2012, 3, 259–263. 10.1021/jz201509h. [DOI] [Google Scholar]
- Panzer F.; Bässler H.; Köhler A. Temperature Induced Order–Disorder Transition in Solutions of Conjugated Polymers Probed by Optical Spectroscopy. J. Phys. Chem. Lett. 2017, 8, 114–125. 10.1021/acs.jpclett.6b01641. [DOI] [PubMed] [Google Scholar]
- Wang R.; Yang X.; Hu S.; Zhang Y.; Yan X.; Wang Y.; Zhang C.; Sheng C. Effect of Thermal Annealing on Aggregations in MEH-PPV Films. J. Phys. Chem. C 2019, 123, 11055–11062. 10.1021/acs.jpcc.8b11991. [DOI] [Google Scholar]
- Hu D.; Yu J.; Barbara P. F. Single-Molecule Spectroscopy of the Conjugated Polymer MEH-PPV. J. Am. Chem. Soc. 1999, 121, 6936–6937. 10.1021/ja990139d. [DOI] [Google Scholar]
- Kobrak M. N.; Bittner E. R. A dynamic model for exciton self-trapping in conjugated polymers. II. Implementation. J. Chem. Phys. 2000, 112, 5410–5419. 10.1063/1.481126. [DOI] [Google Scholar]
- Khan A. L. T.; Sreearunothai P.; Herz L. M.; Banach M. J.; Köhler A. Morphology-dependent energy transfer within polyfluorene thin films. Phys. Rev. B 2004, 69, 085201 10.1103/PhysRevB.69.085201. [DOI] [Google Scholar]
- Xu B.; Holdcroft S. Molecular control of luminescence from poly(3-hexylthiophenes). Macromolecules 1993, 26, 4457–4460. 10.1021/ma00069a009. [DOI] [Google Scholar]
- Banerji N.; Cowan S.; Vauthey E.; Heeger A. J. Ultrafast Relaxation of the Poly(3-hexylthiophene) Emission Spectrum. J. Phys. Chem. C 2011, 115, 9726–9739. 10.1021/jp1119348. [DOI] [Google Scholar]
- Baghgar M.; Labastide J. A.; Bokel F.; Hayward R. C.; Barnes M. D. Effect of Polymer Chain Folding on the Transition from H- to J-Aggregate Behavior in P3HT Nanofibers. J. Phys. Chem. C 2014, 118, 2229–2235. 10.1021/jp411668g. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.