Abstract
Background
Locally advanced rectal cancer (LARC) poses a significantly challenge in clinical management, requiring a multimodal treatment approach. Among innovative strategies, Total Neoadjuvant Therapy (TNT) has emerged, delivering all planned chemotherapy before surgery.
Objective
Our aim was to evaluate the real-world application and efficacy of TNT and to compare it with the non-TNT standard strategy.
Methods
This retrospective study compared locally advanced rectal adenocarcinoma patients treated with Total Neoadjuvant Therapy (TNT) in 2022 with those who underwent traditional chemoradiotherapy (CRT) in 2020–2021. The primary endpoints were the pathologic complete response rate and the sustained clinical complete response rate in patients under W&W.
Results
Among 107 patients (54.2% male, mean age 62.48 years), non-TNT (67 patients) and TNT (40 patients) mean follow-ups were 26.7 and 8.2 months, respectively. No differences in gender(p = 0.163), staging (p = 0.707), or location (p = 0.727) were noted. TNT patients received more short-course radiotherapy (42.5% vs1.5%, p < 0.001). Clinical responses favored TNT (p = 0.030) with no significant differences in pathological responses, recurrence rates, or survival. TNT exhibited higher chemotherapy completion (p = 0.007) and lower adverse events (p < 0.001). Post-surgery events showed no significant differences (p = 0.470). Single center with retrospective design and carries limitations that may restrict the generalizability of the findings and the relatively short follow-up duration are our main limitations.
Conclusion
Our data add to the body of literature favoring the TNT treatment strategy for locally advanced rectal cancer, aiming to achieve comparable complete response rates with less adverse events.
Keywords: Total neoadjuvant therapy, Locally advanced rectal cancer, Chemoradiotherapy, Complete clinical response, Adjuvant chemotherapy, Treatment completion rates
Simple summary
Locally advanced rectal cancer (LARC) presents a clinical challenge requiring a multimodal treatment approach. Total Neoadjuvant Therapy (TNT), delivering all planned chemotherapy pre-surgery, emerges as an innovative strategy. Our retrospective study (n=107) compared TNT to traditional chemoradiotherapy in LARC patients. Follow-ups were 811 (non-TNT) and 249 days (TNT). TNT showed superior clinical responses (p=0.030) with comparable pathological responses, recurrence, and survival rates. TNT exhibited higher chemotherapy completion (p=0.007) and lower adverse events (p<0.001). This adds to existing literature favoring TNT for LARC, describing higher complete response rates with fewer adverse events.
Background
Colorectal cancer stands as the third most prevalent cancer globally, ranking as the second leading cause of cancer-related mortality [1]. Within the spectrum of colorectal malignancies, rectal cancer assumes importance, particularly when presenting as a locally advanced disease, necessitating a multimodal treatment approach that integrates chemotherapy, radiotherapy, and surgery [2]. The imperative goals in managing locally advanced rectal cancer encompass achieving effective local disease control without compromising the patient's quality of life and preventing the onset of distant metastases—known to be the primary contributor to rectal cancer-related mortality [3].
In the pursuit of enhancing therapeutic strategies, numerous novel approaches have been investigated, each designed to optimize outcomes for rectal cancer patients [4, 5]. Among these, Total Neoadjuvant Therapy (TNT) has emerged as a promising contender [5–7]. This innovative approach involves the administration of all planned chemotherapy before surgery, either following or preceding radiotherapy. The sequence of treatments in TNT allows the early delivery of systemic therapy, potentially amplifying control over distant disease while minimizing associated toxicity [7–9]. Additionally, TNT holds the potential to increase rates of complete clinical responses, thereby offering opportunities for organ preservation through Watch & Wait programs, and improving surgical outcomes and pathological responses [5, 9]. However, after 5 years of follow-up, the RAPIDO trial demonstrated that, within its specific TNT protocol, there was an observed increase in long-term local recurrence rates [10].
Nonetheless, the implementation of any neoadjuvant strategy, including TNT, requires a cautious balance to mitigate concerns related to overtreatment and the possibility of delaying potentially curative surgery [11–13].
In this study, we aim to investigate the effectiveness and clinical significance of TNT in the management of advanced rectal cancer. Our goal is to compare TNT with the conventional approach of neoadjuvant chemoradiotherapy (CRT)/short-course radiotherapy (RT) followed by adjuvant chemotherapy. By providing a thorough analysis of our findings, we aim to contribute valuable insights into the real-world applicability and efficacy of TNT.
Materials and methods
This retrospective study included all adult patients diagnosed with locally advanced [stage II or III, TNM classification by the American Joint Committee on Cancer, version 8 (2017)] rectal adenocarcinoma (World Health Organization Classification, 2019) discussed in our institution’s multidisciplinary team meeting who were then submitted to curative intent treatment including chemotherapy and radiotherapy between 01/01/2020 and 31/12/2022. Inclusion criteria were: adult patients and histologically-proven locally advanced rectal adenocarcinoma with distal margin of 15 cm or less from the anal verge on magnetic resonance imaging. This study was approved by the local institutional Ethical Committee board (Comissão de Etica para a Saúde of Instituto Português de Oncologia de Lisboa Francisco Gentil, with the protocol code UIC/1649).
Treatment
The treatment protocol consisted of a systematic approach based on the clinical staging by thoracic and abdominal (TA) computerized tomography (CT) and pelvic magnetic resonance imaging (MRI). Rectal ultrasound was added whenever there were doubts between cT1 and cT2 in MRI. Staging was done according to the TNM classification by the American Joint Committee on Cancer, version 8 (2017).
Patients were assigned to either the TNT or non-TNT group based on the treatment protocol in place at our institution, which shifted to the TNT approach starting on January 1, 2022. Thus, all patients treated before this date received the non-TNT approach, and those treated after this date received TNT.
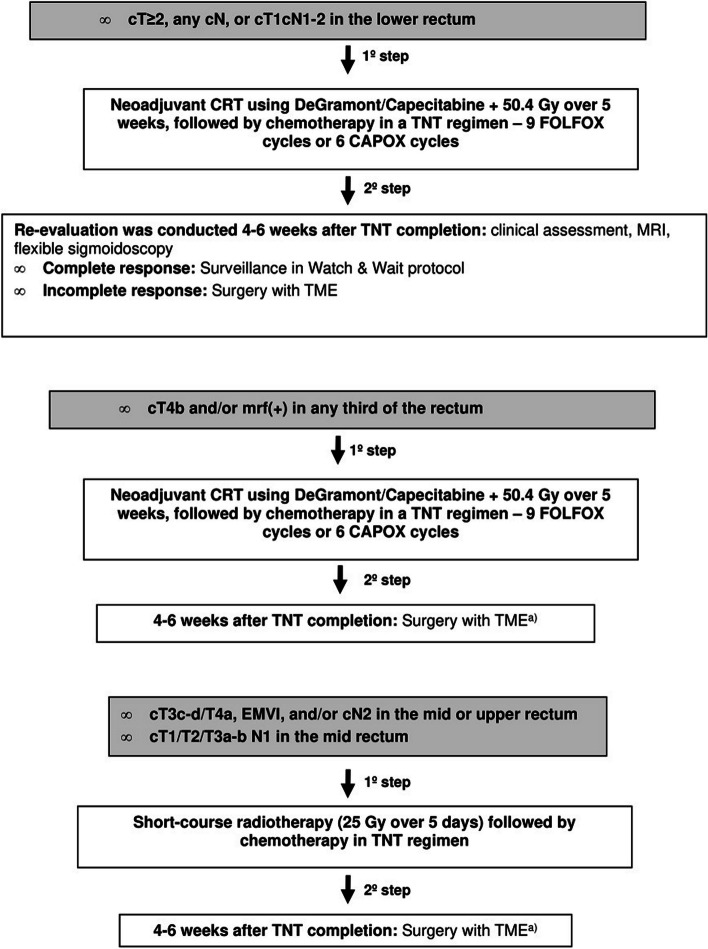
From 01/01/2022 (Fig. 1):
Fig. 1.
(a) Without re-evaluation. TNT: Total Neoadjuvant Therapy, TME: Total Mesorectal Excision, mrf: Mesorectal fascia; EMVI: Extramural Venous Invasion
For patients presenting with cT ≥ 2, any cN, or cT1cN1-2 in the lower rectum, the initial step involved neoadjuvant CRT using DeGramont/Capecitabine plus 50.4 Gy over 5 weeks, followed by chemotherapy in a TNT regimen – 9 FOLFOX cycles or 6 CAPOX cycles. Reevaluation was conducted 4–6 weeks after TNT completion, incorporating clinical assessment, pelvic MRI, and flexible sigmoidoscopy. A clinical complete response led to surveillance under a Watch & Wait (W&W) protocol, while an incomplete response led to surgery with Total Mesorectal Excision (TME).
For cases staged as cT4b and/or with less than a 1 mm margin from the mesorectal fascia [mrf ( +)] in any third of the rectum, the protocol initiated with the same neoadjuvant CRT and TNT regimen. Surgery with TME was performed 4–6 weeks after the completion of TNT, without reevaluation.
For cT3c-d/T4a, extramural venous invasion (EMVI), and/or cN2 cases in the mid or upper rectum, and for cT1/T2/T3a-b cN1 cases in the mid rectum, the protocol incorporated short-course radiotherapy (25 Gy over 5 days) followed by chemotherapy in a total neoadjuvant chemotherapy scheme. Surgery with TME was conducted 4–6 weeks after completing TNT, without reevaluation.
This scheme was compared with the traditional treatment protocol used between 01/01/2020 e 31/12/2021:
For patients presenting with any cT stage and cN2; cT3c-d/T4 and/or mrf( +) and/or EMVI, and any cN stage; cT3a-b N0 or N1, mrf(-), no EMVI, in the lower rectum; and cT2 N0 in the lower rectum at risk of abdominoperitoneal amputation, the initial step involved neoadjuvant chemoradiotherapy (CRT) using DeGramont/Capecitabine plus 50.4 Gy over 5 weeks. This was followed by surgery with Total Mesorectal Excision (TME) after 8–10 weeks and adjuvant chemotherapy with DeGramont or FOLFOX after 4–12 weeks.
For patients staged as cT1/T2/T3a-b N1, mrf(-), and no EMVI, in the mid rectum, the initial step involved neoadjuvant short-course radiotherapy (25 Gy over 5 days), followed by surgery with TME and adjuvant chemotherapy.
In both groups:
For patients with rectal tumours at any distance with significant comorbidities or aged over 80 years, the advantage of adjuvant chemotherapy was not conclusively demonstrated. Thus, it was deemed optional and the decision was made by the patients’ oncologists. Patients in this group who were referred for neoadjuvant therapy underwent short-course radiotherapy (25 Gy over 5 days) alone, followed by surgery 8–10 weeks later.
Patients with tumours in the lower rectum exhibiting a clinical complete response to neoadjuvant therapy, entered the Watch & Wait protocol (WW). In case of near complete response, the patients were then revaluated with MRI and endoscopic examination 4 to 6 weeks later. The W&W protocol involved surveillance with regular follow-ups: pelvic MRI, proctological examination, and flexible sigmoidoscopy every 3 months for the first 2 years, and then at 6-month intervals until 5–10 years. Additionally, annual TAP CT surveillance was conducted for 5–10 years, and a total colonoscopy was performed 1 year after deciding on Watch & Wait, followed by subsequent examinations after 3-years and then every 5 years if no risk adenomas were found. Surgery with TME was proposed for all cases of regrowth.
Endpoints
The primary objective of this study was to assess and compare the pathologic complete response (pCR) rate (defined as ypT0N0) and the sustained clinical (radiologic and endoscopic) complete response (cCR) rate in patients managed with a Watch and Wait (W&W) strategy. Secondary objectives included evaluating treatment tolerability (based on toxicity rates) and completion rates for both CRT/RT with TNT and traditional neoadjuvant plus adjuvant therapy (non-TNT) in rectal cancer treatment.
Additional secondary outcomes included surgery-related outcomes and adverse events, categorized using the Clavien-Dindo classification and the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0.
Collectively, these endpoints aimed to provide a comprehensive understanding of the comparative effectiveness and tolerability rates associated with TNT versus non-TNT in rectal cancer management.
Statistical analyses
The statistical analyses were conducted using IBM SPSS Statistics software, version 27.0 (IBM Corp., Armonk, NY, USA). Patient and treatment parameters underwent comparison through the χ2 test or Fisher’s exact test for categorical variables and Student’s t-test for continuous variables. Data distribution normality was assessed using graphical analysis. In instances where the expected count was below 5 in cells with categorical variables, a likelihood-ratio test was executed, as appropriate. Statistical significance was defined at a P value of < 0.05. The presentation of frequencies and percentages in the tables herein is standard, unless otherwise specified.
To account for potential confounding factors in evaluating the impact of TNT on patient outcomes, a multivariate analysis was performed using binary and multimodal logistic regression. This analysis adjusted for key variables, including treatment group (TNT vs. non-TNT), clinical features (age, gender, tumor stage), pathological characteristics (tumor grade, lymph node involvement), and treatment-related factors (chemotherapy completion rate, radiotherapy dosage).
The Kaplan–Meier method was employed to analyze survival and disease-free survival outcomes in our study. This non-parametric statistical approach is particularly suited for estimating and visualizing time-to-event data, such as the disease-free outcome and survival. The log-rank test was applied to assess potential differences between survival curves, contributing to a comprehensive analysis of the impact of different treatment approaches on patient survival and disease-free intervals.
Results
A total of 107 patients were included, 58 (54,2%) were male and the mean age at diagnosis was 62.48 years (ranging from 29 to 83 years). The mean follow-up duration was 26.66 months for non-TNT and 8.19 months for TNT therapies; overall, the patients’ mean follow-up was 21.05 months. Among these patients, 67 underwent the non-TNT treatment protocol, while 40 underwent TNT. Patients’ characteristics were summarized in Table 1.
Table 1.
Demographics
| Characteristic | Patients, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| All patients | TNT | Standard therapy | P value | ||||
| N = 107 | N = 40 | N = 67 | |||||
| Age (years) | |||||||
| Median [IQR] | 63.0 | [54–71] | 61.5 | [50–70] | 63.0 | [55–73] | |
| Mean [SD] | 61.9 | [11.6] | 60.6 | [11.0] | 62.7 | [12.0] | |
| Range | 29–83 | 36–77 | 29–83 | ||||
| Sex | |||||||
| Male | 58 | (54.2) | 18 | (45.0) | 40 | (59.7) | 0.163 |
| Female | 49 | (45.8) | 22 | (55.0) | 27 | (40.3) | |
| Location | |||||||
| Lower | 50 | (46.7) | 19 | (47.5) | 31 | (46.3) | 0.727 |
| Middle | 37 | (34.6) | 15 | (37.5) | 22 | (32.8) | |
| Upper | 20 | (18.7) | 6 | (15.0) | 14 | (20.9) | |
| Clinical T | |||||||
| cT2 | 9 | (8.4) | 4 | (10.0) | 5 | (7.5) | |
| cT3 | 68 | (63.6) | 26 | (65.0) | 42 | (62.7) | |
| cT4 | 30 | (28.0) | 10 | (25.0) | 20 | (29.9) | |
| Clinical N | |||||||
| cN0 | 8 | (7.5) | 2 | (5.0) | 6 | (9.0) | |
| cN1 | 29 | (27.1) | 9 | (22.5) | 20 | (39.9) | |
| cN2 | 70 | (65.4) | 29 | (72.5) | 41 | (61.2) | |
| Clinical stage | |||||||
| II | 8 | (7.5) | 2 | (5.0) | 6 | (9.0) | 0.707 |
| III | 99 | (92.5) | 38 | (95.0) | 61 | (91.0) | |
| Histologic grade | |||||||
| G1 | 29 | (27.1) | 16 | (40.0) | 13 | (19.4) | 0.067 |
| G2 | 68 | (63.6) | 21 | (52.5) | 47 | (70.1) | |
| G3 | 10 | (9.3) | 3 | (7.5) | 7 | (10.4) | |
TNT Total Neoadjuvant Therapy
The mean time between diagnosis and the multidisciplinary team meeting treatment decision was 53 days, while the period from this meeting to the beginning of treatment was 82 days. The mean follow-up time until local recurrence was 9.73 months (3 patients – 2 from TNT group and 1 non-TNT) and for distant recurrence it was 8.28 months (7 patients – 3 from TNT group and 4 non-TNT). Patients in the TNT-treatment protocol were significantly more likely to receive short-course radiotherapy (42,5% vs 1.5%; p < 0.001 in both univariate and multivariate analyses) (Table 2).
Table 2.
Radiotherapy
| Therapy | Patients, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| All patients | TNT | Standard therapy | P value | ||||
| N = 107 | N = 40 | N = 67 | |||||
| RT | |||||||
| CRT | 89 | (83.2) | 23 | (57.5) | 66 | (0.99) | < 0.001 |
| SCRT | 18 | (16.8) | 17 | (42.5) | 1 | (0.01) | |
CRT Chemoradiotherapy, RT Radiotherapy, SCRT Short-Course Radiotherapy, TNT Total Neoadjuvant Therapy
Statistical analysis revealed no significant differences between the two treatment groups concerning gender (p = 0.163), clinical staging (p = 0.707), tumour grade (p = 0.067), and tumour location (p = 0.727). Multivariate analysis confirmed these findings with no significant differences for gender (p = 0.140), clinical staging (p = 0.823), tumour grade (p = 0.072) and tumour location (p = 0.724). There were no tumours with microsatellite Instability in our cohort (Table 3).
Table 3.
Clinical Response
| Characteristic | Patients, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| All patients | TNT | Standard therapy | P value | ||||
| N = 59 | N = 22 | N = 37 | |||||
| Clinical response at first evaluation | |||||||
| Complete | 9 | −15.3 | 6 | −27.3 | 3 | −8.1 | 0.03 |
| Near complete | 16 | −27.1 | 8 | −36.4 | 8 | −21.6 | |
| No | 34 | −57.6 | 8 | −36.4 | 26 | −70.3 | |
| Complete response at the final evaluation | |||||||
| Yes | 21 | −35.6 | 11 | −50 | 10 | −27 | 0.096 |
| No | 38 | −64.4 | 11 | −50 | 27 | −73 | |
| Patients, n (%) | |||||||
| All patients | TNT | Standard therapy | P value | ||||
| N = 105 | N = 40 | N = 65 | |||||
| Watch & Wait | 22 | −21 | 12 | −30 | 10 | −15.3 | 0.088 |
| N = 14 | N = 9 | N = 5 | |||||
| Regrowth | 3 | −21.4 | 2 | −22.2 | 1 | −20 | 1 |
There were also no significant differences between groups regarding lymphovascular invasion (p = 0.545), extramural venous invasion (p = 0.557) or tumor budding (p = 0.053), nor in the presence of at least 12 lymph nodes in the surgical specimen. Multivariate analysis corroborated these results for lymphovascular invasion (p = 0.425), extramural venous invasion (p = 0.589), and tumor budding (p = 0.051). All patients had an R0 resection (Table 4).
Table 4.
Pathological Response
| Patients, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| All patients | TNT | Standard therapy | P value | ||||
| N = 81 | N = 29 | N = 52 | |||||
| Pathological complete response | |||||||
| Yes | 14 | (17.3) | 5 | (17.2) | 9 | (17.3) | 0.835 |
| No | 67 | (82.7) | 24 | (82.8) | 43 | (82.7) | |
| Lymphovascular invasion | |||||||
| Yes | 14 | (17.5) | 6 | (21.4) | 8 | (15.4) | 0.545 |
| No | 66 | (82.5) | 22 | (78.6) | 44 | (84.6) | |
| Missing | 1 | 1 | 0 | ||||
| Extramural venous invasion | |||||||
| Yes | 15 | (18.8) | 4 | (14.3) | 11 | (21.2) | 0.557 |
| No | 65 | (81.3) | 24 | (85.7) | 41 | (78.8) | |
| Missing | 1 | 1 | 0 | ||||
| Tumor budding | |||||||
| No | 36 | (48.6) | 7 | (29.2) | 29 | (58.0) | 0.053 |
| Low | 26 | (35.1) | 13 | 54.2) | 13 | (26.0) | |
| Intermediate | 1 | (1.4) | 0 | (0.0) | 1 | (2.0) | |
| High | 11 | (14.9) | 4 | (16.7) | 7 | (14.0) | |
| Missing | 7 | 5 | 2 | ||||
| Lymph nodes | |||||||
| ≥ 12 | 16 | (21.3) | 5 | (20.0) | 11 | (22.0) | 1.000 |
| < 12 | 59 | (78.7) | 20 | (80.0) | 39 | (78.0) | |
| Missing | 6 | 4 | 2 | ||||
Clinical response
Patients undergoing TNT, as opposed to non-TNT, when reevaluated after CRT/RT, exhibited a statistically significant increase in the proportion of clinical complete responses at the first evaluation (p = 0.030; multivariate analysis p = 0.027) and were more likely to have a clinical complete response at the final evaluation (after near complete response patients were reevaluated), without reaching statistical significance in either univariate (p = 0.096) or multivariate analysis (p = 0.076) (Table 5).
Table 5.
Relapse
| Relapse | Patients, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| All patients | TNT | Standard therapy | P value | ||||
| N = 98 | N = 36 | N = 62 | |||||
| Distant | 10 | (10.2) | 2 | (6.9) | 8 | (12.9) | 0.317 |
| Local | 2 | (2.0) | 0 | (0.0) | 2 | (3.2) | 0.530 |
No significant differences were found regarding pathological complete responses and sustained clinical complete responses (p = 0.835).
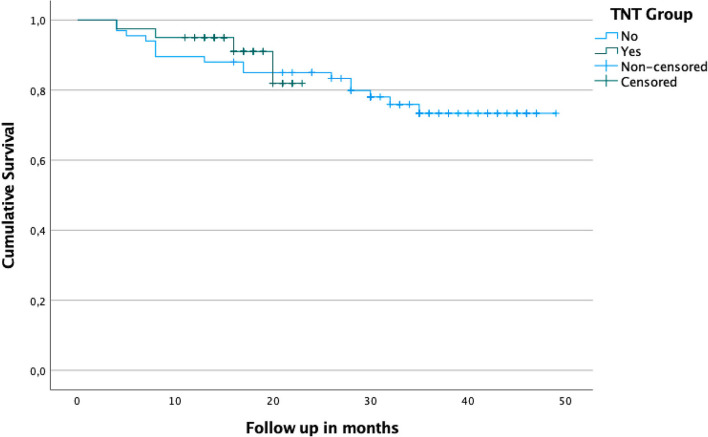
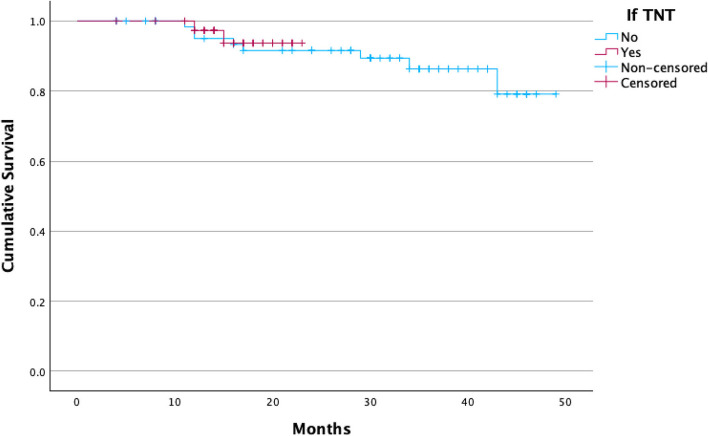
No significant differences were found between groups regarding local (p = 0.530) or distant recurrences (p = 0.317), global or disease-free survival, or regrowth rates in patients under W&W. There were no significant differences on Kaplan Meier curves either for the global survival curve (p = 0.684) or for disease-free survival (p = 0.741) between therapeutic modalities, both shown on Fig. 2 and 3.
Fig. 2.
Estimated survival curve with Kaplan–Meier. There’s no difference regarding disease-free and estimated survival between both groups. TNT: Total Neoadjuvant Treatment
Fig. 3.
Disease-free curve with Kaplan–Meier. There's no difference regarding disease-free and estimated survival between both groups. TNT: Total Neoadjuvant Treatment
Compliance and toxicity
In evaluating compliance and toxicity, statistically significant differences emerged between the two treatment cohorts regarding the overall completion rates of both neoadjuvant and adjuvant therapies. Patients undergoing TNT demonstrated a significantly higher completion rate of the prescribed chemotherapy regimen compared to those in the non-TNT group (p = 0.007), corroborated on multivariate analysis (p = 0.003). When examining adverse events of at least moderate severity, as per the Common Terminology Criteria for Adverse Events (CTCAE), they were significantly more common in the non-TNT group (p < 0.001 in both univariate and multivariate analyses). Most of the adverse events observed were gastrointestinal in nature, affecting 17 patients in the TNT group (47% of the TNT group) compared to 27 patients in the non-TNT group (56% of the non-TNT group). Neurologic symptoms, including paresthesia, were reported in 2 patients in the TNT group versus 10 in the non-TNT group. Hematologic adverse events occurred in 2 patients in the TNT group compared to 8 in the non-TNT group, while dermatologic reactions were noted in 4 patients in the TNT group and 3 in the non-TNT group (Table 6).
Table 6.
Toxicity
| Chemotherapy toxicity | Patients, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| All patients | TNT | Standard therapy | P value | ||||
| N = 86 | N = 40 | N = 46 | |||||
| CTCAE ≥ 2 | 26 | (30.2) | 5 | (12.5) | 21 | (45.7) | < 0.001 |
CTCAE Common Terminology Criteria for Adverse Events, TNT Total Neoadjuvant Therapy
An analysis of post-surgery adverse events, assessed through the Clavien score, revealed no statistically significant differences between the TNT and non-TNT groups (p = 0.470; multivariate analysis p = 0.498).
Discussion
The observed homogeneity among the study groups, characterized by gender, age, tumour location, stage, and histopathological features of the operative specimen, establishes a solid foundation for a valid comparison. This consistency ensures that any differences in outcomes can be reasonably attributed to the distinct treatment approaches rather than confounding variables.
In similarity with other studies, ours demonstrated that the introduction of the new treatment scheme has yielded noteworthy advantages. As shown in the literature [6, 9], our data showed that the TNT protocol has resulted in a higher incidence of complete clinical responses at first evaluation with a statistically significant value (p = 0.030) but not at final evaluation (p= 0.096). This enables the inclusion of a greater number of patients in the Watch & Wait (W&W) protocol (12/40; 30,0% vs 10/65; 15,4%). This shift to a more conservative approach through W&W not only helps patients avoid major surgery-related morbidity and mortality but also yields substantial cost savings [14, 15]. By reducing hospitalization stays, and lowering rehabilitation and outpatient visit requirements, the healthcare system stands to benefit financially [14–17]. Regarding surgical outcomes, consistent with expectations from the current body of literature [6, 8], no significant differences were observed between the two groups in terms of complications or achieving R0 resections.
Upon careful examination of our data regarding treatment completion, a significant disparity emerged between the two groups, with the TNT cohort exhibiting a markedly higher completion rate (80.0% vs. 52.2%; p< 0.001), aligning with findings in the existing literature [18]. In the traditional protocol group, several factors may contribute to the lower completion rates compared to TNT. Firstly, the specific timing of chemotherapy administration in the non-TNT group, within 12 weeks following surgery, could pose a hindrance. Postoperative complications might render some patients unfit for chemotherapy, as observed in our cohort. Additionally, factors such as frailty secondary to surgery, extensively investigated in numerous studies [19, 20], manifested as postoperative weight loss stemming from surgical stress, diminished appetite, or complications; lower levels of hemoglobin, leading to fatigue and reduced overall well-being, could further impede the ability to adhere to the complete course of adjuvant therapy.
Upon examining adverse events, a notable discrepancy was observed, with a higher occurrence of at least moderate adverse events in the non-TNT group compared to the TNT treatment group (45.7% vs. 12.5%; p< 0.001). The literature exhibits conflicting findings on this matter, with some studies corroborating our results [5, 18, 21], while others present contradictory outcomes [2, 6]. However, the elevated rate of adverse events attributed to chemotherapy in the non-TNT group may be rationalized using the same explanations noted for the completion rate.
The lack of distinctions between both groups in terms of Kaplan–Meier survival and disease-free curves, as shown in the Fig. 1 and 2, can be attributed to a short follow-up period, but it seems to show that the new TNT strategy is at least as safe and efficacious as the traditional approach. Although no statistically significant differences were observed between the groups, a noteworthy finding emerged: the incidence of local recurrence was notably higher in the TNT group, accounting for approximately 75% of cases, echoing findings from the 5 years follow-up RAPIDO trial [10]. This observation suggests the presence of viable subclinical tumor cells with proliferative potential. Conversely, concerning distant recurrence, the majority (90%) occurred in the non-TNT group, potentially attributed to incomplete chemotherapy regimens.
Limitations
This study is retrospective and conducted at a single center, which inherently carries limitations that may restrict the generalizability of the findings. The relatively short follow-up duration in the TNT group (8.9 months) compared to the timeframes for local and distant relapse (9.73 months and 8.28 months, respectively) could potentially impact the assessment of disease-free survival in the TNT group relative to the non-TNT group.
It is also noteworthy to mention that in control group SCRT was only performed in 1 patient in the non-TNT group, which could have positively influenced the percentage of complete responses in this group, being a possible bias. However, we believe that this did not negatively impact the adverse events observed in this group, as the adverse events considered in this analysis were primarily due to CT and were thus systemic rather than local.
Conclusions
In summary, our study contributes to the existing literature endorsing the TNT paradigm for locally advanced rectal cancer. By prioritizing similar complete response rates alongside mitigated adverse events, our findings underscore the therapeutic promise of TNT. These results emphasize the imperative for continued investigation and incorporation of TNT protocols into clinical standards, with the aim of refining therapeutic outcomes for patients with locally advanced rectal cancer.
Authors’ contributions
Writing and Manuscript Preparation: Luís Correia Gomes, Bernardo Alves Pereira, and Isadora Rosa were responsible for drafting the manuscript and coordinating revisions based on all authors’ input. Data Collection and Management: Isália Miguel, Ana Luís, Ana Pina, Cátia Pedro, Daniela Cavadas, Daniela Pereira, Joana Lemos, João Maciel, João Oliveira, José Venâncio, Madalena Santos, Manuel Limbert, Miguel Braga, Miriam Abdulrehman, Pedro Freitas, Ricardo Fonseca, and Teresa Ferreira contributed to data acquisition, analysis, and verification. Review and Approval: All authors reviewed the final manuscript draft, contributed critical insights, and approved the final version for submission.
Funding
There’s no funding to declare.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the local institutional Ethical Committee board (Comissão de Etica para a Saúde of Instituto Português de Oncologia de Lisboa Francisco Gentil, with the protocol code UIC/1649).
Consent for publication
The manuscript does not contain any individual person’s data in any form, so inform consent is not necessary.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021May;71(3):209–49. 10.3322/caac.21660. (Epub 2021 Feb 4 PMID: 33538338). [DOI] [PubMed] [Google Scholar]
- 2.Oronsky B, Reid T, Larson C, Knox SJ. Locally advanced rectal cancer: The past, present, and future. Semin Oncol. 2020Feb;47(1):85–92. 10.1053/j.seminoncol.2020.02.001. (Epub 2020 Feb 21 PMID: 32147127). [DOI] [PubMed] [Google Scholar]
- 3.Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef M, Leijtens JW, Hulsewé KW, Buijsen J, Beets GL. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011D 10;29(35):4633–40. 10.1200/JCO.2011.37.7176. Epub 2011 Nov 7 PMID: 22067400. [DOI] [PubMed] [Google Scholar]
- 4.van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, Habr-Gama A, Perez RO, Renehan AG, van de Velde CJH; IWWD Consortium. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018 Jun 23;391(10139):2537–2545. 10.1016/S0140-6736(18)31078-X. PMID: 29976470. [DOI] [PubMed]
- 5.Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021 Jan;22(1):29–42. 10.1016/S1470-2045(20)30555-6. Epub 2020 Dec 7. Erratum in: Lancet Oncol. 2021 Feb;22(2):e42. PMID: 33301740.
- 6.van der Valk MJM, Marijnen CAM, van Etten B, Dijkstra EA, Hilling DE, Kranenbarg EM, Putter H, Roodvoets AGH, Bahadoer RR, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes AMR, de Groot DJA, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; Collaborative investigators. Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer - Results of the international randomized RAPIDO-trial. Radiother Oncol. 2020 Jun;147:75–83. 10.1016/j.radonc.2020.03.011. Epub 2020 Mar 30. Erratum in: Radiother Oncol. 2020 Jun;147:e1. PMID: 32240909.
- 7.Liberale G, Vankerckhove S, Caldon MG, et al. Patterns of response and progression to oLAR in patients undergoing TNT for rectal cancer: individual risk factors and critical considerations. Ann Surg. 2021;273(5):944–51. [Google Scholar]
- 8.Moyer AM, Vogel JD, Lai SH, Kim H, Chin RI, Moskalenko M, Olsen JR, Birnbaum EH, Silviera ML, Mutch MG, Chapman BC. Total Neoadjuvant Therapy in Rectal Cancer: Multi-center Comparison of Induction Chemotherapy and Long-Course Chemoradiation Versus Short-Course Radiation and Consolidative Chemotherapy. J Gastrointest Surg. 2023May;27(5):980–9. 10.1007/s11605-023-05601-3. (Epub 2023 Feb 9 PMID: 36759387). [DOI] [PubMed] [Google Scholar]
- 9.Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, Kuhnt T, Staib L, Brunner T, Grosu AL, Schmiegel W, Jacobasch L, Weitz J, Folprecht G, Schlenska-Lange A, Flentje M, Germer CT, Grützmann R, Schwarzbach M, Paolucci V, Bechstein WO, Friede T, Ghadimi M, Hofheinz RD, Rödel C; German Rectal Cancer Study Group. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019 Dec 1;37(34):3212–3222. 10.1200/JCO.19.00308. Epub 2019 May 31. PMID: 31150315. [DOI] [PubMed]
- 10.Dijkstra EA, Nilsson PJ, Hospers GAP, Bahadoer RR, Meershoek-Klein Kranenbarg E, Roodvoets AGH, Putter H, Berglund Å, Cervantes A, Crolla RMPH, Hendriks MP, Capdevila J, Edhemovic I, Marijnen CAM, van de Velde CJH, Glimelius B, van Etten B; Collaborative Investigators. Locoregional Failure During and After Short-course Radiotherapy Followed by Chemotherapy and Surgery Compared With Long-course Chemoradiotherapy and Surgery: A 5-Year Follow-up of the RAPIDO Trial. Ann Surg. 2023 Oct 1;278(4):e766-e772. 10.1097/SLA.0000000000005799. Epub 2023 Jan 20. PMID: 36661037; PMCID: PMC10481913. [DOI] [PMC free article] [PubMed]
- 11.Schrag D, Shi Q, Weiser MR, Gollub MJ, Saltz LB, Musher BL, Goldberg J, Al Baghdadi T, Goodman KA, McWilliams RR, Farma JM, George TJ, Kennecke HF, Shergill A, Montemurro M, Nelson GD, Colgrove B, Gordon V, Venook AP, O’Reilly EM, Meyerhardt JA, Dueck AC, Basch E, Chang GJ, Mamon HJ. Preoperative Treatment of Locally Advanced Rectal Cancer. N Engl J Med. 2023Jul 27;389(4):322–34. 10.1056/NEJMoa2303269. (Epub 2023 Jun 4 PMID: 37272534). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, Morand C, de la Fouchardière C, Lamfichekh N, Juzyna B, Jouffroy-Zeller C, Rullier E, Marchal F, Gourgou S, Castan F, Borg C; Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021 May;22(5):702–715. 10.1016/S1470-2045(21)00079-6. Epub 2021 Apr 13. PMID: 33862000. [DOI] [PubMed]
- 13.Liu S, Jiang T, Xiao L, Yang S, Liu Q, Gao Y, Chen G, Xiao W. Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis. Oncologist. 2021 Sep;26(9):e1555-e1566. 10.1002/onco.13824. Epub 2021 Jun 7. PMID: 33987952; PMCID: PMC8417863. [DOI] [PMC free article] [PubMed]
- 14.Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, Guillem JG, Paty PB, Avila K, Garcia-Aguilar J; Rectal Cancer Consortium. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015 Oct 23;15:767. 10.1186/s12885-015-1632-z. PMID: 26497495; PMCID: PMC4619249. [DOI] [PMC free article] [PubMed]
- 15.Justiniano CF, Becerra AZ, Xu Z, Aquina CT, Boodry CI, Schymura MJ, Boscoe FP, Noyes K, Temple LK, Fleming FJ. A Population-Based Study of 90-Day Hospital Cost and Utilization Associated With Robotic Surgery in Colon and Rectal Cancer. J Surg Res. 2020Jan;245:136–44. 10.1016/j.jss.2019.07.052. (Epub 2019 Aug 13 PMID: 31419638). [DOI] [PubMed] [Google Scholar]
- 16.Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004 Oct;240(4):711–7; discussion 717–8. 10.1097/01.sla.0000141194.27992.32. PMID: 15383798; PMCID: PMC1356472. [DOI] [PMC free article] [PubMed]
- 17.Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol. 2008;47(1):20–31. 10.1080/02841860701697720. (PMID: 17957502). [DOI] [PubMed] [Google Scholar]
- 18.Johnson GGRJ, Park J, Helewa RM, Goldenberg BA, Nashed M, Hyun E. Total neoadjuvant therapy for rectal cancer: a guide for surgeons. Can J Surg. 2023Apr 21;66(2):E196–201. 10.1503/cjs.005822. PMID:37085291;PMCID:PMC10125160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaud Maturana M, English WJ, Nandakumar M, Li Chen J, Dvorkin L. The impact of frailty on clinical outcomes in colorectal cancer surgery: a systematic literature review. ANZ J Surg. 2021Nov;91(11):2322–9. 10.1111/ans.16941. (Epub 2021 May 20 PMID: 34013571). [DOI] [PubMed] [Google Scholar]
- 20.Niemeläinen S, Huhtala H, Ehrlich A, Kössi J, Jämsen E, Hyöty M. Surgical and functional outcomes and survival following Colon Cancer surgery in the aged: a study protocol for a prospective, observational multicentre study. BMC Cancer. 2021Jun 14;21(1):698. 10.1186/s12885-021-08454-8. PMID:34126949; PMCID:PMC8201898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuta M, Boc N, Brecelj E, Peternel M, Velenik V. Total neoadjuvant therapy vs standard therapy of locally advanced rectal cancer with high-risk factors for failure. World J Gastrointest Oncol. 2021Feb 15;13(2):119–30. 10.4251/wjgo.v13.i2.119. PMID:33643528; PMCID:PMC7896420 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.