Abstract
Background
Salt stress is one of the major environmental factors affecting plant growth and productivity. BRI1-EMS suppressor 1/brassinazole-resistant 1 ((BES1/BZR1) plays an important role in responding to abiotic stress in plants. Although the impacts of BES1/BZR1 on plant growth and resistance have been documented, the potential mechanisms are not fully elucidated in Betula platyphylla. This work contributes to the understanding of how BES1/BZR1 promotes stress tolerance in woody plants.
Results
Six BES1/BZR1 family members were identified from Betula platyphylla. Cis-element analysis showed that the promoters of six genes were rich in ABA-responsive element (ABRE), MYB and MBS cis-acting elements, which are reported to be involved in abiotic stress responses. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis indicated that BpBZR1-6 (BPChr10G06000) could be induced by salt stress, ABA and BRs. BpBZR1-6 was localized in the nucleus and had transactivation activity. Ectopic expression of BpBZR1-6 enhanced Arabidopsis tolerance and decreased abscisic acid (ABA) sensitivity under salt treatment. Specifically, the seed germination rate, root length, fresh weight and chlorophyll content were significantly higher in BpBZR1-6-overexpressing (OE) transgenic plants than in wild-type (WT) plants after salt stress (P < 0.05). Additionally, BpBZR1-6 overexpression showed enhanced the reactive oxygen species (ROS) scavenging capability under salt stress, including increasing the activities of antioxidant enzyme, resulting in a decrease in O2− and H2O2 accumulation, and reducing malondialdehyde (MDA) content. Meanwhile, the expression levels of six antioxidant enzyme genes were higher in OE plants than in WT plants after stress.
Conclusion
BpBZR1-6 overexpression enhanced the salt tolerance of transgenic plants by modulating antioxidant enzyme gene expression and ROS scavenging, which may provide underlying strategy for breeding of salt-tolerant plants.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05738-6.
Keywords: Betula platyphylla, BpBZR1-6, Salt stress, Transgenic A. thaliana, ROS scavenging
Background
Plants are coordinately regulated by various hormones to improve their resistance to abiotic stress environments such as drought, salinity, high temperature and low temperature [1]. Brassinosteroids (BRs) is the sixth natural plant hormone newly identified in recent years and plays an important role in plant growth and development, signal transduction, biotic and abiotic stress response and other processes [2–5]. The molecular determinants of the BR signaling pathway start with the receptor BRI1 and coreceptor BAK1 located on the membrane and ultimately lead to activation of the BES1/BZR1 family of transcription factors [6]. BES1/BZR1 is a plant-specific transcription factor that regulates the expression of BR target genes and ultimately regulates the growth, development and resilience of plants [7, 8].
BES1/BZR1 plays important role in plant adaptation to environmental stress. BES1/BZR1 is involved in drought, heat and freeze stress response by regulating glutathione S-transferase 1 (GST1) gene expression, responding to dry RD26 and CBF genes, and interacting with RD26 and WRKY transcription factors [9–13]. In transgenic tomato (Solanum lycopersicum) and A. thaliana, it was found that BES transcription factors positively regulate BR signaling and are simultaneously tolerant to salt stress [14]. Using a transient transformation system, it was found that the overexpression of BpBZR1 improved the tolerance of birch to salt stress by removing the accumulation of H2O2 and malondialdehyde (MDA) and enhancing the activities of superoxide dismutase (SOD) and peroxidase (POD/Prx) [15]. Studies on the relationship between BRs and salt stress have recently revealed the mechanisms that may control BR-ABA antagonism at multiple regulatory levels from the receptor complex to downstream transcription factors [6]. In addition, BES1 transcription factors can also participate in primary phloem morphogenesis, inhibit the ABA insensitive genes ABI3 and ABI5, attenuate ABA signaling in seedling development, accelerate flowering in the reproductive stage, and regulate hypocotyl elongation [16]. The regulation of downstream genes by the BES1/BZR1 gene is achieved through binding to cis-elements such as E-box (CANNTG), appearing in many BR-induced promoters. BES1/BZR1 interacts with a basic helix-loop-helix protein, BIM1, to synergistically bind to the E-box [17]. BES1/BZR1 transcription factors can bind BRRE (CGTGT/CG) and E-box in the promoter of many genes that regulate plant growth and development in Arabidopsis [18, 19]. Taken together, the critical role of BES/BZR family in developmental processes and stress responses, has contributed to the functional research and their regulation mechanisms in plants becoming a hot research topic. In addition, there are few studies on the stress resistance function of BES/BZR family genes in woody plants. Therefore, it is of great significance to identify the key genes of BES/BZR family stress resistance in woody plants and explore their functions for studying the molecular mechanism of plant stress resistance and molecular breeding.
Betula platyphylla Suk (birch) is an important afforestation and landscaping tree species in Northeast China. There is a large area of saline alkali land in the western part of Northeast China. Therefore, cultivating salt-tolerant birch has important value for expanding afforestation areas. Previous studies have shown that five BES/BZR genes are involved in salt tolerance in birch [15]. In this study, a novel BES1/BZR1 gene, BpBZR1-6, was identified, which was upregulated significantly under salt stress and responses to ABA and BR treatment. The salt tolerance function of BpBZR1-6 was proven using transgenic A. thaliana. This study provides insight regarding the functions of BpBZR1 under salt stress and provides a theoretical basis for exploring the mechanism of BZR transcription factors in birch.
Results
Identification and sequence analysis of BpBZRs in birch
A new BZR1 gene was identified from birch and named BpBZR1-6, along with five previously identified BpBZR1 genes, and a total of 6 BpBZR1 family members were identified (Supplementary Table S1). To investigate the structural relationship between BpBZR1-6 and the other five BpBZR1 genes sequences, sequence alignment, intron distribution, and phylogenetic analysis were performed. The results showed that all six members had BZR conserved domains (Fig. 1A). The N-terminus of BpBZR1-6 had a typical BZR domain and was a typical DNA-binding motif of the basic helix-loop-helix (bHLH) (Fig. 1E). The intron and exon gene structures showed that BpBZR1-1, BpBZR1-4, and BpBZR1-6 all contained one intron, and BpBZR1-2 and BpBZR1-5 contained 2–3 introns, however, BpBZR1-3 had no introns (Fig. 1C). The phylogenetic tree indicated that BpBZR1-1 and BpBZR1-4 were clustered into one group, BpBZR1-6 was clustered into an individual group and closely related to ZmBZR1-2 (Fig. 1D). The multiple sequence alignment indicated that the amino acid sequences of BpBZR1-6, AtBZR and ZmBZR were relatively conserved.
Fig. 1.
Gene structure, cis-acting elements, phylogenetic analysis and multiple sequence alignments of BpBZRs. A Protein structure analysis of six BpBZRs. The yellow and green boxes represent the BES/BZR family conserved domains. B The region 2000 bp upstream sequence of the start codon of the BpBZRs was selected for cis-acting element analysis of BpBZR promoters. Boxes in different colors represent different cis-acting elements. C Intron-exon structures of six BpBZRs. The filled boxes and lines represent exons and introns. Green and yellow boxes represent UTR sequences and CDSs. D Phylogenetic relationships of BpBZRs with Arabidopsis thaliana, Zea mays and Camellia sinensis, and the phylogenetic tree was produced by MEGA5.0 software based on these complete protein sequences. E Multiple sequence alignment analysis of the BpBZR1-6 protein with AtBES1 (NM_202134), AtBEH1 (NM_114935), AtBEH2 (NM_119842), CsBES1-1 (TEA003951), CsBES1-2 (TEA004217), CsBES1-3 (TEA012036), ZmBZR1-2 (ONM28854) and ZmBZR1-5 (AQK60894). The black background represents a similarity of 100%. The dark gray background represents the similarity of 75 -100%. The light gray background represents a similarity of 35-75%
Cis-acting element analysis of six BpBZR promoters
The cis-acting element analysis of six BpBZR promoters showed that the stress-related elements, light response elements, hormone response elements and other elements were located on the BpBZR promoters. All six BpBZR genes promoters contained MYB and ABA-responsive element (ABRE) cis-acting elements, and most of the BpBZR genes had MBS, low-temperature responsive element (LTR), dehydration response element (DRE), and GT1-motif cis-acting elements (Fig. 1B). These results suggested that most BpBZR genes play important roles in abiotic stress responses.
BpBZR1-6 responses to abiotic stress
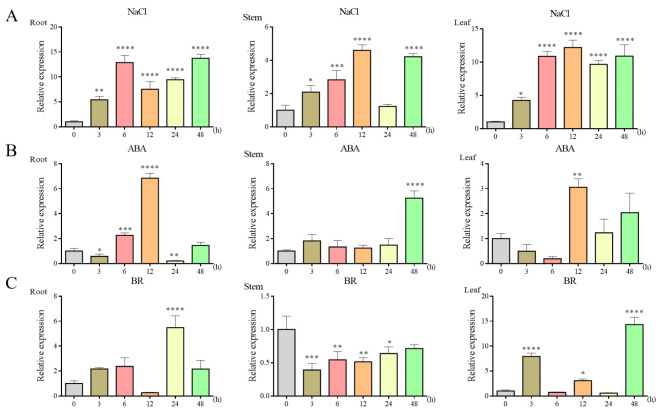
To analyze the expression pattern of BpBZR1-6 under stress, the expression levels of BpBZR1-6 in birch seedlings treated with NaCl, ABA and BRs were examined by quantitative real-time polymerase chain reaction (qRT-PCR). Under salt treatment, BpBZR1-6 showed significant upregulation in the roots and leaves and was gradually induced more than 12-fold, peaking at 48 h and 12 h, respectively. In the stems, the expression of BpBZR1-6 was gradually upregulated in the early stage of treatment and reached the highest level at 12 h (Fig. 2A). We also conducted qRT‒PCR testing again to compare the response of six BpBZRs to salt stress, the expression levels of BpBZR1-6 in the roots and leaves were significantly upregulated at all time points and were higher than that of the other five BpBZR genes (Supplementary Fig. S1). Under ABA treatment, obvious upregulation of BpBZR1-6 expression was observed in the roots and stems at 12 h and 48 h after treatment (Fig. 2B). The expression of BpBZR1-6 was significantly upregulated more than 10-fold in the leaves compared with the control treated with BR for 48 h, with a peak at 24 h in the roots (Fig. 2C). These results indicated that the expression of BpBZR1-6 can be induced by salt, ABA and BRs.
Fig. 2.
Analysis of expression patterns of BpBZR1-6 in roots, stems and leaves under NaCl, ABA and BR stress. The seedlings were exposed to a water solution supplemented with 200 mM NaCl and sprayed with 100 µM ABA and 1 µM BR. Root, stem and leaf samples were collected at 0, 3, 6, 12, 24, and 48 h. A NaCl, B ABA, C BR. The 0 h time point was used as control. All values are the means (± SE) of three biological replicates. Student’s t-test, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
BpBZR1-6 is localized in the nucleus
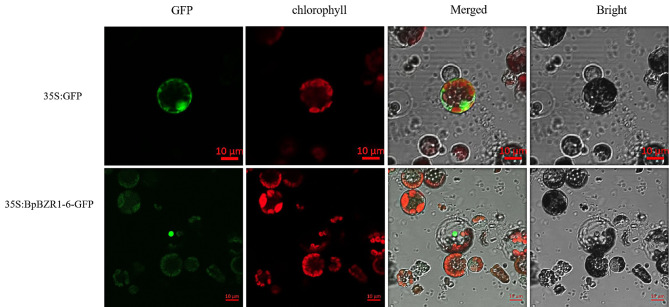
To explore the subcellular localization, green fluorescent protein (GFP) was fused to the C-terminus of the BpBZR1-6 protein. The recombinant vector 35 S:BpBZR1-6-GFP and 35 S:GFP as controls were transiently transformed into the protoplasts of P. trichocarpa leaves. The observation of GFP fluorescence showed that the control GFP was localized to the plasma membrane, nucleus and cytosol. However, 35 S:BpBZR1-6-GFP was localized in the nucleus only (Fig. 3). This result indicates that BpBZR1-6 is a nuclear-localized protein.
Fig. 3.
Subcellular localization of BpBZR1-6. The ORF of BpBZR1-6 was fused to 35 S:pBI121-GFP, and 35 S:GFP and 35 S:BpBZR1-6-GFP were transiently expressed in poplar protoplasts for 18 h. The scale bar was 10 μm
BpBZR1-6 shows transactivation activity
To determine the transactivation activity of BpBZR1-6, the complete BpBZR1-6 CDS and its fragments were fused into the pGBKT7 vector and transformed into yeast-two hybrid (Y2H) yeast strain. Yeast cells carrying BpBZR1-6 grew on SD/-Trp/-his/X-α-Gal and appeared blue colonies, indicating that the BpBZR1-6 protein had transactivation activity. Furthermore, the amino acid sequence of BpBZR1-6 was divided into two fragments, dC1 (1-106 aa) and dC2 (107–212 aa), to test transactivation activity. The results showed that yeasts containing fragment of dC2 grew on SD/-Trp/-his/X-α-Gal and appeared blue colonies, implying that dC2 had transactivation activity, in contrast, dC1 had no transactivation activity. Next, dC2 was divided into two fragments, dC3 (107–172 aa) and dC4 (173–212 aa), the yeasts containing fragment of dC3 showed blue colonies on SD/-Trp/-his/X-α-Gal medium (Fig. 4). The yeast experiments suggested that the minimum transactivation activity domain was located on a fragment of dC3 (107–172 aa).
Fig. 4.
Identification of the minimal transactivation activity domain in BpBZR1-6. The full-length (FL) and different fragments (dC1-4) of BpBZR1-6 were fused into the GAL4 DNA-binding domain. They were transformed into yeast cells and allowed to grow on SD/-Trp or SD/-Trp/-His/X-α-gal medium to determine the active region
BpBZR1-6 binds to E-box elements
E-box elements are considered to be BES/BZR transcription factor binding elements [20]. In our study, a yeast-one hybrid (Y1H) assay was performed to test whether BpBZR1-6 binds to the E-box elements. The results showed that yeast cells co-transformed with pGADT7- BpBZR1-6/pHIS2-E-box grew on SD/-Leu/-Trp/-His with 50 mM 3-AT medium. In the negative control, yeast cells co-transformed with pGADT7- BpBZR1-6-pHIS2-p53 did not grow normally (Fig. 5). These results indicated that BpBZR1-6 can bind to the E-box elements in yeasts.
Fig. 5.
Y1H assay analyses of binding between BpBZR1-6 and the E-box. pGADT7-p53 and pHIS2-p53 were co-transformed as positive controls. pGADT7-BpBZR1-6-pHIS2-p53 was the negative control
Ectopic expression of BpBZR1-6 facilitates seed germination under salt stress and reduces the sensitivity of germination to ABA
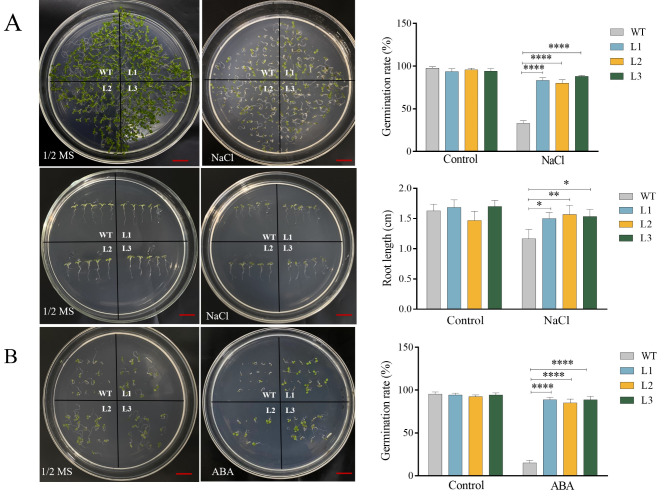
To investigate the function of BpBZR1-6 in the plant response to salt stress, three independent homozygous T3 transgenic Arabidopsis lines (L1, L2 and L3) transformed with 35 S:BpBZR1-6 were selected and used for stress treatment analysis. The transgenic and WT seeds were sterilized on normal 1/2 MS culture medium and 1/2 MS culture medium containing 100 mM NaCl and 3 µM ABA to observe germination. Subsequently, on the 1/2 MS culture medium without treatment, the seedlings displayed a vigorous phenotype, and root lengths showed no significant difference. However, on the 1/2 MS culture medium supplemented with 100 mM NaCl, the germination rates and root lengths of the three lines were markedly higher or longer than those of the WT (Fig. 6A). At the same time, on 1/2 MS culture medium containing 3 µM ABA, the germination rates of transgenic Arabidopsis lines were dramatically higher than those of WT and exhibited insensitivity to ABA (Fig. 6B). The results showed that the expression of BpBZR1-6 accelerates seed germination rates and root lengths under salt stress and decreases their ABA sensitivity.
Fig. 6.
Analysis of the germination rate and root lengths of WT and BpBZR1-6-overexpressing transgenic plants under NaCl and ABA stress. A Germination percentage and root length experiment under 100 mM NaCl treatment. B Germination percentage experiment under 3 µM ABA treatment. WT stands for wild type plant. The L1-L3 were BpBZR1-6 transgenic plants. Values are the mean ± SD, Student’s t-test, * P < 0.05, ** P < 0.01, **** P < 0.0001
BpBZR1-6 improves the salt tolerance of transgenic Arabidopsis
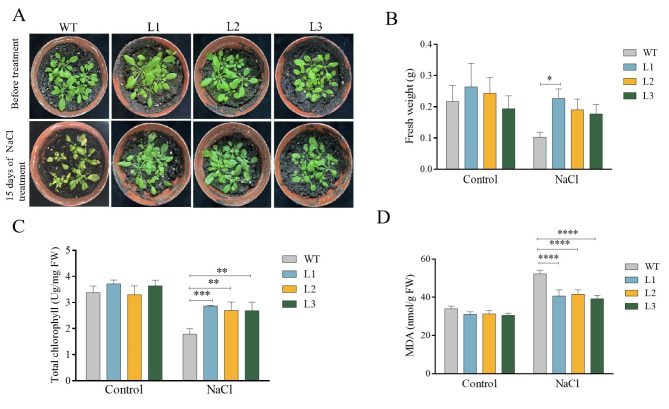
To further investigate the tolerance of BpBZR1-6 transgenic Arabidopsis to salt stress in soil, seedlings of BpBZR1-6 OE and WT plants were treated with 100 mM NaCl for 15 d. There were no obvious differences between transgenic and WT plants under control conditions. However, after 15 days of treatment with NaCl, the growth of both BpBZR1-6 OE transgenic plants and WT plants was inhibited, but the vegetative growth of transgenic plants was better than that of WT plants (Fig. 7A). Meanwhile, the fresh weight of transgenic plants was higher than that of WT plants after salt treatment (Fig. 7B).
Fig. 7.
Phenotype of the WT and transgenic Arabidopsis plants under salt stress. A Growth states of transgenic and WT seedlings after 15 days of salt stress. B-D Fresh weight, chlorophyll content, and MDA content of transgenic and WT Arabidopsis seedlings under salt stress. WT stands for wild type plant. The L1-L3 were BpBZR1-6 transgenic plants. Vertical bars in B-D indicate ± SE of three replicates. Student’s t-test, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
To examine how transgenic Arabidopsis expressing BpBZR1-6 improve their salt tolerance, the contents of chlorophyll and MDA were measured. The chlorophyll contents in the transgenic lines were significantly higher than those in the WT after salt stress, however, the MDA contents in the transgenic lines were significantly lower than those in the WT after salt stress (Fig. 7C, D). These findings indicated that the ectopic expression of the BpBZR1-6 gene improves the salt tolerance of transgenic Arabidopsis by reducing chlorophyll loss and MDA accumulation.
BpBZR1-6 enhances reactive oxygen species (ROS) scavenging capacity under salt stress
To measure the accumulation of O2− and H2O2 in the WT and BpBZR1-6 OE plants under control and salt conditions, nitro blue tetrazolium (NBT) staining (for O2−) and 3,3’-diaminobenzidine (DAB) staining (for H2O2) were performed. Under control conditions, there were no obvious differences in the accumulation of O2− and H2O2 between WT and transgenic Arabidopsis plants. Under salt stress, the accumulation of O2− and H2O2 in the leaves of the BpBZR1-6 OE plants was lower than that in the leaves of the WT plants (Fig. 8A). These results suggested that OE plants improved their salt tolerance by reducing the accumulation of ROS and that BpBZR1-6 plays an important role in enhancing ROS-scavenging capacity under salt stress.
Fig. 8.
BpBZR1-6-overexpressing Arabidopsis plants exhibited enhanced antioxidant activity. A DAB staining and NBT staining were observed in WT and transgenic Arabidopsis leaves under normal conditions or 200 mM NaCl for 12 h. B-C SOD and POD activity. D-E Analysis of SOD and POD genes expression under normal conditions or salt treatment. WT stands for wild type plant. The L1-L3 were BpBZR1-6 transgenic plants. Values are the mean ± SD, Student’s t-test, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
To uncover the potential molecular mechanisms underlying the enhanced tolerance of the BpBZR1-6 OE plants under stress, superoxide dismutase (SOD) and peroxidase (POD/Prx) activities and relative expression levels of SOD (AtCSD3, AtSOD1, AtSOD3) and POD/ Prx (AtPrx4, AtPrx7, AtPrx8) genes in Arabidopsis were investigated. The results showed that the SOD and POD activities were significantly higher in the transgenic plants than in the WT plants under salt stress (Fig. 8B, C). Compared with the WT plants, the SOD and POD genes showed significantly higher expression levels in OE plants under salt stress, under control conditions, the expression levels of AtCSD3, AtSOD1, AtSOD3, AtPrx4 and AtPrx8 showed no differences between WT and transgenic plants. (Fig. 8D, E). Further analysis demonstrated that these SOD and POD genes promoters were rich in E-box elements (Supplementary Fig. S2). Taken together, these results indicated that the BpBZR1-6 OE plants suffered less stress-induced damage than the WT plants due to the increased expression of antioxidant enzyme genes and their enzyme activities.
Discussion
Plants often encounter biotic and abiotic stresses that seriously affect their growth, resulting in decreased yield and quality. Moreover, the plants is regulated by a variety of different signaling pathways, including BR signaling. Transcription factors can be rapidly induced to express, reaching a peak within a few hours and then decreasing under abiotic stress conditions [21]. BZR TF genes of Eucalyptus grandis (EgrBZR) showed differential transcript abundance levels in response to exogenously applied BR, MeJA, and SA and salt and cold stresses [22]. The BES/BZR family includes plant-specific transcription factors involved in the BR reaction pathway, it is not only involved in plant growth and development but also responds to drought, high salt, cold and heat stresses [14, 23, 24]. Overexpression of TaBZR2 from wheat (Triticum aestivum L.) in plants exhibited drought-tolerant phenotypes [10]. Therefore, identification of the functions of BES/BZR transcription factors is crucial for understanding the molecular mechanisms of plant adaptation to biotic and abiotic stress environments. Members of the BES/BZR family have been identified in at least 149 plant species over the past decade [25]. In previous studies, it has been demonstrated that there are eleven ZmBES1/BZR1 TFs in maize (Zea mays L.) [26, 27], 20 BES/BZR gene family members were identified in wheat (Triticum aestivum L.) [28], seven in foxtail millet (Setaria italica (L.) P. Beauv.) [28], and six in E. grandis [22]. In the present study, six BES1/BZR1 gene family members were identified in B. platyphylla, and all have BES1/BZR1 conserved domains (Fig. 1A). The intron and exon gene structure of BpBZRs showed that there are differences in the number of introns of family members, which indicates the differences in the evolution of the BES/BZR family (Fig. 1C). The multiple sequences alignment of BZR1 proteins from birch, A. thaliana, Zea mays and Camellia sinensis, showed that BpBZR1-6 contains vital helix-loop-helix (bHLH) structures that are typical of target gene binding (Fig. 1E).
The analysis of cis-acting elements showed that the promoter regions of the six BpBZR genes were rich in elements related to stress responses, such as DRE, ABRE, MYB, LTR and TGACG-motif elements. The number of plant hormone response elements and stress response elements in the promoter of different BES/BZR members was variable. For instance, the promoter of BpBZR1-6 contains four ABREs, which are ABA response elements, while the promoters of BpBZR1-1, BpBZR1-2 and BpBZR1-3 contain only one (Fig. 1B). The phylogenetic analysis indicated that BpBZR1-6 was closely related to ZmBZR1-2 (Fig. 1D). Studies have shown that ZmBES1/BZR1-2 and ZmBES1/BZR1-5 functionally participate in regulating seed size and stress response [20, 29]. BES/BZR members of the same family may respond differently to plant hormones or environmental stresses, suggesting differences in their functions in hormone signaling pathways and stress responses [28]. In previous studies, five BpBZR1 family members were upregulated or downregulated in response to abiotic stress [15]. Likewise, our results showed that the six different family members had different responses to salt stress, implying their various biological functions (Supplementary Fig. S1). The present results further clarified that the expression of BpBZR1-6 was upregulated in three tissues under salt stress and was higher than other five BpBZR1 genes. BpBZR1-6 also responds to ABA and BR treatment in birch (Supplementary Fig. S1 and Fig. 2). Therefore, all these results suggested that BpBZR1-6 played roles in the salt stress response of birch and might be involved in the ABA and BR signaling pathways.
Plants are constantly subjected to multiple abiotic stresses that have effects on plant growth, and they respond to these abiotic stresses in many ways, from gene expression to physiology [30, 31]. To further validate the salt tolerance function of BpBZR1-6 in plants, the growth, physiology, and gene expression changes of transgenic and wild-type plants under salt stress were compared using Arabidopsis transgenic experiments. In our study, BpBZR1-6 transgenic Arabidopsis lines exhibited salt tolerance, reflected in a greater germination rate of roots, root length, fresh weight and chlorophyll content of seedlings compared to those of the WT under salt stress (Fig. 7A-C), indicating that the expression of the BZR gene reduces the impact of salt stress on plant growth. Salt stress can cause oxidative damage to proteins and nucleic acids due to excessive accumulation of ROS [32]. MDA is one of the most important products of membrane lipid peroxidation, and abiotic stress can aggravate its production and thus aggravate membrane damage [33]. Hence, the MDA content is widely used to evaluate plant tolerance to abiotic stresses. Meanwhile, plants use some complex antioxidant strategies to address damage caused by ROS [34]. DAB and NBT staining can be used to determine the amount of H2O2 and O2− [35]. The results showed that BpBZR1-6 transgenic plants accumulated less MDA than WT plants to resist damage (Fig. 7D). DAB and NBT staining showed that there was less ROS in transgenic lines of BpBZR1-6 (Fig. 8A). The enzyme activities of SOD and POD were higher in BpBZR1-6 OE plants than in WT plants after salt stress treatment (Fig. 8B-C). These results indicate that BpBZR1-6 can enhance plant salt tolerance by increasing antioxidant enzyme activity to scavenge ROS and reduce membrane damage.
POD and SOD are important genes for scavenging ROS. Studies have shown that POD genes can improve cold tolerance by removing ROS [36]. Further investigation revealed that the expression levels of AtCSD3 and AtCSS were upregulated under salt stress [37]. These studies demonstrated that SODs and PODs, the first line of defense against oxidative damage, are involved in scavenging ROS in response to various abiotic stresses. In this study, the expression levels of the SOD and POD genes AtCSD3, AtSOD1, AtSOD3, AtPrx4, AtPrx7 and AtPrx8 in OE and WT plants were examined, and the results showed a significant increase in POD and SOD transcripts in the transgenic plants compared to WT plants under salt stress (Fig. 8E). These results indicate that BpBZR1-6 can induce the expression of POD and SOD genes to scavenge ROS in transgenic Arabidopsis. Heterologous expression of ZmBES1/BZR1-5 in transgenic Arabidopsis resulted in decreased ABA sensitivity and positively enhanced salt and drought tolerance under osmotic stress by binding to the E-box to induce the expression of the downstream gene RCI2A [20]. Our study also identified that BpBZR1-6 can bind to the E-box (Fig. 5). Further analysis demonstrated that E-box elements appeared in the promoter regions of AtCSD3, AtSOD1, AtSOD3, AtPrx4, AtPrx7 and AtPrx8 (Supplementary Fig. S2). In addition, we analyzed the promoters of the homologous genes of SOD and POD in birch, and the results showed that BpCSD3, BpSOD3, BpPrx4 and BpPrx7 genes were abundant in E-box elements (Supplementary Fig. S3). These results indicated that BpBZR1-6 has a positive role in salt tolerance by regulating the expression of stress-related genes and reducing ROS accumulation.
As mentioned earlier, heterologous expression of ZmBES1/BZR1-5 in transgenic Arabidopsis resulted in decreased ABA sensitivity [20]. Much attention has been drawn to the relationship between BRs and ABA. It was shown that the roots of BR mutants are hypersensitive to ABA treatment, exhibiting an antagonistic relationship [38]. Seeds with reduced BR biosynthesis or signal transduction are more sensitive to ABA [39]. BES1 can antagonize ABA signaling to inhibit the expression of ABI3 by the BES1-TPL-HDAC19 complex [40]. In our study, BpBZR1-6 facilitated plant growth, seed germination rates and seedling root lengths after NaCl treatment (Fig. 6A). Likewise, under ABA treatment, germination rates and root lengths were higher in transgenic Arabidopsis expressing BpBZR1-6 than in WT, exhibiting less sensitivity to ABA (Fig. 6B). We further speculated that reduced sensitivity to ABA based on BpBZR1-6 overexpression may be due to an antagonistic relationship between ABA and BRs. The underlying mechanisms need to be further explored. Adjusting the root development system is an important clue for plants to resist stress because roots are the first defensive line to face undesirable growth environments [41, 42]. Therefore, exploring the mechanism regulating root growth and plant stress resistance based on the interaction between BRs and ABA is of great research significance and deserves further investigation.
Conclusion
A BSE1/BZR1 TF, BpBZR1-6, was identified to respond to salt, ABA and BR treatment. Transgenic experiments in Arabidopsis have shown that BpBZR1-6 can promote seed germination and root development and maintain normal growth under salt stress conditions. By binding to E-box elements to induce resistance gene expression, BpBZR1-6 enhances antioxidant enzyme activity, scavenges ROS, and reduces membrane damage to improve plant salt stress resistance. BpBZR1-6 might mediate the interaction between ABA and BR signals. This study is of great significance for elucidating the regulatory mechanism of the BES/BZR1 family under salt stress in birch.
Materials and methods
Plant materials
To check the expression level of BpBZR1-6 under abiotic stress, tissue culture seedlings of wild-type (WT) birch clone (Betula Platyphylla Suk) were grown for approximately one month and then moved to soil for two months under conditions of 16 h light/8 h dark, 65–75% relative humidity, and an indoor temperature of 25 °C. The seedlings were watered with a solution of 200 mM NaCl and sprayed with 100 µM ABA and 1 µM BR for 3, 6, 12, 24 and 48 h, and well-watered seedlings were used as a control. Three seedlings were pooled into one sample, and three replicates were required for each treatment at each time point. Meanwhile, root, stem, and leaf samples were collected after treatment, frozen in liquid nitrogen and stored at − 80 °C for RNA extraction. Arabidopsis thaliana ecotype Columbia (Col-o) seeds were cultivated in an artificial climate room (25 °C, 80 ~ 1120 µmol∙m− 2∙s− 1, 16 h/8 h day/night cycles).
Identification and sequence analysis of BZRs
The hidden Markov model (HMM) file referring to the BES/BZR domain was downloaded from Pfam. database (http://pfam.xfam.org/) by using Pfam ID PF05687. The BpBZRs protein sequences were searched using HMMER software [43] in birch genome data and the genome of birch comes from the Northeast Forestry University [44]. The conserved domains of the BpBZRs were further confirmed using the NCBI-Conserved Domain Data (CDD) database (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi). The protein sequences of Arabidopsis thaliana, Zea mays and Camellia sinensis were obtained from NCBI and TPIA databases. Phylogenetic tree analysis was constructed using MEGA5 software [45], and protein sequences were aligned using BioEdit software (Isis Pharmaceuticals).
Structure and cis-acting elements analysis of the 6 BZRs in the birch
Phytozome (https://phytozome.jgi.doe.gov/) [44] and TBtools [46] were used to perform structural analysis of exons and introns. The sequences 2000 bp upstream of the start codon for the BZRs were obtained from Phytozome. The cis-acting elements in these promoter sequences were analyzed with the online PlantCARE software (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) and visualized by TBtools.
RNA isolation and qRT‒PCR
pBIOZOL Plant Total RNA extraction reagent (Bioflux, China) was used for RNA isolation. A reverse transcription kit (Takara Japan) was used for cDNA synthesis. For the expression analysis of BZR, the tubulin (GenBank number: FG067376) and ubiquitin (GenBank number: FG065618) genes were used as internal controls. For the expression analysis of downstream genes, resistance-related gene sequences in Arabidopsis CDS3 (NM_121815), SOD1 (NM_111929), SOD3 (NM_122237), Prx4 (NM_101321), Prx7 (NM_102824), and Prx8 (NM_103173) were downloaded fromNCBI database. AtActin (At5G09810) was used as an internal control in Arabidopsis. All primers used for qRT‒PCR are listed in Supplementary Table S2. Takara quantitative PCR enzyme (Takara Japan) was used for the qRT-PCR experiments, and the reaction program was set as follows: 94 °C for 30 s followed by 45 cycles of 94 °C for 12 s, 58 °C for 30 s and 72 °C for 45 s. Detection was carried out using a Roche Light Cycler 480 II (Jena q Tower 3G, Germany). All reactions were performed in triplicate. Expression levels were determined based on the 2−ΔΔCT method [47].
Protoplast isolation and subcellular localization
The full-length coding DNA sequence (CDS) without a stop codon of BpBZR1-6 was cloned from a cDNA sample using the primers 5’-TCTAGACTGGTACCC ATGAAGGAGGTGGCCGGAAA-3’ and 5’-CTAGTCAGTCGACCCCTA ATGGCGACGCAGAGGA-3’ (the underlined sequences are the homologous sequences of SmaI sites) and was inserted into the Sma I sites of the 35 S: pBI121-GFP vector to generate the 35 S-BpBZR1-6-GFP fusion expression vector. Poplar (Populus trichocarpa) was cultured for approximately 3 weeks in tissue culture bottles under conditions of 25 ± 1 ℃, light-dark cycle 14 h/10 h, light intensity 400 µmol·m− 2·s−1, and leaves were collected to isolate protoplasts. Protoplast isolation and transient transfection assays were performed according to previously described methods [48]. The 35 S-BpBZR1-6-GFP fusion construct and 35 S-GFP (control) were transiently expressed in poplar protoplasts. After dark culturing for 18 h at 25 °C, the transfected protoplasts were observed with a fluorescence microscope (LSM700, Zeiss, Jena, Germany).
Transactivation activity analysis
To study whether BpBZR1-6 had transcriptional activation activity and the activation region, we fused the full-length and truncated fragments of BpBZR1-6 into the pGBKT7 vector and transformed them into Y2H yeast cells. All the primers are listed in Supplementary Table S3. The positive control was the co-transformed plasmids of pGADT7-T and pGBKT7-p53, and the negative control was the co-transformed plasmids of pGADT7-T and pGBKT7-lamp. The experiments were performed according to the classical yeast transformation kit (Coolaber, Beijing, China). The transformed yeast cells were cultured on SD/-Trp and SD/-Trp/-His/X-α-gal medium and captured using a camera (Nikon, Japan).
Yeast one-hybrid assays
To verify whether BpBZR1-6 can bind to the E-box, a sequence containing triple tandem copies of the E-box was designed and cloned into the pHIS2 vector with EcoRI and SacI sites. The ORF of BpBZR1-6 was amplified and inserted into the SmaI sites of pGADT7-ADRecII using the primers shown in Supplementary Table S4. All the recombinant vectors of pGADT7-p53 and pHIS-p53 (positive control), pGADT7-BpBZR1-6 and pHIS-p53 (negative control), pGADT7-BpBZR1-6 and pHIS2-E-box were transformed into the yeast strain Y187. Then, the yeast cultures were streaked onto SD/-Trp/-Leu and SD/-Trp/-Leu/-His with 50 mM 3-AT, incubated for 3–5 days and photographed by camera (Nikon, Japan).
Genetic transformation in Arabidopsis
The recombinant plasmid (35S-BpBZR1-6-GFP) was transferred into the Agrobacterium strain EHA105 to infect Arabidopsis using the floral dip method [49]. Seeds from T0 transgenic plants were screened by 1/2 MS medium with 50 mg/L kanamycin and identified by PCR analysis (forward primer 5’- GGGATGACGCACAATCCCAC-3’ and reverse primer 5’-CTGAACTTGTGGCCGTTTAC-3’). The homozygous lines of T3 generation plants were used further study.
Stress treatment of transgenic Arabidopsis
To test the seed germination and root length of the transgenic plants under NaCl and ABA treatment, the seeds of BpBZR1-6-overexpressing transgenic Arabidopsis and WT plants were sterilized and grown on 1/2 MS medium and 1/2 MS medium with 100 mM NaCl and 3 µM ABA, respectively. The phenotype was photographed, and the germination rates and root length were measured. To analyze the salt stress tolerance of transgenic Arabidopsis, 4-week-old BpBZR1-6-overexpressing transgenic Arabidopsis and WT plants were treated with 100 mM NaCl for 15 days. After treatment, the fresh weight and chlorophyll content were determined according to a previous report [50], and MDA in the leaves was determined by using an MDA kit (Jiancheng Nanjing, China). All of these experiments were repeated at least three times.
Statistical analyses
Statistical analyses were carried out using Graphpad prism v6 software. The mean ± standard deviation (SD) was obtained from the average of three biological replicates. Data were compared using Student’s t-test, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- BRs
Brassinosteroids
- MDA
Malondialdehyde
- SOD
Superoxide Dismutase
- POD
Peroxidase
- HMM
Hidden Markov Model
- CDD
Conserved Domain Data
- qRT‒PCR
Quantitative real-time polymerase chain reaction
- ABRE
ABA-responsive element
- WT
Wild type
- GST1
Glutathione S-transferase 1
- CDS
Coding DNA sequence
- ROS
Reactive oxygen species
- Y1H
Yeast-one hybrid
- Y2H
Yeast-two hybrid
Author contributions
The study was conceived and conducted by Chao Wang and Fude Wang. Yao Chi analyzed the data and wrote the manuscript. Mingyu Yu identified BES/BZR gene family and studied the bioinformatics analysis. Zihan Wang and Meiqi Zhou performed the experiments. Leifei Zhao and Jingjing Shi performed the literature searches. All authors approved the final manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (2021YFD2200304), the Fundamental Research Funds for the Central Universities (2572023AW11), the Innovation Project of State Key Laboratory of Tree Genetics and Breeding (Northeast Forestry University) (2021A03) and the Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).
Data availability
The datasets generated and /or analyzed during the current study are available in Phytozome v13 (https://phytozome-next.jgi.doe.gov/), TPIA (http://tpia.teaplants.cn/geneIdConvert.html) and NCBI (https://www.ncbi.nlm.nih.gov/nuccore/) database.
Declarations
Ethics approval and consent to participate
Birch (Betula Platyphylla) and Poplar (Populus trichocarpa) specimens were obtained from Northeast Forestry University, Harbin, China. The voucher specimens were stored in Northeast Forestry University. The plants were identified by Yao Chi. All plant materials used in this study were owned by the authors and/or no permissions are required. All the plants complied with national guidelines and legislation, and did not deposit in a public available herbarium and involve in any endangered or protected species.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fude Wang, Email: wangfude070605@163.com.
Chao Wang, Email: wangchao@nefu.edu.cn.
References
- 1.Tang J, Zhang Y, Zhang M, Wang F. Effects of drought and pathogenic stress on photosynthetic physiology of Larix gmelinii mycorrhizal seedings [J]. For Eng. 2023;39(6):26–35. [Google Scholar]
- 2.Amorim-Silva V, García-Moreno Á, Castillo AG, Lakhssassi N, Esteban Del Valle A, Pérez-Sancho J, Li Y, Posé D, Pérez-Rodriguez J, Lin J, Valpuesta V, Borsani O, Zipfel C, Macho AP, Botella MA. TTL Proteins Scaffold Brassinosteroid Signaling Components at the plasma membrane to Optimize Signal Transduction in Arabidopsis. Plant Cell. 2019;31(8):1807–28. 10.1105/tpc.19.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano-Durán R, Macho AP, Boutrot F, Segonzac C, Somssich IE, Zipfel C. The transcriptional regulator BZR1 mediates trade-off between plant innate immunity and growth. Elife. 2013;2:e00983. 10.7554/eLife.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Joanne C. Brassinosteroid actions in plants. J Exp Bot. 1999;332:275–82. [Google Scholar]
- 5.Hao J, Yin Y, Fei SZ. Brassinosteroid signaling network: implications on yield and stress tolerance. Plant Cell Rep. 2013;32:1017–30. 10.1007/s00299-013-1438-x. [DOI] [PubMed] [Google Scholar]
- 6.Nolan T, Chen J, Yin Y. Cross-talk of Brassinosteroid signaling in controlling growth and stress responses. Biochem J. 2017;474(16):2641–61. 10.1042/BCJ20160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin Y, Vafeados D, Tao Y, Yoshida S, Asami T, Chory J. A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell. 2005;120(2):249–59. 10.1016/j.cell.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, Asami T, Chory J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109(2):181–91. 10.1016/s0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Nolan TM, Ye H, Zhang M, Tong H, Xin P, Chu J, Chu C, Li Z, Yin Y. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in Brassinosteroid-Regulated Plant Growth and Drought responses. Plant Cell. 2017;29(6):1425–39. 10.1105/tpc.17.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui XY, Gao Y, Guo J, Yu TF, Zheng WJ, Liu YW, Chen J, Xu ZS, Ma YZ. BES/BZR transcription factor TaBZR2 positively regulates Drought responses by activation of TaGST1. Plant Physiol. 2019;180(1):605–20. 10.1104/pp.19.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Ye K, Shi Y, Cheng J, Zhang X, Yang S. BZR1 positively regulates freezing Tolerance via CBF-Dependent and CBF-Independent pathways in Arabidopsis. Mol Plant. 2017;10(4):545–59. 10.1016/j.molp.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Ye H, Liu S, Tang B, Chen J, Xie Z, Nolan TM, Jiang H, Guo H, Lin HY, Li L, Wang Y, Tong H, Zhang M, Chu C, Li Z, Aluru M, Aluru S, Schnable PS, Yin Y. RD26 mediates crosstalk between drought and brassinosteroid signalling pathways. Nat Commun. 2017;8:14573. 10.1038/ncomms14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin Y, Qin K, Song X, Zhang Q, Zhou Y, Xia X, Yu J. BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase-mediated reactive oxygen Species Signaling in Tomato. Plant Cell Physiol. 2018;59(11):2239–54. 10.1093/pcp/pcy146. [DOI] [PubMed] [Google Scholar]
- 14.Jia C, Zhao S, Bao T, Zhao P, Peng K, Guo Q, Gao X, Qin J. Tomato BZR/BES transcription factor SlBZR1 positively regulates BR signaling and salt stress tolerance in tomato and Arabidopsis. Plant Sci. 2020;302:110719. 10.1016/j.plantsci.2020.110719. [DOI] [PubMed] [Google Scholar]
- 15.Lv J, Li Y, Liu Z, Li X, Lei X, Gao C. Response of BpBZR genes to abiotic stress and hormone treatment in Betula platyphylla. Plant Physiol Biochem. 2020;151:157–65. 10.1016/j.plaphy.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Ye H, Li L, Yin Y. Recent advances in the regulation of brassinosteroid signaling and biosynthesis pathways. J Integr Plant Biol. 2011;53(6):455–68. 10.1111/j.1744-7909.2011.01046. [DOI] [PubMed] [Google Scholar]
- 17.He JX, Gendron JM, Sun Y, Gampala SS, Gendron N, Sun CQ, Wang. ZY. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science. 2005;307:1634–1638. 10.1126/science.1107580 [DOI] [PMC free article] [PubMed]
- 18.Sun Y, Fan XY, Cao DM, Tang W, He K, Zhu JY, He JX, Bai MY, Zhu S, Oh E, Patil S, Kim TW, Ji H, Wong WH, Rhee SY, Wang ZY. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell. 2010;19(5):765–77. 10.1016/j.devcel.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, Li L, Zola J, Aluru M, Ye H, Foudree A, Guo H, Anderson S, Aluru S, Liu P, Rodermel S, Yin Y. A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J. 2011;65(4):634–46. 10.1111/j.1365-313X.2010.04449. [DOI] [PubMed] [Google Scholar]
- 20.Sun F, Yu H, Qu J, Cao Y, Ding L, Feng W, Bin Khalid MH, Li W, Fu F. Maize ZmBES1/BZR1-5 decreases ABA sensitivity and confers tolerance to osmotic stress in transgenic Arabidopsis. Int J Mol Sci. 2020;21:996. 10.3390/ijms21030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai X, Xu Y, Ma Q, Xu W, Tai W, Xue Y, Kang C. Overexpression of a R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, Drought, and salt stress in transgenic Arabidopsis. Plant Physiol. 2007;143:1739–51. 10.1104/pp.106.094532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan C, Guo G, Yan H, Qiu Z, Liu Q, Zeng B. Characterization of Brassinazole resistant (BZR) gene family and stress induced expression in Eucalyptus grandis. Physiol Mol Biol Plants. 2018;24(5):821–31. 10.1007/s12298-018-0543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha G, Park JI, Jung HJ, Ahmed NU, Kayum MA, Kang JG, Nou IS. Molecular characterization of BZR transcription factor family and abiotic stress induced expression profiling in Brassica rapa. Plant Physiol Biochem. 2015;92:92–104. 10.1016/j.plaphy.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Feng W, Liu Y, Cao Y, Zhao Y, Zhang H, Sun F, Yang Q, Li W, Lu Y, Zhang X, Fu F, Yu H. Maize ZmBES1/BZR1-3 and – 9 transcription factors negatively regulate Drought Tolerance in Transgenic Arabidopsis. Int J Mol Sci. 2022;23(11):6025. 10.3390/ijms23116025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin J, Tian F, Yang DC, Meng YQ, Kong L, Luo J, Gao G. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017;45(D1):D1040–5. 10.1093/nar/gkw982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu H, Feng W, Sun F, Zhang YY, Qu JT, Liu B, Lu F, Yang L, Fu F, Li W. Cloning and characterization of BES1/BZR1 transcription factor genes in maize. Plant Growth Regul. 2018;86:235–49. [Google Scholar]
- 27.Manoli A, Trevisan S, Quaggiotti S, Varotto S. Identification and characterization of the BZR transcription factor family and its expression in response to abiotic stresses in Zea mays L. Plant Growth Regul. 2018;84:423–36. [Google Scholar]
- 28.Liu D, Cui Y, Zhao Z, Li S, Liang D, Wang C, Feng G, Wang J, Liu Z. Genome-wide identification and characterization of the BES/BZR gene family in wheat and foxtail millet. BMC Genomics. 2021;22(1):682. 10.1186/s12864-021-08002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Guo W, Du D, Pu L, Zhang C. Overexpression of a maize BR transcription factor ZmBZR1 in Arabidopsis enlarges organ and seed size of the transgenic plants. Plant Sci. 2020;292:110378. 10.1016/j.plantsci.2019.110378. [DOI] [PubMed] [Google Scholar]
- 30.Mareri L, Parrotta L, Cai G. Environmental stress and plants. Int J Mol Sci. 2022;23(10):5416. 10.3390/ijms23105416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Q, Liu B, Dong W, Li J, Wang D, Liu Z, Gao C. Comparative transcriptomic and metabolomic analyses provide insights into the responses to NaCl and cd stress in Tamarix Hispida. Sci Total Environ. 2023;884:163889. 10.1016/j.scitotenv.2023.163889. [DOI] [PubMed] [Google Scholar]
- 32.Wang LQ, Wen SS, Wang R, Wang C, Gao B, Lu MZ. PagWOX11/12a activates PagCYP736A12 gene that facilitates salt tolerance in poplar. Plant Biotechnol J. 2021;19(11):2249–60. 10.1111/pbi.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breusegem FV, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol. 2016;141:384–90. 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30. [DOI] [PubMed] [Google Scholar]
- 35.Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. Imaging of photo-oxidative stress responses in leaves. J Exp Bot. 2002;53:1249–54. [PubMed] [Google Scholar]
- 36.Kim BH, Kim SY, Nam KH. Genes encoding plant-specific class III peroxidases are responsible for increased cold tolerance of the brassinosteroid-insensitive 1 mutant. Mol Cells. 2012;34(6):539–48. 10.1007/s10059-012-0230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou G, Liu C, Cheng Y, Ruan M, Ye Q, Wang R, Yao Z, Wan H. Molecular evolution and functional divergence of stress-responsive Cu/Zn superoxide dismutases in plants. Int J Mol Sci. 2022;23(13):7082. 10.3390/ijms23137082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Nam KH, Vafeados D, Chory J. BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol. 2001;127(1):14–22. 10.1104/pp.127.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steber CM, McCourt P. A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 2001;125:763–9. 10.1104/pp.125.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryu H, Cho H, Bae W, Hwang I. Control of early seedling development by BES1/TPL/HDA19-mediated epigenetic regulation of ABI3. Nat Commun. 2014;5:4138. 10.1038/ncomms5138. [DOI] [PubMed] [Google Scholar]
- 41.Amtmann A. Learning from evolution: Thellungiella generates new knowledge on essential and critical components of abiotic stress tolerance in plants. Mol Plant. 2009;2:3–12. 10.1093/mp/ssn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karlova R, Boer D, Hayes S, Testerink C. Root plasticity under abiotic stress. Plant Physiol. 2021;187:1057–70. 10.1093/plphys/kiab392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson LS, Eddy SR, Portugaly E. Hidden Markov model speed heuristic and iterative HMM search procedure. BMC Bioinformatics. 2010;11:431. 10.1186/1471-2105-11-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen S, Wang Y, Yu L, Zheng T, Wang S, Yue Z, Jiang J, Kumari S, Zheng C, Tang H, Li J, Li Y, Chen J, Zhang W, Kuang H, Robertson JS, Zhao PX, Li H, Shu S, Yordanov YS, Huang H, Goodstein DM, Gai Y, Qi Q, Min J, Xu C, Wang S, Qu GZ, Paterson AH, Sankoff D, Wei H, Liu G, Yang C. Genome sequence and evolution of Betula platyphylla. Hortic Res. 2021;8(1):37. 10.1038/s41438-021-00481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–9. 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–7. 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆Ct method. Methods. 2001;25:402–8. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 48.Guo J, Morrell-Falvey JL, Labbé JL, Muchero W, Kalluri UC, Tuskan GA, Chen JG. Highly efficient isolation of Populus mesophyll protoplasts and its application in transient expression assays. PLoS ONE. 2012;7(9):e44908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clough SJ, Bent AF. Floral dip: a simplifified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–43. 10.1046/j.1365-313x. [DOI] [PubMed] [Google Scholar]
- 50.Lightenthaler H. Chlorophylls and carotenoids: pigments of photo synthetic biomembranes. Methods Enzymol. 1987;148:350–82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and /or analyzed during the current study are available in Phytozome v13 (https://phytozome-next.jgi.doe.gov/), TPIA (http://tpia.teaplants.cn/geneIdConvert.html) and NCBI (https://www.ncbi.nlm.nih.gov/nuccore/) database.