Abstract
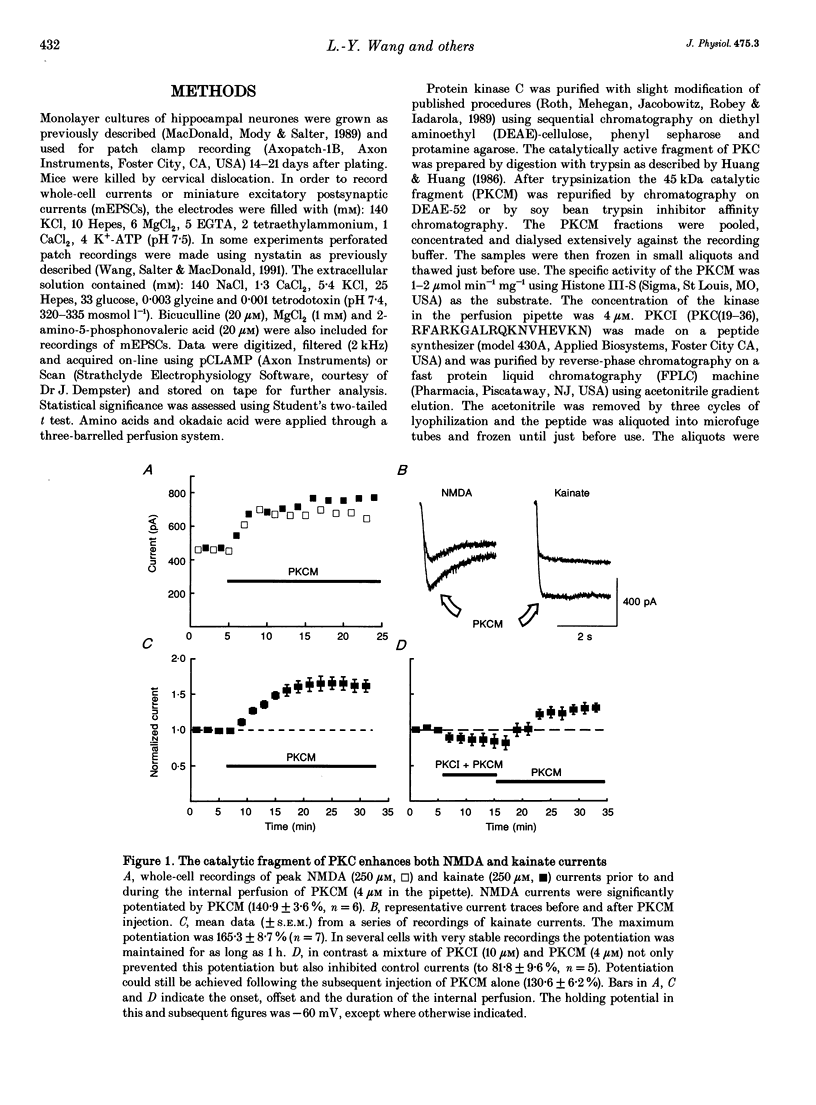
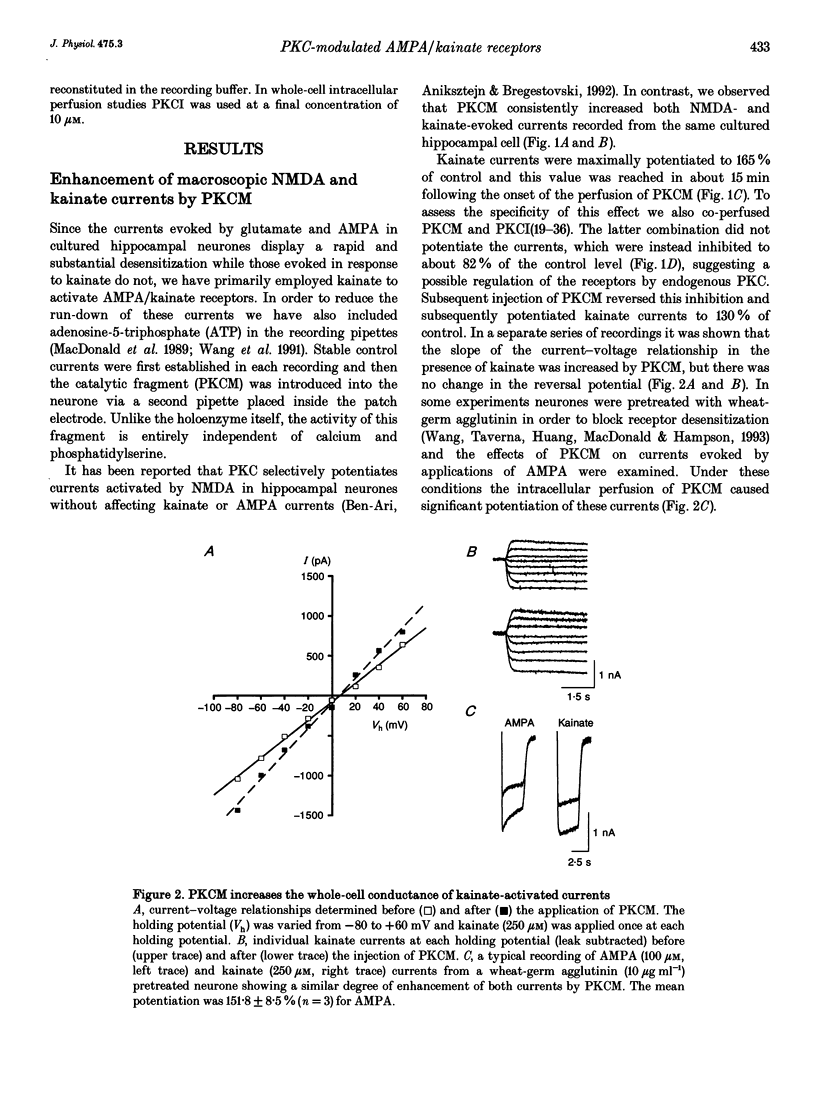
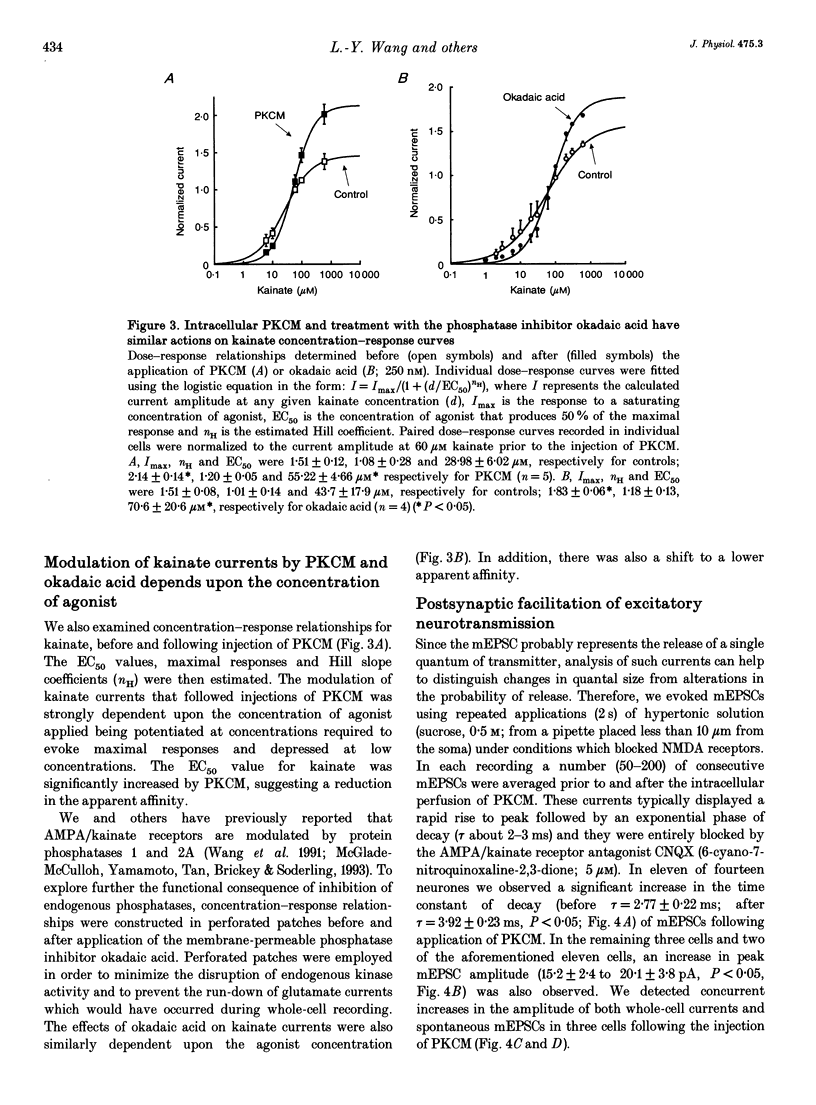
1. The patch clamp technique, together with intracellular perfusion of the catalytic fragment of protein kinase C (PKCM), was employed to investigate the role of this enzyme in the intracellular regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate receptors in cultured hippocampal neurones. 2. The responses evoked by near-maximal concentrations of kainate (250 microM) and AMPA (100 microM) were potentiated by the introduction of PKCM, whilst co-application of the inhibitory peptide fragment PKCI(19-36) prevented this action. 3. Modulation of kainate responses by PKCM was dependent upon the concentration of agonist applied. Currents evoked by kainate were potentiated at concentrations above those which caused 50% of the maximal response (EC50) and depressed at lower concentrations. Furthermore, okadaic acid, a specific inhibitor of phosphatases 1 and 2A, had a similar effect upon concentration-response relationships when currents activated by kainate were recorded using the perforated patch technique. 4. In addition, the mean amplitude and/or time constant of decay of miniature excitatory synaptic currents (mediated by AMPA/kainate receptors) was increased by the intracellular injection of PKCM. 5. These observations suggest that the function of postsynaptic excitatory amino acid receptors can be modulated by the activity of PKC as well as by endogenous phosphatases. This regulation may contribute to some forms of synaptic plasticity within the central nervous system.
Full text
PDF
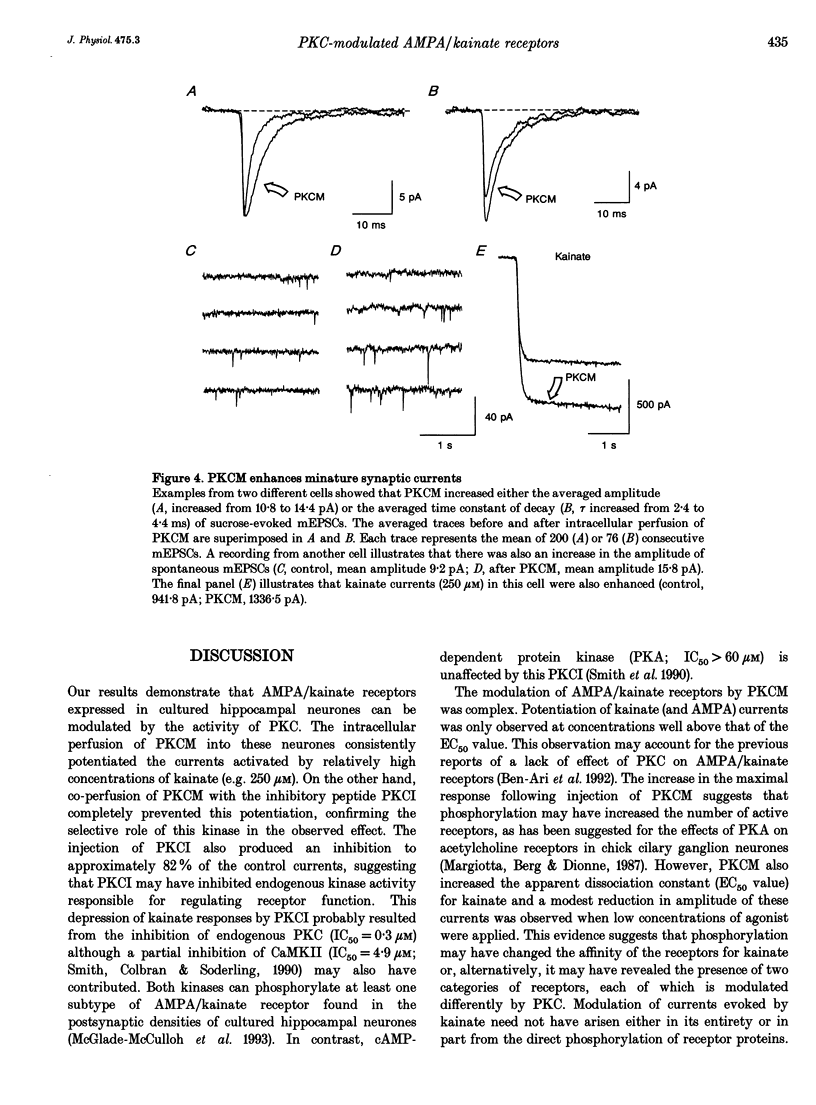


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ari Y., Aniksztejn L., Bregestovski P. Protein kinase C modulation of NMDA currents: an important link for LTP induction. Trends Neurosci. 1992 Sep;15(9):333–339. doi: 10.1016/0166-2236(92)90049-e. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Browning M. D., Dudek E. M. Activators of protein kinase C increase the phosphorylation of the synapsins at sites phosphorylated by cAMP-dependent and Ca2+/calmodulin-dependent protein kinase in the rat hippocampal slice. Synapse. 1992 Jan;10(1):62–70. doi: 10.1002/syn.890100109. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Lester R. A., Tong G., Jahr C. E., Westbrook G. L. The time course of glutamate in the synaptic cleft. Science. 1992 Nov 27;258(5087):1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Davies S. N., Lester R. A., Reymann K. G., Collingridge G. L. Temporally distinct pre- and post-synaptic mechanisms maintain long-term potentiation. Nature. 1989 Apr 6;338(6215):500–503. doi: 10.1038/338500a0. [DOI] [PubMed] [Google Scholar]
- Eriksson J. E., Brautigan D. L., Vallee R., Olmsted J., Fujiki H., Goldman R. D. Cytoskeletal integrity in interphase cells requires protein phosphatase activity. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11093–11097. doi: 10.1073/pnas.89.22.11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E., Chen S. J., Sweatt J. D. Mechanism of protein kinase C activation during the induction and maintenance of long-term potentiation probed using a selective peptide substrate. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8337–8341. doi: 10.1073/pnas.90.18.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Mody I., Salter M. W. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol. 1989 Jul;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margiotta J. F., Berg D. K., Dionne V. E. Cyclic AMP regulates the proportion of functional acetylcholine receptors on chicken ciliary ganglion neurons. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8155–8159. doi: 10.1073/pnas.84.22.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlade-McCulloh E., Yamamoto H., Tan S. E., Brickey D. A., Soderling T. R. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature. 1993 Apr 15;362(6421):640–642. doi: 10.1038/362640a0. [DOI] [PubMed] [Google Scholar]
- Prat A. G., Bertorello A. M., Ausiello D. A., Cantiello H. F. Activation of epithelial Na+ channels by protein kinase A requires actin filaments. Am J Physiol. 1993 Jul;265(1 Pt 1):C224–C233. doi: 10.1152/ajpcell.1993.265.1.C224. [DOI] [PubMed] [Google Scholar]
- Roth B. L., Mehegan J. P., Jacobowitz D. M., Robey F., Iadarola M. J. Rat brain protein kinase C: purification, antibody production, and quantification in discrete regions of hippocampus. J Neurochem. 1989 Jan;52(1):215–221. doi: 10.1111/j.1471-4159.1989.tb10919.x. [DOI] [PubMed] [Google Scholar]
- Sacktor T. C., Osten P., Valsamis H., Jiang X., Naik M. U., Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. K., Colbran R. J., Soderling T. R. Specificities of autoinhibitory domain peptides for four protein kinases. Implications for intact cell studies of protein kinase function. J Biol Chem. 1990 Feb 5;265(4):1837–1840. [PubMed] [Google Scholar]
- Swope S. L., Moss S. J., Blackstone C. D., Huganir R. L. Phosphorylation of ligand-gated ion channels: a possible mode of synaptic plasticity. FASEB J. 1992 May;6(8):2514–2523. [PubMed] [Google Scholar]
- Vyklicky L., Jr, Patneau D. K., Mayer M. L. Modulation of excitatory synaptic transmission by drugs that reduce desensitization at AMPA/kainate receptors. Neuron. 1991 Dec;7(6):971–984. doi: 10.1016/0896-6273(91)90342-w. [DOI] [PubMed] [Google Scholar]
- Wang L. Y., Salter M. W., MacDonald J. F. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science. 1991 Sep 6;253(5024):1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]
- Wang L. Y., Taverna F. A., Huang X. P., MacDonald J. F., Hampson D. R. Phosphorylation and modulation of a kainate receptor (GluR6) by cAMP-dependent protein kinase. Science. 1993 Feb 19;259(5098):1173–1175. doi: 10.1126/science.8382377. [DOI] [PubMed] [Google Scholar]


