Abstract
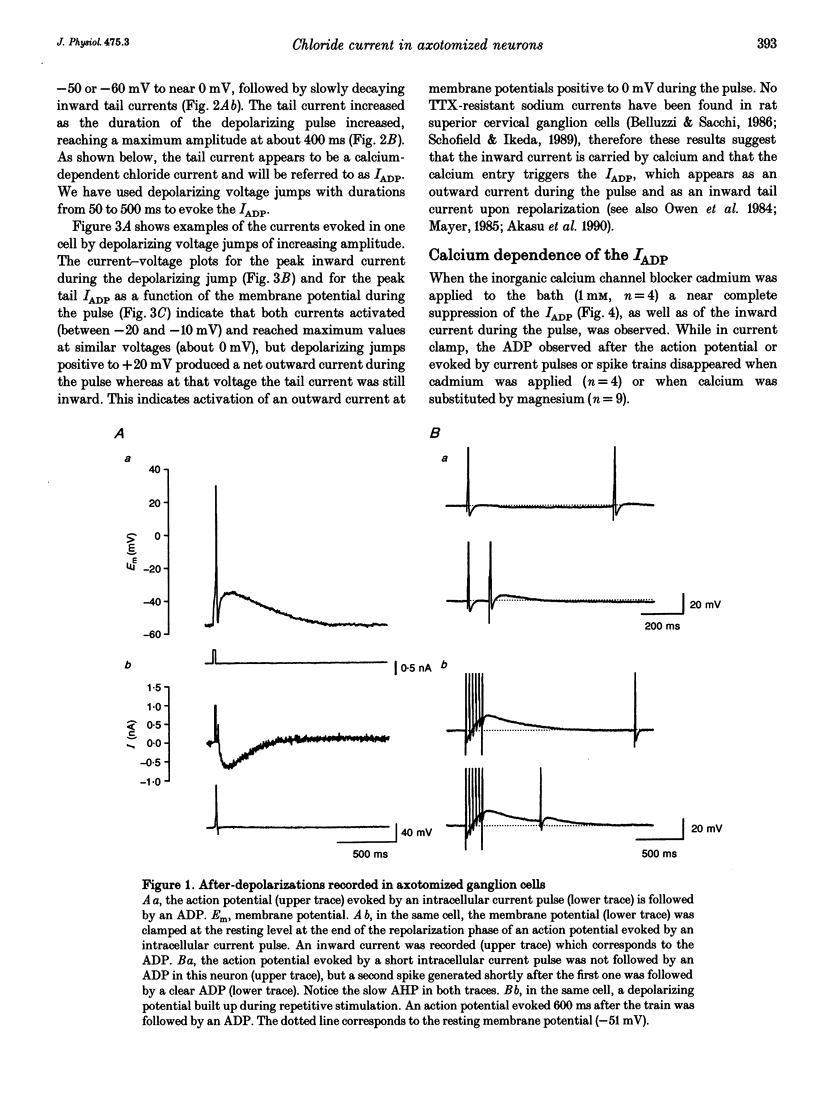
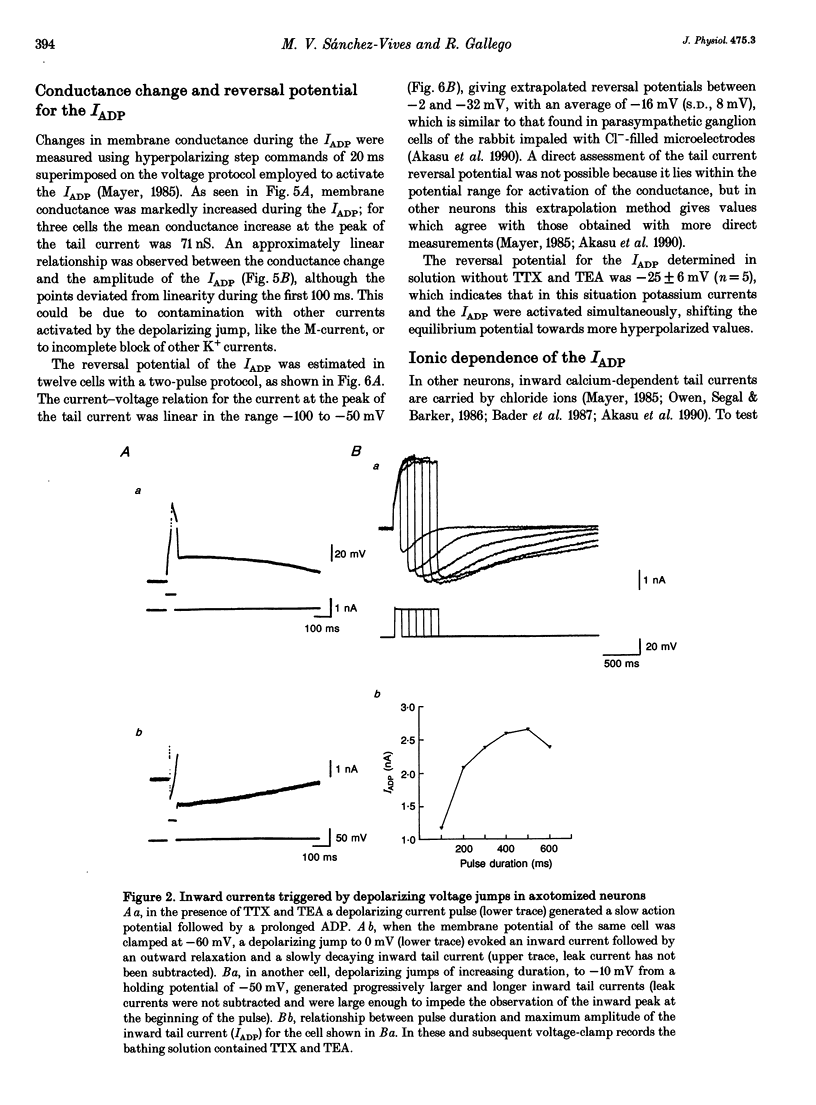
1. Seven to ten days after sectioning their axons, rat sympathetic neurons were studied using intracellular recording techniques in an in vitro preparation of the superior cervical ganglion. 2. In 75% of axotomized cells, an after-depolarization (ADP) was observed following spike firing or depolarization with intracellular current pulses. Discontinuous single-electrode voltage-clamp techniques were employed to study the ADP. When the membrane potential was clamped at the resting level just after an action potential, a slow inward current was recorded in cells that showed an ADP. 3. In the presence of TTX and TEA, inward peaks and outward currents were recorded during depolarizing voltage jumps, followed by slowly decaying inward tail currents accompanied by large increases in membrane conductance. The inward peak and tail currents activated between -10 and -20 mV and reached maximum amplitudes around 0 mV. With depolarizing jumps to between +40 and +50 mV, net outward currents were recorded during the depolarizing jumps but inward tail currents were still activated. 4. In the presence of the Ca2+ channel blocker cadmium, or when Ca2+ was substituted by Mg2+, the ADP disappeared. In voltage-clamped cells, cadmium blocked the inward tail currents. The reversal potential for the inward tail current was approximately -15 mV. Substitution of the extracellular NaCl by sucrose or sodium isethionate increased the amplitude of the inward tail current, and displaced its equilibrium potential to more positive values. Changes in extracellular [K+] did not appreciably affect the inward tail current amplitude or equilibrium potential. Niflumic acid, a blocker of chloride channels activated by Ca2+, almost completely blocked the tail current. 5. No ADPs were observed in non-axotomized neurons, and when depolarizing pulses were applied while in voltage clamp no inward tail currents were evoked in these normal cells. 6. It is concluded that axotomy of sympathetic ganglion cells produces the appearance of a Ca(2+)-dependent chloride current responsible for the ADP observed following spike firing.
Full text
PDF

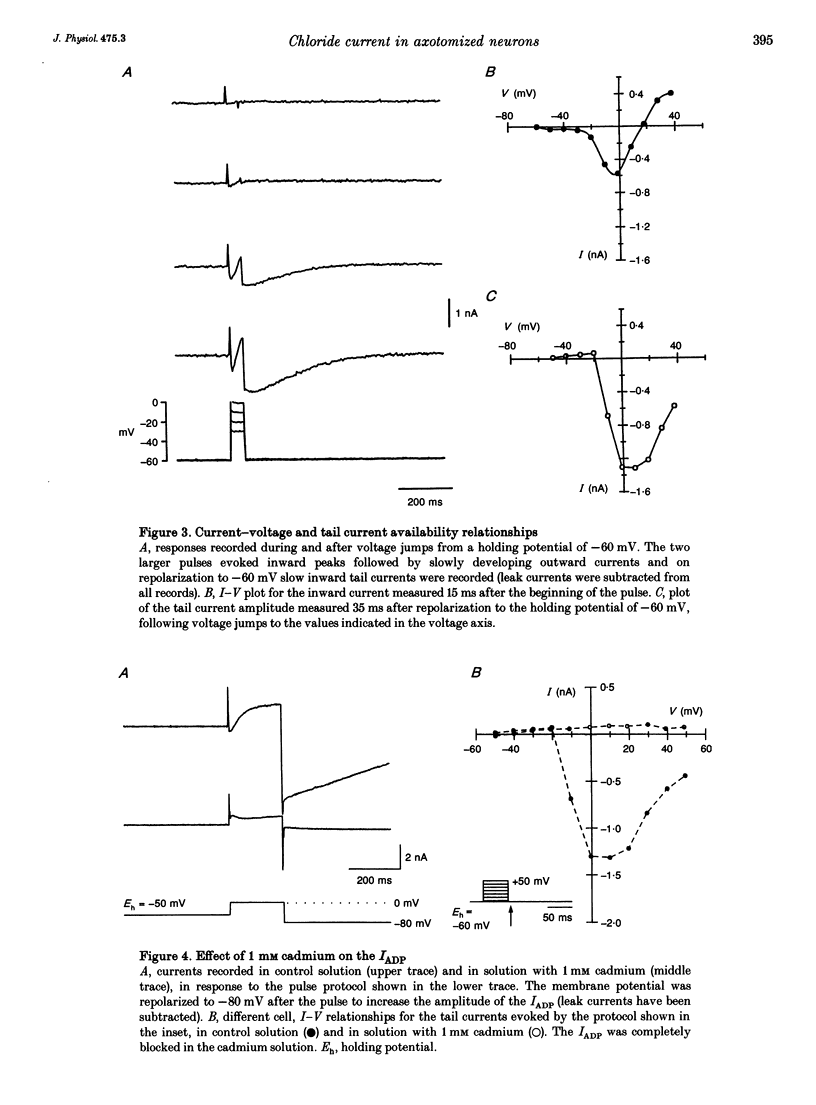
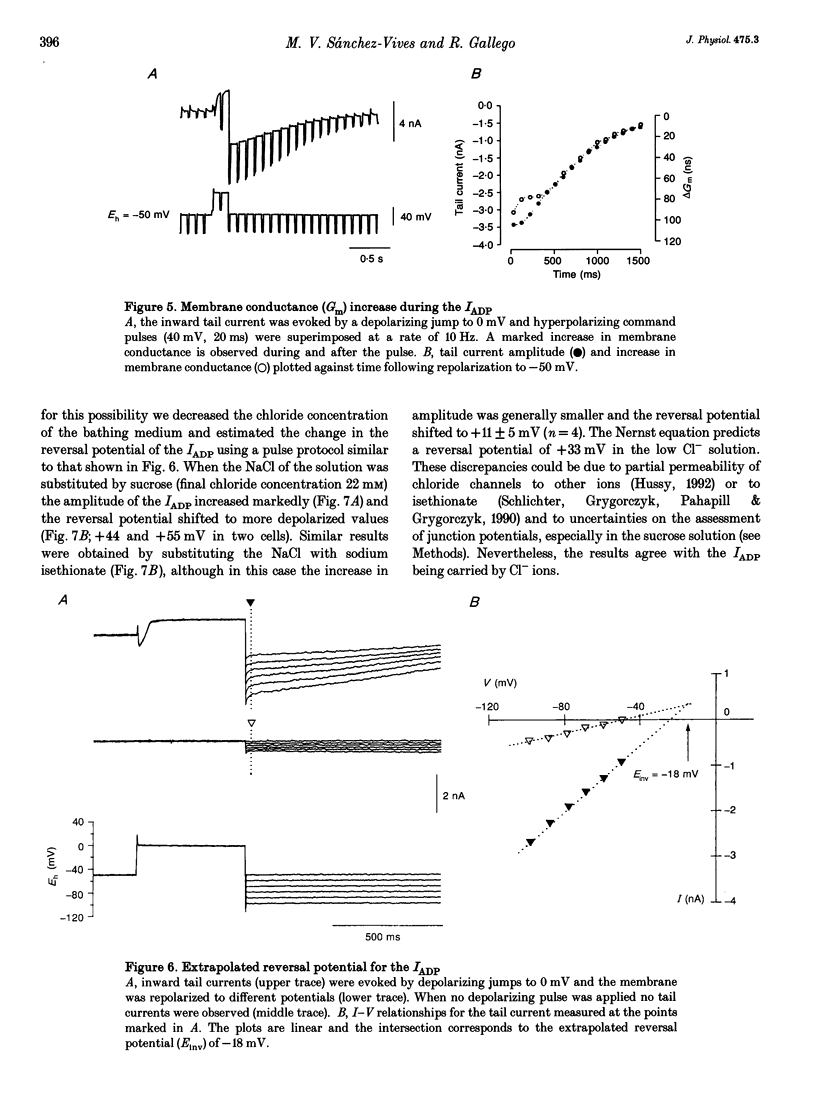
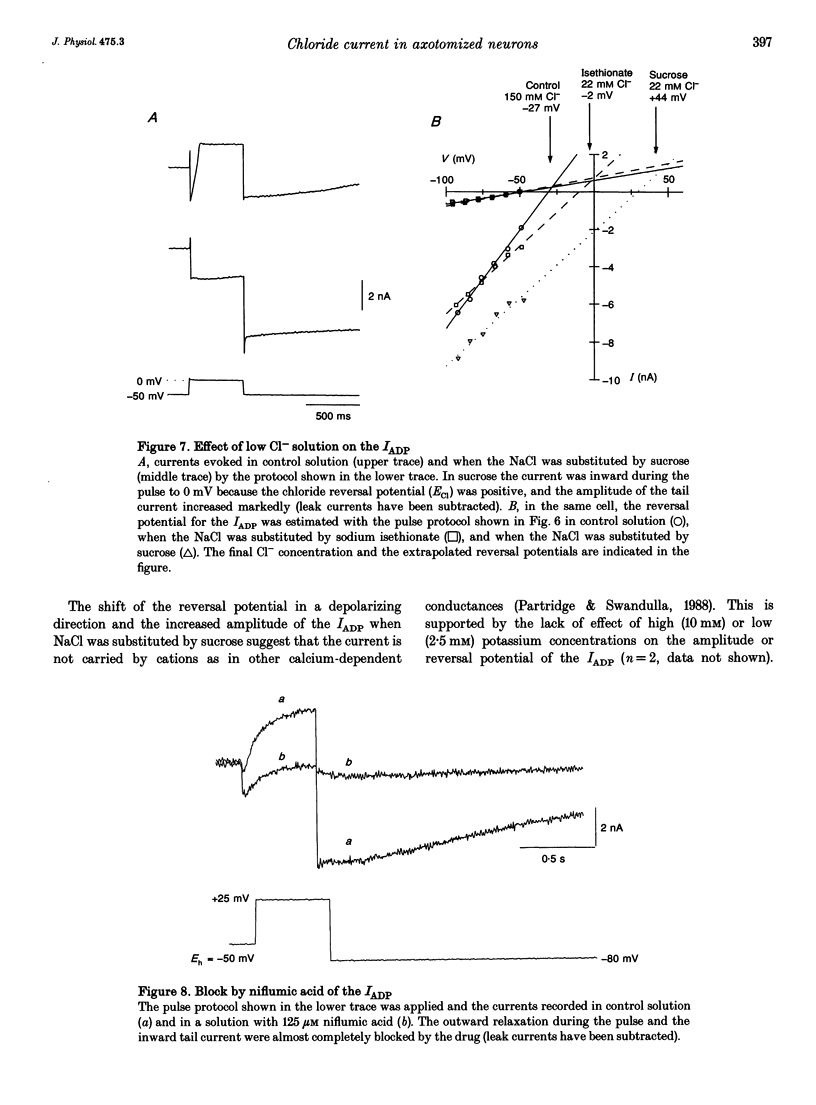
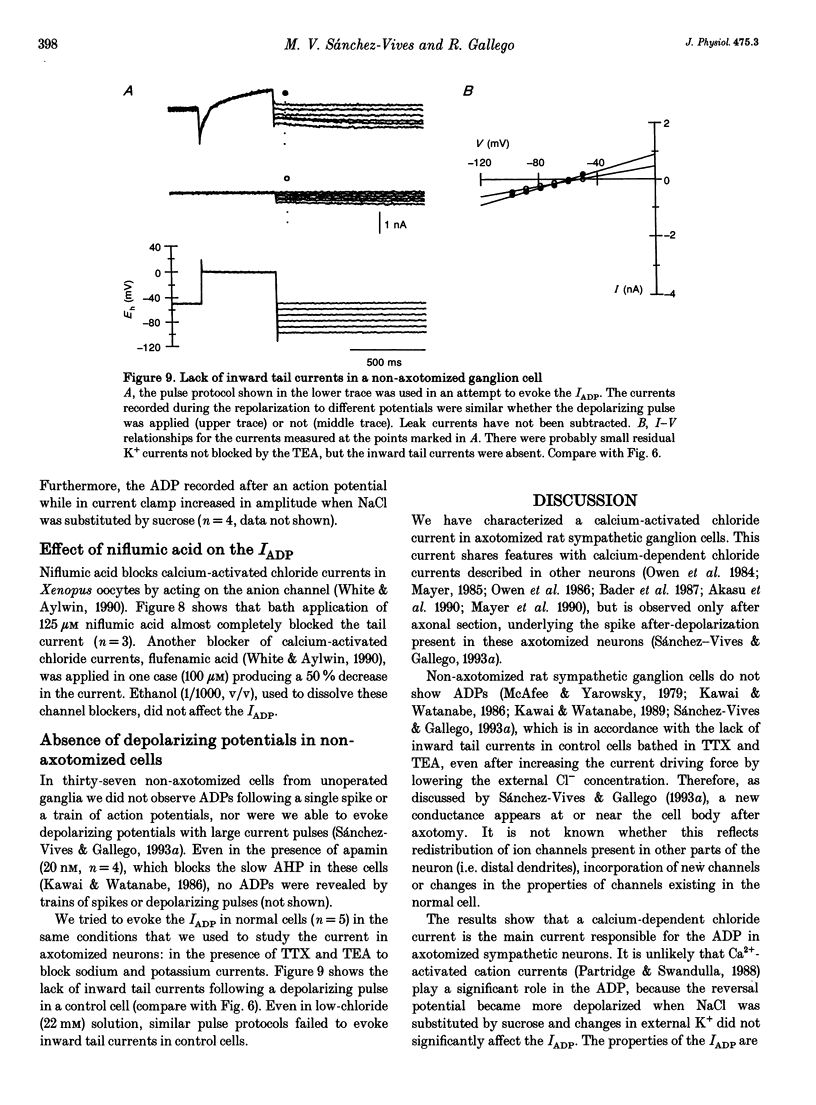


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agin D., Holtzman D. Glass microelectrodes: the origin and elimination of tip potentials. Nature. 1966 Sep 10;211(5054):1194–1195. doi: 10.1038/2111194a0. [DOI] [PubMed] [Google Scholar]
- Akasu T., Nishimura T., Tokimasa T. Calcium-dependent chloride current in neurones of the rabbit pelvic parasympathetic ganglia. J Physiol. 1990 Mar;422:303–320. doi: 10.1113/jphysiol.1990.sp017985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Leefmans F. J., Gamiño S. M., Giraldez F., Noguerón I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988 Dec;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schlichter R. Calcium-activated chloride current in cultured sensory and parasympathetic quail neurones. J Physiol. 1987 Dec;394:125–148. doi: 10.1113/jphysiol.1987.sp016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K., Grafe P., Reddy M. M., ten Bruggencate G. Different types of potassium transport linked to carbachol and gamma-aminobutyric acid actions in rat sympathetic neurons. Neuroscience. 1984 Jul;12(3):917–927. doi: 10.1016/0306-4522(84)90179-9. [DOI] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. A quantitative description of the sodium current in the rat sympathetic neurone. J Physiol. 1986 Nov;380:275–291. doi: 10.1113/jphysiol.1986.sp016285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. The calcium-dependent potassium conductance in rat sympathetic neurones. J Physiol. 1990 Mar;422:561–583. doi: 10.1113/jphysiol.1990.sp018001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Dörge A., Beck F., Rick R. Intracellular electrolyte concentrations in rat sympathetic neurones measured with an electron microprobe. Pflugers Arch. 1984 Mar;400(3):274–279. doi: 10.1007/BF00581559. [DOI] [PubMed] [Google Scholar]
- Hasuo H., Phelan K. D., Twery M. J., Gallagher J. P. A calcium-dependent slow afterdepolarization recorded in rat dorsolateral septal nucleus neurons in vitro. J Neurophysiol. 1990 Dec;64(6):1838–1846. doi: 10.1152/jn.1990.64.6.1838. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Hussy N. Calcium-activated chloride channels in cultured embryonic Xenopus spinal neurons. J Neurophysiol. 1992 Dec;68(6):2042–2050. doi: 10.1152/jn.1992.68.6.2042. [DOI] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Blockade of Ca-activated K conductance by apamin in rat sympathetic neurones. Br J Pharmacol. 1986 Jan;87(1):225–232. doi: 10.1111/j.1476-5381.1986.tb10175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Effects of ryanodine on the spike after-hyperpolarization in sympathetic neurones of the rat superior cervical ganglion. Pflugers Arch. 1989 Mar;413(5):470–475. doi: 10.1007/BF00594175. [DOI] [PubMed] [Google Scholar]
- Lieberman A. R. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee D. A., Yarowsky P. J. Calcium-dependent potentials in the mammalian sympathetic neurone. J Physiol. 1979 May;290(2):507–523. doi: 10.1113/jphysiol.1979.sp012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty N. A., O'Neil R. G. Calcium signaling in cell volume regulation. Physiol Rev. 1992 Oct;72(4):1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. A Ca-dependent Cl- conductance in cultured mouse spinal neurones. Nature. 1984 Oct 11;311(5986):567–570. doi: 10.1038/311567a0. [DOI] [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. Voltage-clamp analysis of a Ca2+- and voltage-dependent chloride conductance in cultured mouse spinal neurons. J Neurophysiol. 1986 Jun;55(6):1115–1135. doi: 10.1152/jn.1986.55.6.1115. [DOI] [PubMed] [Google Scholar]
- Partridge L. D., Swandulla D. Calcium-activated non-specific cation channels. Trends Neurosci. 1988 Feb;11(2):69–72. doi: 10.1016/0166-2236(88)90167-1. [DOI] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Parker J. C. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991 Dec 12;1071(4):407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- Schlichter L. C., Grygorczyk R., Pahapill P. A., Grygorczyk C. A large, multiple-conductance chloride channel in normal human T lymphocytes. Pflugers Arch. 1990 Jun;416(4):413–421. doi: 10.1007/BF00370748. [DOI] [PubMed] [Google Scholar]
- Schofield G. G., Ikeda S. R. Sodium and calcium currents of acutely isolated adult rat superior cervical ganglion neurons. Pflugers Arch. 1988 May;411(5):481–490. doi: 10.1007/BF00582368. [DOI] [PubMed] [Google Scholar]
- Sánchez-Vives M. V., Gallego R. Effects of axotomy or target atrophy on membrane properties of rat sympathetic ganglion cells. J Physiol. 1993 Nov;471:801–815. doi: 10.1113/jphysiol.1993.sp019929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titmus M. J., Faber D. S. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog Neurobiol. 1990;35(1):1–51. doi: 10.1016/0301-0082(90)90039-j. [DOI] [PubMed] [Google Scholar]
- White M. M., Aylwin M. Niflumic and flufenamic acids are potent reversible blockers of Ca2(+)-activated Cl- channels in Xenopus oocytes. Mol Pharmacol. 1990 May;37(5):720–724. [PubMed] [Google Scholar]


