Abstract
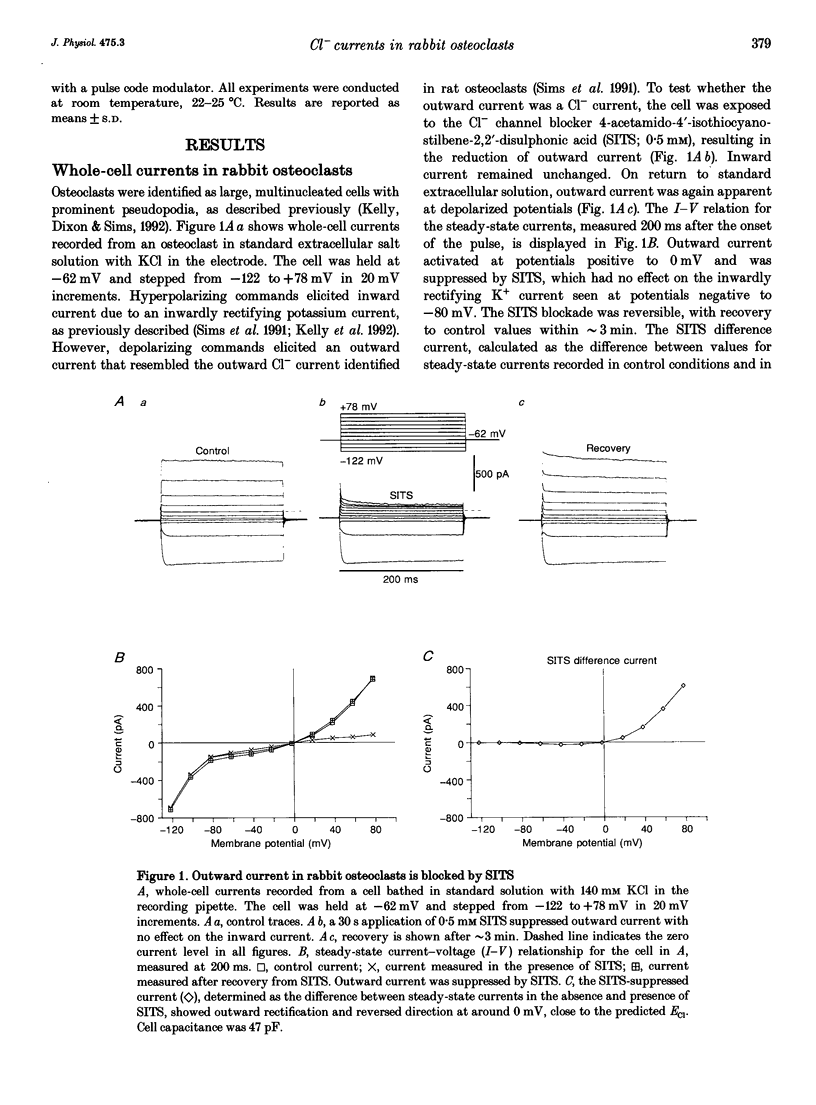
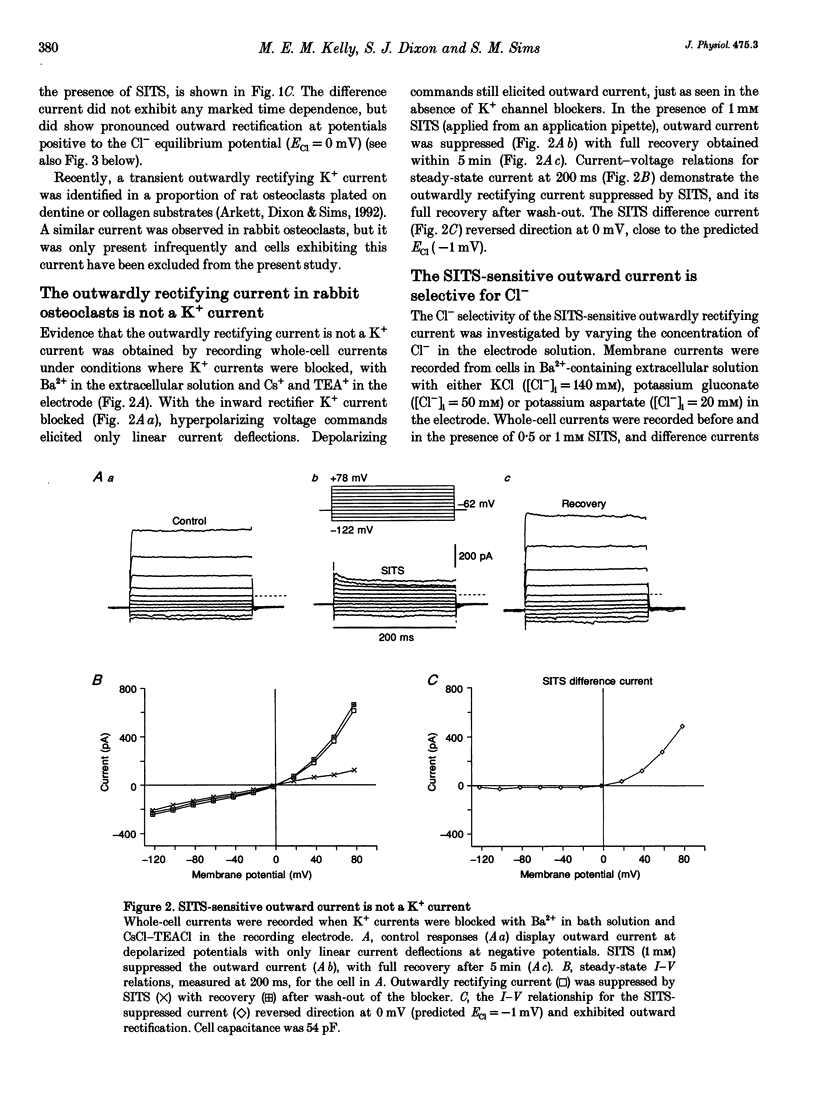
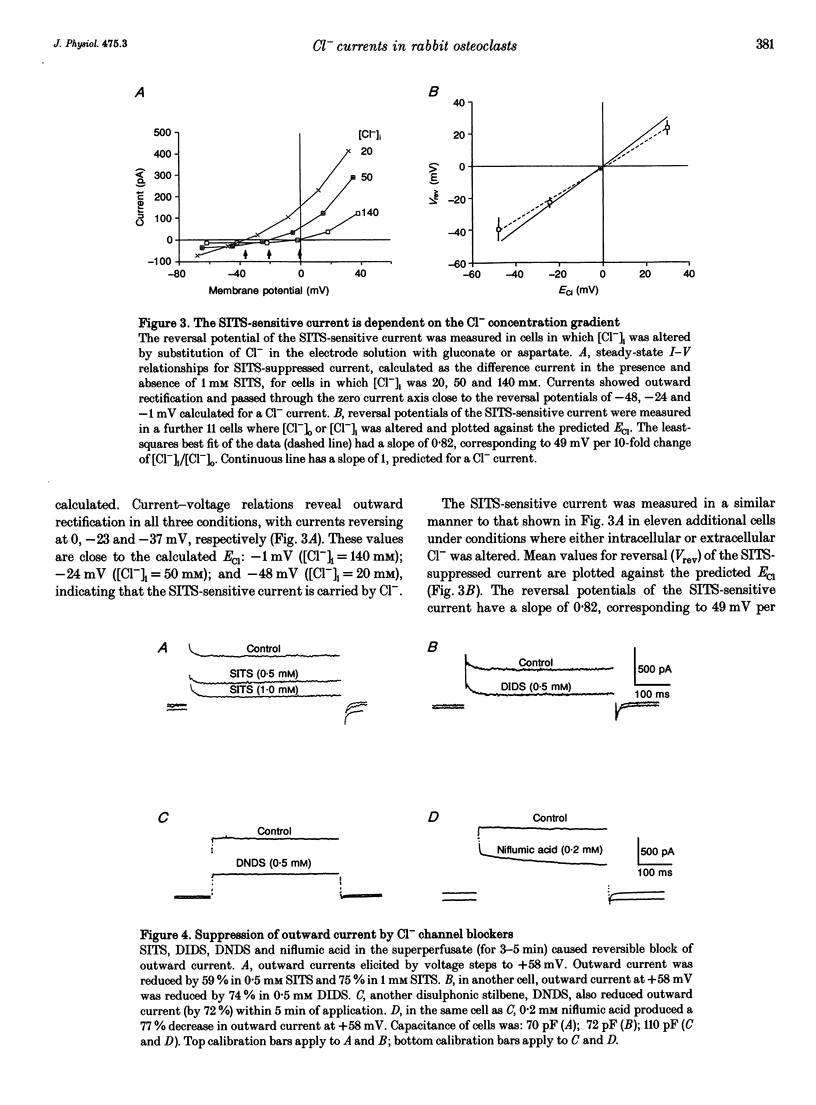
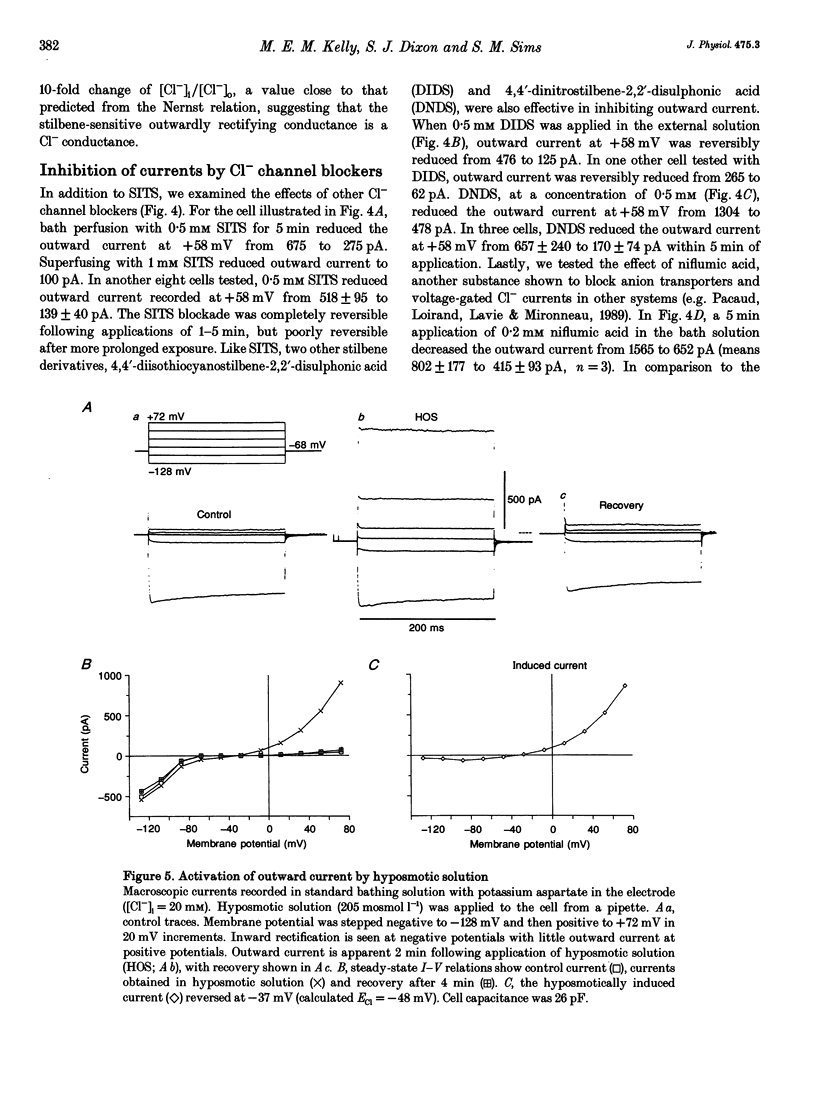
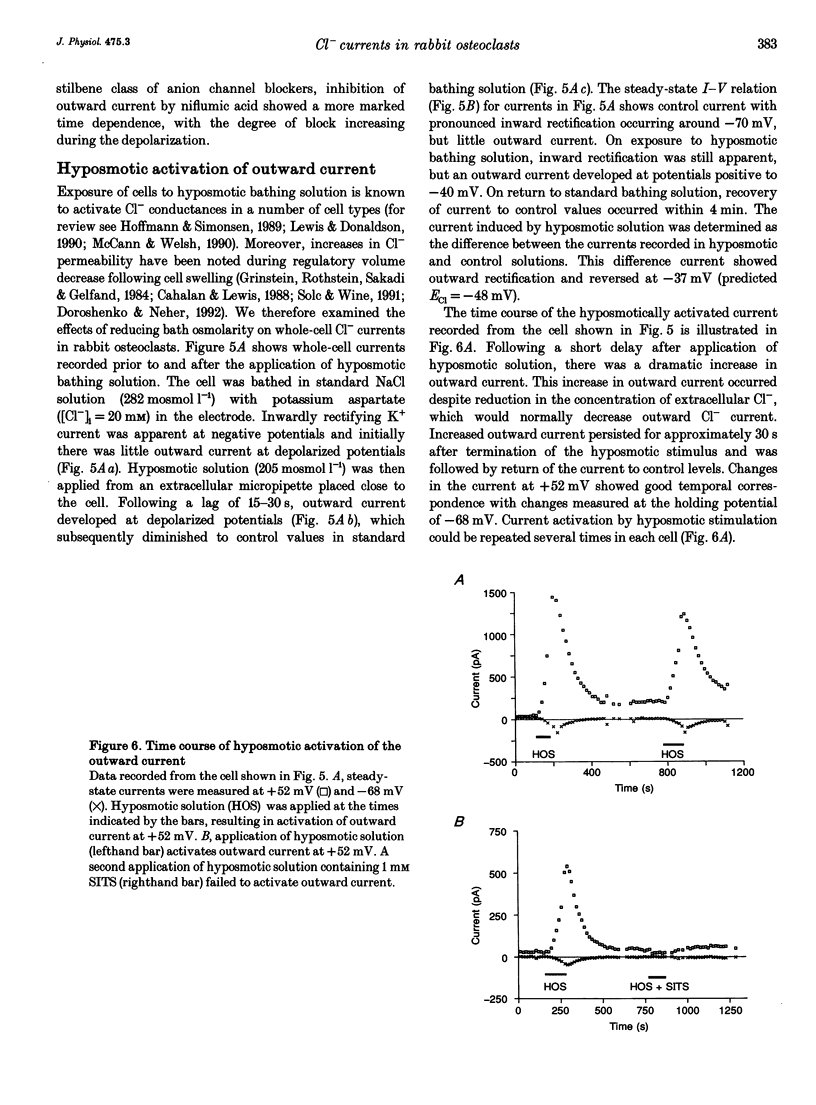
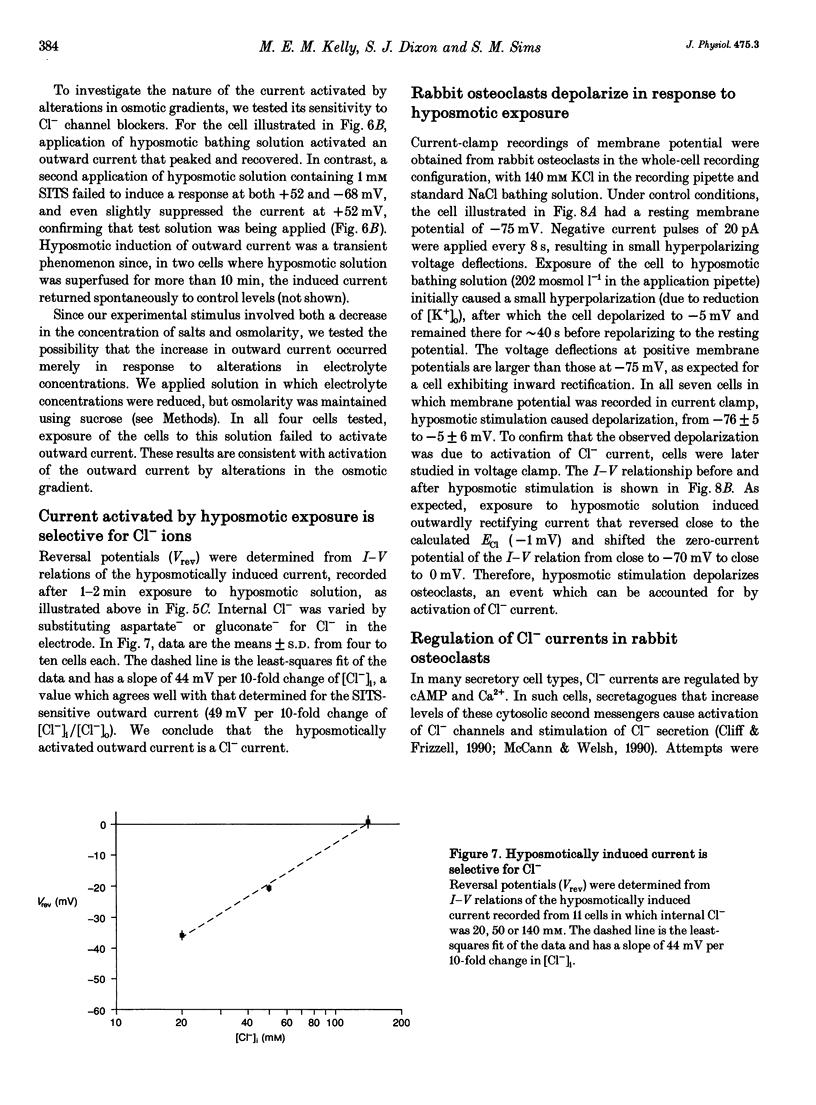
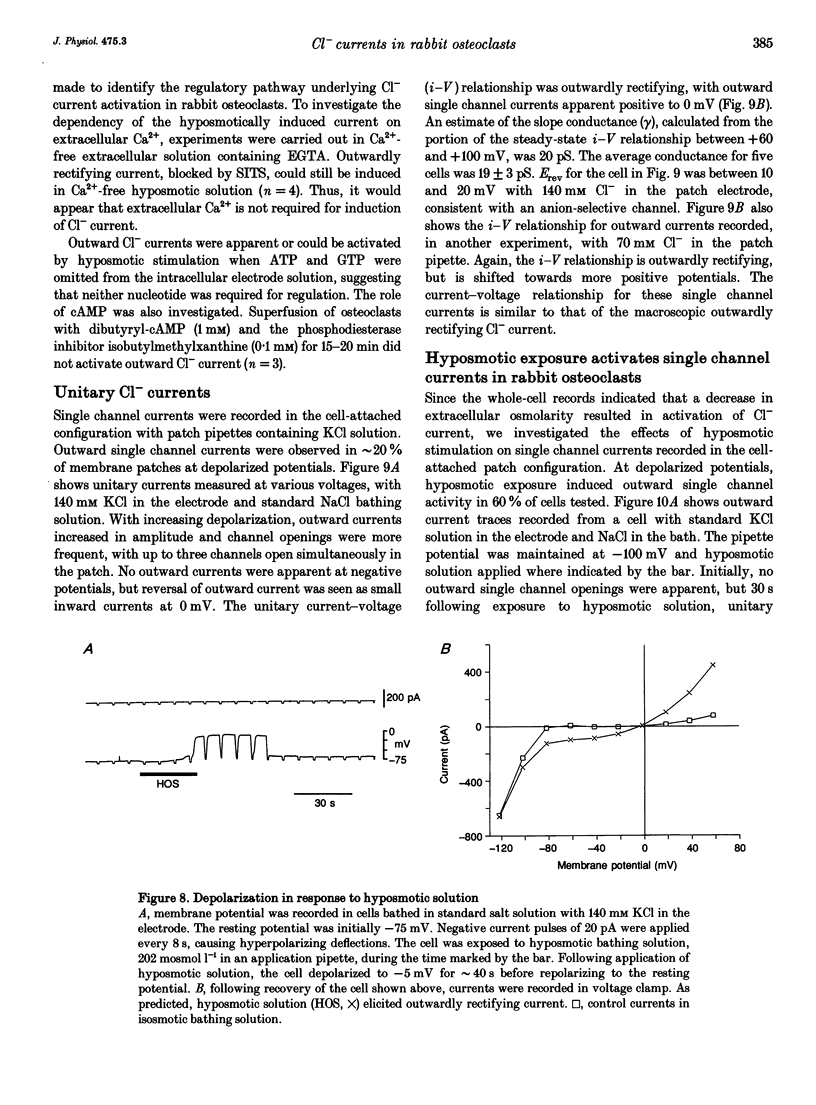
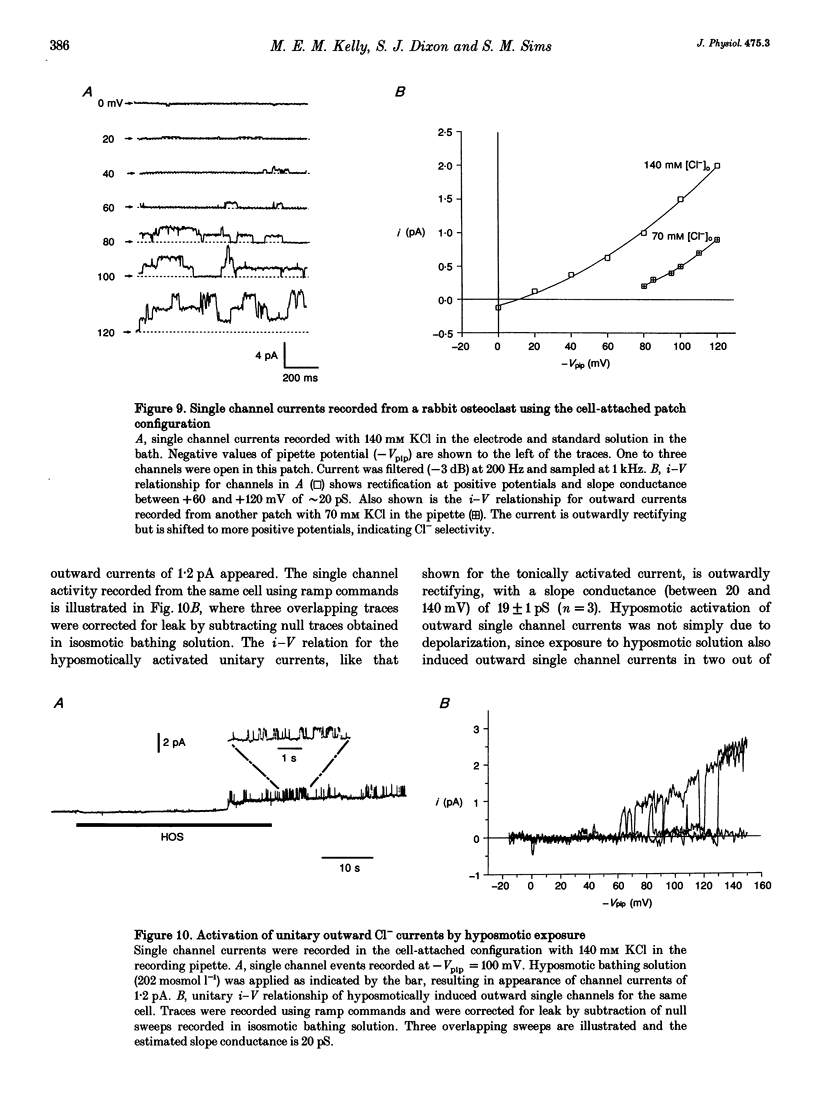
1. We characterized chloride currents in freshly isolated rabbit osteoclasts using whole-cell and single channel patch-clamp recording configurations. Depolarization activated an outwardly rectifying current in 40-50% of cells, distinct from the inwardly rectifying K+ current we have previously reported in osteoclasts. 2. The outwardly rectifying current persisted under conditions where all K+ currents were blocked. Furthermore, the outward current was reversibly inhibited by Cl- transport blockers 4-acetamido-4'-isothiocyanostilbene-2,2'-disulphonic acid (SITS); 4,4'-diisothiocyanostilbene-2,2'-disulphonic acid (DIDS); 4,4'-dinitrostilbene-2,2'-disulphonic acid (DNDS); and niflumic acid. The blocked current had a reversal potential close to the predicted chloride equilibrium potential and was dependent on the chloride concentration gradient. 3. In those osteoclasts in which outwardly rectifying current was not initially apparent, exposure to hyposmotic extracellular solution resulted in its reversible activation. The induced current was due to Cl-, based on its reversal close to the chloride equilibrium potential and sensitivity to blockade by Cl- channel inhibitors. The hyposmotically induced current could be activated in Ca(2+)-free solutions containing 0.2 mM EGTA. 4. When studied in the current-clamp configuration, hyposmotic stimulation caused depolarization from -76 +/- 5 to -5 +/- 6 mV (mean +/- S.D., n = 7). 5. Unitary Cl- currents were recorded in the cell-attached patch configuration at positive potentials. Single channels had a slope conductance of 19 +/- 3 pS (n = 5). Reduction of the external [Cl-] shifted the current-voltage relationship in the positive direction, supporting the conclusion that these were Cl- currents. Like the whole-cell currents, single channel Cl- currents were activated by exposure of cells to hyposmotic bathing solution. 6. We conclude that rabbit osteoclasts express an outwardly rectifying Cl- current that can be activated by osmotic stress. Cl- channels may play a role in cell volume regulation and may also provide conductive pathways for dissipating the potential difference that arises from electrogenic proton transport during bone resorption.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkett S. A., Dixon S. J., Sims S. M. Substrate influences rat osteoclast morphology and expression of potassium conductances. J Physiol. 1992 Dec;458:633–653. doi: 10.1113/jphysiol.1992.sp019438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banderali U., Roy G. Activation of K+ and Cl- channels in MDCK cells during volume regulation in hypotonic media. J Membr Biol. 1992 Mar;126(3):219–234. doi: 10.1007/BF00232319. [DOI] [PubMed] [Google Scholar]
- Bekker P. J., Gay C. V. Biochemical characterization of an electrogenic vacuolar proton pump in purified chicken osteoclast plasma membrane vesicles. J Bone Miner Res. 1990 Jun;5(6):569–579. doi: 10.1002/jbmr.5650050606. [DOI] [PubMed] [Google Scholar]
- Blair H. C., Schlesinger P. H. Purification of a stilbene sensitive chloride channel and reconstitution of chloride conductivity into phospholipid vesicles. Biochem Biophys Res Commun. 1990 Sep 28;171(3):920–925. doi: 10.1016/0006-291x(90)90771-e. [DOI] [PubMed] [Google Scholar]
- Blair H. C., Teitelbaum S. L., Ghiselli R., Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989 Aug 25;245(4920):855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- Blair H. C., Teitelbaum S. L., Tan H. L., Koziol C. M., Schlesinger P. H. Passive chloride permeability charge coupled to H(+)-ATPase of avian osteoclast ruffled membrane. Am J Physiol. 1991 Jun;260(6 Pt 1):C1315–C1324. doi: 10.1152/ajpcell.1991.260.6.C1315. [DOI] [PubMed] [Google Scholar]
- Bridges R. J., Worrell R. T., Frizzell R. A., Benos D. J. Stilbene disulfonate blockade of colonic secretory Cl- channels in planar lipid bilayers. Am J Physiol. 1989 Apr;256(4 Pt 1):C902–C912. doi: 10.1152/ajpcell.1989.256.4.C902. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Lewis R. S. Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser. 1988;43:281–301. [PubMed] [Google Scholar]
- Chatterjee D., Chakraborty M., Leit M., Neff L., Jamsa-Kellokumpu S., Fuchs R., Baron R. Sensitivity to vanadate and isoforms of subunits A and B distinguish the osteoclast proton pump from other vacuolar H+ ATPases. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6257–6261. doi: 10.1073/pnas.89.14.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen O., Hoffmann E. K. Cell swelling activates K+ and Cl- channels as well as nonselective, stretch-activated cation channels in Ehrlich ascites tumor cells. J Membr Biol. 1992 Jul;129(1):13–36. doi: 10.1007/BF00232052. [DOI] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshenko P., Neher E. Volume-sensitive chloride conductance in bovine chromaffin cell membrane. J Physiol. 1992 Apr;449:197–218. doi: 10.1113/jphysiol.1992.sp019082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol Rev. 1989 Jul;69(3):765–796. doi: 10.1152/physrev.1989.69.3.765. [DOI] [PubMed] [Google Scholar]
- Fuller C. M., Benos D. J. CFTR! Am J Physiol. 1992 Aug;263(2 Pt 1):C267–C286. doi: 10.1152/ajpcell.1992.263.2.C267. [DOI] [PubMed] [Google Scholar]
- García-Díaz J. F. Whole-cell and single channel K+ and Cl- currents in epithelial cells of frog skin. J Gen Physiol. 1991 Jul;98(1):131–161. doi: 10.1085/jgp.98.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A., Sarkadi B., Gelfand E. W. Responses of lymphocytes to anisotonic media: volume-regulating behavior. Am J Physiol. 1984 Mar;246(3 Pt 1):C204–C215. doi: 10.1152/ajpcell.1984.246.3.C204. [DOI] [PubMed] [Google Scholar]
- Gründer S., Thiemann A., Pusch M., Jentsch T. J. Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature. 1992 Dec 24;360(6406):759–762. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- Hall T. J., Chambers T. J. Optimal bone resorption by isolated rat osteoclasts requires chloride/bicarbonate exchange. Calcif Tissue Int. 1989 Dec;45(6):378–380. doi: 10.1007/BF02556011. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Kelly M. E., Dixon S. J., Sims S. M. Inwardly rectifying potassium current in rabbit osteoclasts: a whole-cell and single-channel study. J Membr Biol. 1992 Mar;126(2):171–181. doi: 10.1007/BF00231915. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J., Raisz L. G. Effects of two inhibitors of anion transport on bone resorption in organ culture. Endocrinology. 1989 Aug;125(2):1019–1024. doi: 10.1210/endo-125-2-1019. [DOI] [PubMed] [Google Scholar]
- Kubo M., Okada Y. Volume-regulatory Cl- channel currents in cultured human epithelial cells. J Physiol. 1992 Oct;456:351–371. doi: 10.1113/jphysiol.1992.sp019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J. D., Li M., Welsh M. J. Identification and regulation of whole-cell chloride currents in airway epithelium. J Gen Physiol. 1989 Dec;94(6):1015–1036. doi: 10.1085/jgp.94.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Regulation of Cl- and K+ channels in airway epithelium. Annu Rev Physiol. 1990;52:115–135. doi: 10.1146/annurev.ph.52.030190.000555. [DOI] [PubMed] [Google Scholar]
- Morris C. E. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Lavie J. L., Mironneau C., Mironneau J. Calcium-activated chloride current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1989 Apr;413(6):629–636. doi: 10.1007/BF00581813. [DOI] [PubMed] [Google Scholar]
- Ravesloot J. H., Ypey D. L., Vrijheid-Lammers T., Nijweide P. J. Voltage-activated K+ conductances in freshly isolated embryonic chicken osteoclasts. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6821–6825. doi: 10.1073/pnas.86.17.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F. Baroreceptor mechanisms at the cellular level. Fed Proc. 1987 Jan;46(1):12–16. [PubMed] [Google Scholar]
- Schwarze W., Kolb H. A. Voltage-dependent kinetics of an anionic channel of large unit conductance in macrophages and myotube membranes. Pflugers Arch. 1984 Nov;402(3):281–291. doi: 10.1007/BF00585511. [DOI] [PubMed] [Google Scholar]
- Sims S. M., Dixon S. J. Inwardly rectifying K+ current in osteoclasts. Am J Physiol. 1989 Jun;256(6 Pt 1):C1277–C1282. doi: 10.1152/ajpcell.1989.256.6.C1277. [DOI] [PubMed] [Google Scholar]
- Sims S. M., Kelly M. E., Dixon S. J. K+ and Cl- currents in freshly isolated rat osteoclasts. Pflugers Arch. 1991 Oct;419(3-4):358–370. doi: 10.1007/BF00371118. [DOI] [PubMed] [Google Scholar]
- Solc C. K., Wine J. J. Swelling-induced and depolarization-induced C1-channels in normal and cystic fibrosis epithelial cells. Am J Physiol. 1991 Oct;261(4 Pt 1):C658–C674. doi: 10.1152/ajpcell.1991.261.4.C658. [DOI] [PubMed] [Google Scholar]
- Tabcharani J. A., Jensen T. J., Riordan J. R., Hanrahan J. W. Bicarbonate permeability of the outwardly rectifying anion channel. J Membr Biol. 1989 Dec;112(2):109–122. doi: 10.1007/BF01871272. [DOI] [PubMed] [Google Scholar]
- Teti A., Blair H. C., Teitelbaum S. L., Kahn A. J., Koziol C., Konsek J., Zambonin-Zallone A., Schlesinger P. H. Cytoplasmic pH regulation and chloride/bicarbonate exchange in avian osteoclasts. J Clin Invest. 1989 Jan;83(1):227–233. doi: 10.1172/JCI113863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubl J., Murer H., Kolb H. A. Ion channels activated by osmotic and mechanical stress in membranes of opossum kidney cells. J Membr Biol. 1988 Sep;104(3):223–232. doi: 10.1007/BF01872324. [DOI] [PubMed] [Google Scholar]
- Vaes G. Cellular biology and biochemical mechanism of bone resorption. A review of recent developments on the formation, activation, and mode of action of osteoclasts. Clin Orthop Relat Res. 1988 Jun;(231):239–271. [PubMed] [Google Scholar]
- Vänänen H. K., Karhukorpi E. K., Sundquist K., Wallmark B., Roininen I., Hentunen T., Tuukkanen J., Lakkakorpi P. Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J Cell Biol. 1990 Sep;111(3):1305–1311. doi: 10.1083/jcb.111.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Liedtke C. M. Chloride and potassium channels in cystic fibrosis airway epithelia. 1986 Jul 31-Aug 6Nature. 322(6078):467–470. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]
- Worrell R. T., Butt A. G., Cliff W. H., Frizzell R. A. A volume-sensitive chloride conductance in human colonic cell line T84. Am J Physiol. 1989 Jun;256(6 Pt 1):C1111–C1119. doi: 10.1152/ajpcell.1989.256.6.C1111. [DOI] [PubMed] [Google Scholar]


