Abstract
Background
We present the treatment and follow-up of a rare patient with acute retinal necrosis (ARN) with optic neuropathy as the first manifestation.
Case presentation
A 57-year-old male patient first presented with reduced vision and optic neuropathy in his right eye.After 20 days, the vision in the left eye decreased, and the slit-lamp examination showed no remarkable changes in the anterior segment. The right eye showed 2 + cells and flares in the anterior chamber. The iris texture was good, the pupils were round (light reflex), the crystalline lens was slightly opaque, and the vitreous was turbid. An anterior chamber paracentesis was performed to collect aqueous humor for polymerase chain reaction testing. The PCR test showed positive detection of VZV in the right eye, while the content of VZV in the left eye was lower than the reference value. Both eyes of the patient were successively diagnosed with acute retinal necrosis (ARN) due to VZV infection. The patient was treated with topical antiviral and anti-inflammatory eye medication, oral steroids, and general antiviral drugs. After three months, the vision and condition were relatively stable.
Conclusions
Clinicians should realize that accurate diagnosis and timely antiviral therapy are crucial for treating ARN.
Keywords: Acute retinal necrosis, Optic neuropathy, Virus, Antiviral treatment, Steroids
Background
Acute retinal necrosis (ARN) is a type of uveitis syndrome caused by a viral infection. ARN can be caused by varicella zoster virus (VZV), herpes simplex viruses 1 and 2 (HSV-1, HSV-2), cytomegalovirus (CMV), and Epstein-Barr virus (EBV), with a relatively low prevalence of approximately [1–4]. Based on two nationwide UK surveys, the annual incidence of ARN is estimated to be 0.5 to 0.63 new cases per million population [5, 6]. The typical clinical manifestations of ARN are granulomatous inflammation, retinal arteriolar vascular occlusion, and peripheral retinitis [7]. We report a rare case of ARN with optic neuropathy as the initial manifestation.
Case presentation
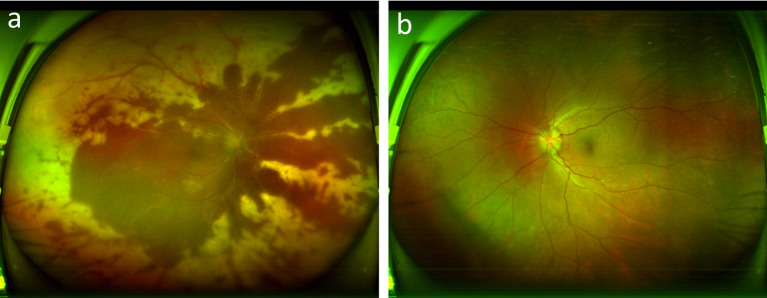
A 57-year-old Asian male patient came to our hospital with visual acuity decreased for 20 days in the right eye and two days in his left eye. Twenty days ago, the patient experienced unexplained vision loss in their right eye. He went to a regional hospital where tests revealed optic disc edema, and he was diagnosed with ischemic optic neuropathy (Fig. 1). He was treated with drugs such as nourishing nerves and improving circulation and systemic hormones (intravenous dexamethasone, unknown dose, continuous use for ten days, changed to prednisone 30 mg orally, Once a day for a week), the patient ‘s treatment details are not know.
Fig. 1.
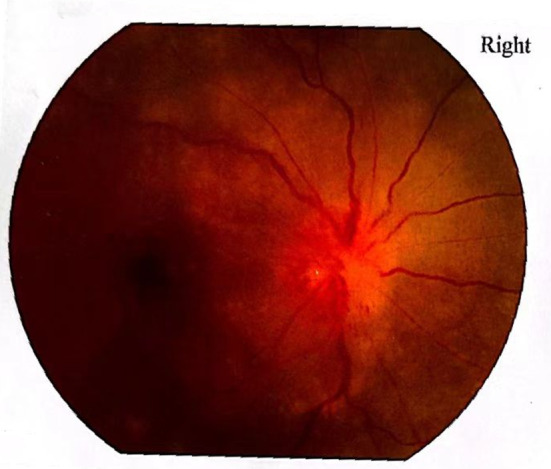
Right eye fundus photography
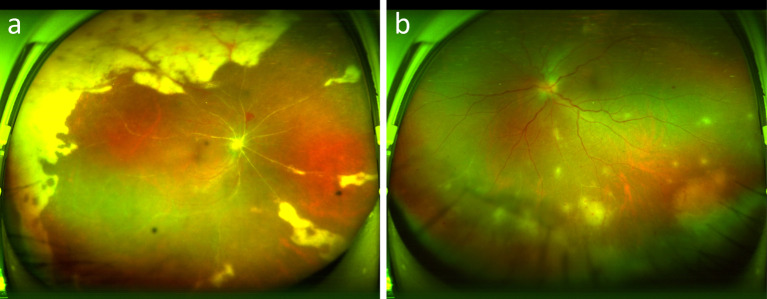
The patient presented to our hospital with a sudden decrease in vision in his left eye two days ago. The patient had a 1-year history of diabetes mellitus and no other medical history. His best-corrected visual acuity (BCVA) was 20/200 in his right eye and 20/30 in his left eye. The IOP was 13 mmHg and 14.7 mmHg, respectively. The slit lamp examination of the right eye showed light ciliary congestion, keratic precipitates (KPs) and 2 + anterior chamber cells, mild lens opacity, and mild vitreous opacities. The left eye was mild congested, shallow anterior chamber with vitreous opacity. Fundus photography of his right eye revealed an unclear optic disc boundary, extensive arterial occlusion, large yellow-white lesions around the retina, and patchy hemorrhage in the upper retina. His left eye showed an indistinct optic disc border with vascular alignment and a reddish-brown retina (Fig. 2).
Fig. 2.
Fundus photography of the patient on the day of hospitalization. Right eye (a), left eye (b)
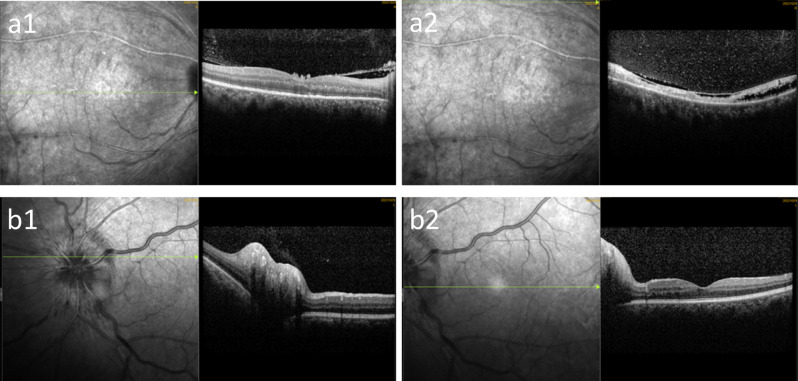
Optical coherence tomography (OCT) showed that there was a cord-like reflection in the vitreous cavity of the right eye, the central concave curve of the macular area was flat, the light reflection of the neuroepithelial layer was uneven, the thickness was different, the local reflection of the outer layer of the retina was discontinuous, and the low reflection area was visible between the neuroepithelial layers above the fovea. The foveal curve of the macular region in his left eye was good, and the mild reflection of the neuroepithelial layer was not uniform (Fig. 3).
Fig. 3.
Optical coherence tomography of the patient on the day of hospitalization. Right eye (a), left eye (b)
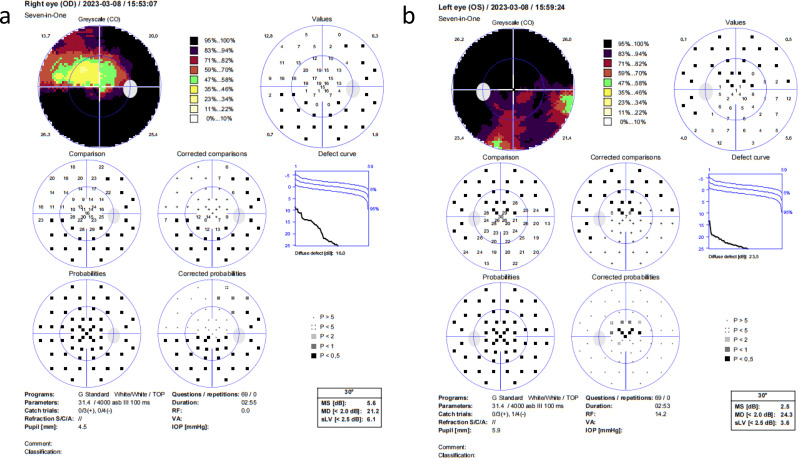
The field of view revealed a decrease in diffuse light sensitivity in the central visual field of the right eye, with a large area of visual field defect temporal and inferiorly connected to the physiological blind spot and central involvement. The left eye had reduced diffuse light sensitivity in the central visual field and a large area of visual field defect connected to the physiological blind spot superiorly (Fig. 4).
Fig. 4.
Visual field examination of the patient on the day of hospitalization. Right eye (a), left eye (b)
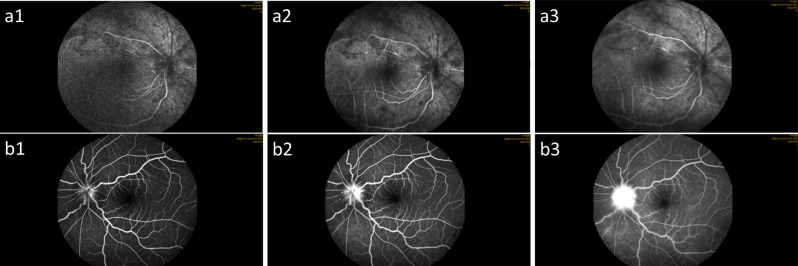
Fluorescein fundus angiography showed that the right eye’s arteriovenous filling time was delayed, some blood vessels had not been filled, flocculent fluorescence in front of the retina was covered, the retina was scattered in patchy high fluorescence, the left eye’s optic disc boundary was blurred, and the surrounding vascular wall gradually showed fluorescent staining and leakage (Fig. 5).
Fig. 5.
Fluorescein fundus angiography of the patient on the day of hospitalization. Right eye (a), left eye (b)
On the second day after admission, his left eye’s BCVA dropped to 20/167. The anterior chamber was shallow, and vitreous opacity was present. Fundus photographs of his left eye showed optic disc edema and yellow-white exudation (Fig. 6).
Fig. 6.
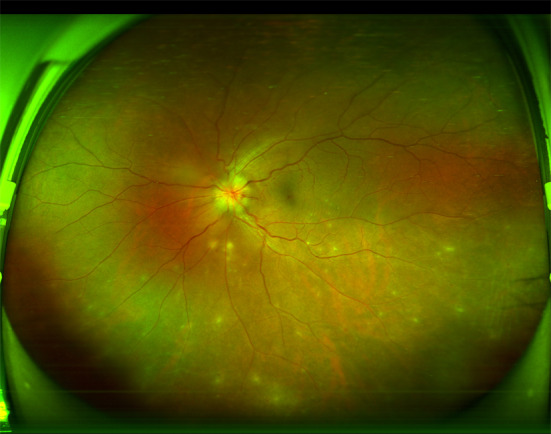
Fundus photography of the patient left eye on the second day after admission
The results of the interferon-gamma release test were negative. An anterior chamber paracentesis collected aqueous humor for viral testing. The PCR detection of VZV in the intraocular fluid of the right eye was positive, and the PCR detection of VZV virus in the intraocular fluid of the left eye was 1.2 × 102copies/mL but less than the reference value of 5 × 102copies/mL.
To further exclude intracranial lesions, the patient underwent brain and orbital MRI and central nervous demyelination series detection. MRI of the brain and orbital did not reveal any positive findings. Laboratory tests of T-spot, rheumatism series antibodies, and central nervous system demyelination series were negative.
The right eye had posterior corneal deposits, inflammatory cells in the anterior chamber, large yellow-white lesions around the retina, retinal vascular occlusion, and VZV positive detected by PCR in intraocular fluid. In 2015, Japanese experts proposed new diagnostic criteria for ARN [8]: (1) Ocular findings: (a) anterior chamber cells or amniotic corneal deposits; (b) single or multiple foci of yellowish-white necrosis in the periphery of the retina; (c) retinal arteritis; (d) congestion of the optic disc; (e) inflammatory clouding of the vitreous body; and (f) elevated intraocular pressure. (2) Characteristic disease progression: (a) rapid progression of retinal necrotic foci; (b) retinal tears or retinal detachment; (c) retinal vascular occlusion; (d) optic nerve atrophy; (e) effectiveness of antiviral therapy; (3) virological testing of intraocular fluid, including analysis of HSV-1, HSV-2, or VZV by PCR or Goldman-Whitmer coefficients; (4) Intraocular fluid virology, including analysis of HSV-1, HSV-2, or VZV by PCR or Goldman-Whitmer coefficient; (5) Disease progression. or Goldman-Whitmer coefficient analysis of HSV-1, HSV-2 or VZV. This patient met the criteria; his right eye was diagnosed as ARN, but his left eye showed a progressive visual decline optic disc, and the VZV virus was detected by intraocular fluid PCR. Considering the disease characteristics, medical history, clinical presentation, and ocular examination, ARN was suspected in his left eye.
Differential diagnosis
The case was differentiated from ischemic optic neuropathy(ION) and progressive outer retinal necrosis (PRN / PORN).
The patient was differentiated between ARN with optic neuropathy as the first manifestation and ischemic optic neuropathy: both had sudden vision loss, optic disc edema, optic neuropathy, and visual field defect. The difference between the two is that the PCR detection of VZV in the fluid of ARN patients is positive, and the brain and orbital MRI and central nervous demyelination series are normal; in ischemic optic neuropathy, MRI and ultrasound can identify temporal artery abnormalities, combined with the symptoms and signs of GCA and blood tests for diagnosis.
Anterior ischemic optic neuropathy (AION) results in decreased visual acuity, mild to moderate optic disc edema, longitudinal distribution of visual field defects, and more severe visual field defects in the lower half [9]. The patient suddenly had decreased strength, optic disc edema, quadrantal visual field defects connected to physiological blind spots, and high-risk factors for diabetes. There was no obvious manifestation of retinal necrosis in the early stage of the disease, which was consistent with the characteristics of ischemic optic neuropathy.
Progressive outer layer retinal necrosis (PRN/PORN) is a rare and disruptive disease whose etiology is primarily related to VZV. This disease is typical of patients with immune eye disease and has an exceedingly fast clinical course, with both eyes frequently involved. It begins with a sudden but painless loss of vision. The inflammatory process occurs in the outer layer of the retina and manifests itself as ill-defined multiple inflammatory foci that spread from the center to the periphery, with early involvement of the macula and the posterior pole of the eye. As the disease progresses, the inner layers also become damaged. Inflammatory infiltration in the anterior chamber and intravitreous is minimal compared to ARN. Retinal vascular damage and intraretinal are not characteristic [10].
Treatments and follow-up
The patients received local anti-inflammatory and antiviral therapy along with systemic antiviral treatment. Ganciclovir 0.5 mg/kg bid was injected. Ganciclovir 3 mg was injected into the vitreous cavity of both eyes twice a week. Intravenous infusion of blood circulation drugs, aspirin 50 mg oral qn.
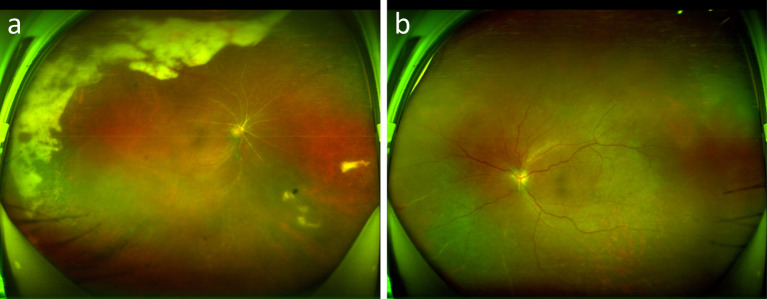
One week after he had been treated, the BCVA in his left eye dropped again, from 20/167 to a finger count. The slit lamp examination indicated mild conjunctival congestion, keratic precipitates, anterior chamber flash (+), and inflammatory response in the anterior chamber and anterior vitreous of the eye. His fundus photographs displayed optic disc pallor, vascular atresia, and peripheral retinal atrophy (Fig. 7).
Fig. 7.
Fundus photography of the patient on one week day after admission.Right eye (a), left eye (b)
The results of the interferon-gamma release test showed that the PCR test value of the VZV virus in the intraocular fluid of the left eye was 1.03 × 102copies/mL. At this time, the left eye was diagnosed with ARN. The patient was diagnosed with binocular ARN and was a VZV-related ARN with optic neuropathy as the first manifestation.
The treatment plan was adjusted to local and systemic antiviral treatment of the eye, and oral glucocorticoid 25 mg qd; ganciclovir 3 mg was injected into the vitreous cavity of both eyes twice a week and reduced to once a week after four weeks. Ganciclovir 0.5 mg/kg intravenous injection for three weeks, then changed to acyclovir 0.4 g orally.
After three weeks of medical therapy, the BCVA increased from 20/200 to 20/70 in his right eye and from FC to 20/200 in his left eye. PCR detection of aqueous humor in patients showed that IL8 decreased from 662.2 to 64pg/ml and VZV decreased from 6.13 × 102 to 2.25 × 102copies/mL.
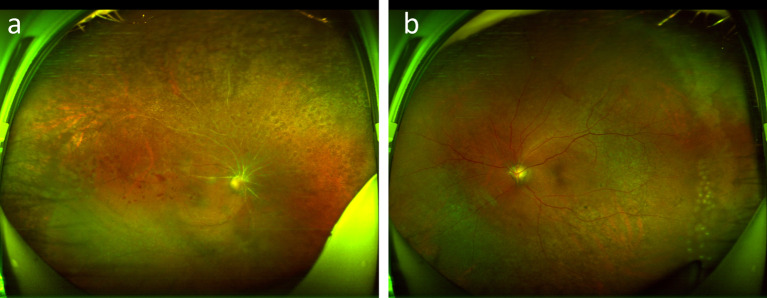
One and a half months after being treated, the BCVA was 20/70 in his right eye and 20/200 in his left eye, and IOP was 10 mmHg and 11 mmHg. There was no obvious conjunctival congestion in the two eyes, the corneas were clear, the anterior chambers were deep, no anterior chamber flare, the lenses were slightly cloudy, and the vitreous humor was cloudy. Fundus photographs revealed unclear borders of the optic discs in both eyes, extensive occlusion of arteries, and yellowish-white peripapillary retinal lesions (Fig. 8). Because the condition was relatively stable, the patient was satisfied and left the hospital.
Fig. 8.
Fundus photography after being treated on one and a half months. Right eye (a), left eye (b)
Three months later, the patient returned to the hospital. The best-corrected visual acuity was 20/70 in the right eye and 20/200 in the left eye, and the intraocular pressures were 6.7 and 11.3 mmHg. There was no significant conjunctival in either eye, the corneas were clear, the anterior chamber was deep without inflammation, and the vitreous was slightly opacity. After the consent, preventive laser retinopexy was given to both eyes to reduce the occurrence of RD. Fundus photography showed a pale optic disc, partial vascular occlusion, and laser spots around the retina (Fig. 9).
Fig. 9.
Fundus photography after restored vision during the follow-up examination. Right eye (a), Left eye (b)
Discussion and conclusions
Characteristic features of ARN include significant vitritis, uveitis, and circumferential progressive necrotizing retinitis. Early diagnosis is crucial for effectively treating ARN. ARN diagnosis is based on clinical symptoms and can be confirmed through viral PCR testing. Several studies indicate that ARN typically begins with periorbital pain, which is often accompanied by elevated intraocular pressure, scleritis, or anterior uveitis. These conditions can lead to vitreous clouding and retinitis. The inflammation of the retina usually develops throughout 4 to 6 weeks. Retinitis manifests as multifocal yellowish-white exudates that primarily originate in the periretinal area. These multiple lesions often merge, covering the peripheral retina and gradually extending toward the posterior pole of the eye, while the macula is generally unaffected. This process is frequently associated with vasculitis and perivascular and arterial thrombosis, which are distinctive features of the disease [7, 11].
However, the ARN patients we reported presented with optic disc lesions as the initial manifestation of the disease. At this stage, PCR testing of the intraocular fluid revealed a negative result for VZV. As the disease progressed, we observed vascular atresia, peripheral retinal atrophy, and inflammation in the anterior chamber and anterior vitreous. During this stage, PCR testing of the intraocular fluid revealed a positive result for VZV, which differs from the incidence and progression of ARN described in the literature. The reasons we considered include the possibility that the virus was latent or confined to the posterior segment of the eye. This could explain why the virus had not yet been fully released into the anterior chamber. As a result, PCR testing of the early anterior chamber puncture fluid showed a low level of VZV virus. Botsford BW et al. reported that acute retinal necrosis caused by different virus types occurred at varying times from the onset of symptoms to PCR detection of virus positivity [12]. The onset of the first symptoms was eight days in the HSV-2 group and fourteen days in the VZV group. Our analysis of this case’s etiology aligns with published literature.
ARN can be treated with topical and systemic corticosteroids to alleviate the ocular inflammatory response. How to choose the timing of glucocorticoids in the treatment of ARN ? This question is worth considering. Alexa Li et al. reported that PCR detection could be used to diagnose suspected ARN. However, patients should receive aggressive antiviral therapy while awaiting a viral diagnosis, as the risk of disease progression increases without treatment [13]. In an ARN study, it was reported that patients with ARN were treated with corticosteroids without antiviral therapy, leading to severe retinopathy and poor visual acuity [14]. Weisman et al. described a case of suspected optic neuritis that was treated with intravenous methylprednisolone. The patient subsequently developed bilateral central retinal artery occlusion, acute retinal necrosis, and meningitis [15]. The report confirmed that glucocorticoid therapy without antiviral treatment may worsen retinal diseases.
Studies have shown that the acute phase of ARN should be treated with medication. Once ARN is diagnosed, intravenous acyclovir should be administered immediately. The total treatment duration is six weeks to reduce the incidence of contralateral eyes [16]. Regarding using corticosteroids during ARN treatment, some retinal experts recommend adding corticosteroids 24 to 48 h after starting antiviral medications [7]. Some uveitis experts recommend topical corticosteroids for cystoid macular edema after resolving active retinitis, considering the potential for recurrence [13]. While corticosteroids can help improve inflammatory markers related to ARN, particularly through local injections in the eye, they should be used cautiously. This is because they may increase the risk of disease progression and vision loss. We reported that the case was givenlocal and systemic antiviral therapies in the early stages of treating of ARN. When the virus was detected through PCR testing, hormone therapy was added to mitigate the severe inflammatory response associated with ARN, aligning with reports from the literature.
In summary, this case serves as a reminder for clinicians to consider the factors associated with ARN when assessing patients who present with atypical optic neuropathy. Accurate diagnosis and timely antiviral therapy are crucial for treating ARN. We reported that this patient with ARN received a combination of local and systemic antiviral therapy at the onset of the disease. When the virus was detected through PCR testing, oral hormone therapy was added to help reduce the severe inflammatory response associated with ARN. This approach provides a valuable treatment strategy for clinicians managing ARN. In addition, there is still no consensus on the treatment of ARN, including the selection of antiviral drugs, the timing and dose of hormone therapy. Further long-term clinical studies are needed to optimize the management of ARN treatment.
Acknowledgements
Not applicable.
Abbreviations
- ARN
Acute retinal necrosis
- VZV
Varicella-zoster virus
- HSV
Herpes simplex viruses
- CMV
Cytomegalovirus
- EBV
Epstein–Barr virus
- BCVA
Best corrected visual acuity
- PCR
Polymerase chain reaction
- PRN
Ischemic optic neuropathy
- PORN
Progressive outer retinal necrosis
Author contributions
Ll L wrote the manuscript, analyzed the data, and reviewed the literature. QX W, QM T and GY H collected ophthalmologic data and assisted in drafting the manuscript. XF X revised the manuscript and discussions. All authors read and approved the final manuscript.
Funding
National Natural Science Foundation of China (82104937). Shandong Province medical health science and technology project (202407021372).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Culbertson WW, Blumenkranz MS, Haines H, Gass DM, Mitchell KB, Norton EW. The acute retinal necrosis syndrome. Part 2: histopathology and etiology. Ophthalmology. 1982;89(12):1317–25. 10.1016/s0161-6420(82)34638-2. [DOI] [PubMed] [Google Scholar]
- 2.Walters G, James TE. Viral causes of the acute retinal necrosis syndrome. Curr Opin Ophthalmol. 2001;12(3):191–5. 10.1097/00055735-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Schaal S, Kagan A, Wang Y, Chan CC, Kaplan HJ. Acute retinal necrosis associated with Epstein-Barr virus: immunohistopathologic confirmation. JAMA Ophthalmol. 2014;132(7):881–2. 10.1001/jamaophthalmol.2014.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvo CM, Khan MA, Mehta S, Garg SJ, Dunn JP. Correlation of clinical outcomes with quantitative polymerase chain reaction DNA copy number in patients with acute retinal necrosis. Ocul Immunol Inflamm. 2017;25(2):246–52. 10.3109/09273948.2015.1115081. [DOI] [PubMed] [Google Scholar]
- 5.Cochrane TF, Silvestri G, McDowell C, Foot B, McAvoy CE. Acute retinalnecrosis in the United Kingdom: results of a prospective surveillance study. Eye. 2012;26(3):370–7. 10.1038/eye.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muthiah MN, Michaelides M, Child CS, Mitchell SM. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol. 2007;91:1452–5. 10.1136/bjo.2007.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shantha JG, Weissman HM, Debiec MR, Albini TA, Yeh S. Advances in the management of acute retinal necrosis. Int Ophthalmol Clin. 2015;55(3):1–13. 10.1097/IIO.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takase H, Okada AA, Goto H, Mizuki N, Namba K, Ohguro N, et al. Development and validation of new diagnostic criteria for acute retinal necrosis. Jpn J Ophthalmol. 2015;59(1):14–20. 10.1007/s10384-014-0362-0. [DOI] [PubMed] [Google Scholar]
- 9.By Mark J, Morrow MD. FAAN Ischemic Optic Neuropathy Continuum (Minneap Minn). 2019;25(5):1215–35. 10.1212/CON.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 10.Kanski J, Bowling B. Kanski’s Clinical Ophthalmology.ISBN: 978-0-7020-5572-0; 8th ed. 2016; 11: 440–442.
- 11.Whitcup SM. Acute retinal necrosis. In: Nussenblatt RB, Whitcup SM, editors. Uveitis fundamentals and clinical practice. 4th ed. Elsevier Inc; 2010. pp. 176–82.
- 12.Botsford BW, Nguyen VQ, Eller AW. Acute retinal necrosis: difference in outcome by viral type and options for antiviral therapy. Retina. 2021;41(7):1547–52. 10.1097/IAE.0000000000003058. [DOI] [PubMed] [Google Scholar]
- 13.Li AL, Fine HF, Shantha JG, Yeh S. Update on the management of Acute Retinal Necrosis. Ophthalmic Surg Lasers Imaging Retina. 2019;50(12):748–51. 10.3928/23258160-20191119-01. [DOI] [PubMed] [Google Scholar]
- 14.Hojjatie SL, Shantha JG, O’Keefe GD, Kraft CS, Voloschin A, Grossniklaus H, et al. Cytopathology of vitreous specimens in Acute Retinal Necrosis. Ocul Immunol Inflamm. 2022;30(7–8):1609–16. 10.1080/09273948.2021.1922926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weissman HM, Biousse V, Schechter MC, Del Rio C, Yeh S. Bilateral central retinal artery occlusion associated with herpes simplex virus-associated acute retinal necrosis and meningitis: case report and literature review. Ophthalmic Surg Lasers Imaging Retina. 2015;46(2):279–83. 10.3928/23258160-20150213-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Usui Y, Goto H. Overview and diagnosis of acute retinal necrosis syndrome [J]. Semin Ophthalmol. 2008;23(4):275–83. 10.1080/08820530802111325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.