Abstract
1. Properties of dorsal horn interneurones that process information from group II muscle afferents in the sacral segments of the spinal cord have been investigated in the cat using both intracellular and extracellular recording. 2. The interneurones were excited by group II muscle afferents and cutaneous afferents but not by group I muscle afferents. They were most effectively excited by group II afferents of the posterior biceps, semitendinosus, triceps surae and quadriceps muscle nerves and by cutaneous afferents running in the cutaneous femoris, pudendal and sural nerves. The earliest synaptic actions were evoked monosynaptically and were very tightly locked to the stimuli. 3. EPSPs evoked monosynaptically by group II muscle afferents and cutaneous afferents of the most effective nerves were often cut short by disynaptic IPSPs. As a consequence of this negative feedback the EPSPs gave rise to single or double spike potentials and only a minority of interneurones responded with repetitive discharges. However, the neurones that did respond repetitively did so at a very high frequency of discharges (0.8-1.2 ms intervals between the first 2-3 spikes). 4. Sacral dorsal horn group II interneurones do not appear to act directly upon motoneurones because: (i) these interneurones are located outside the area within which last order interneurones have previously been found and (ii) the latencies of PSPs evoked in motoneurones by stimulation of the posterior biceps and semitendinosus, cutaneous femoris and pudendal nerves (i.e. the main nerves providing input to sacral interneurones) are compatible with a tri- but not with a disynaptic coupling. Spatial facilitation of EPSPs and IPSPs following synchronous stimulation of group II and cutaneous afferents of these nerves shows, however, that sacral interneurones may induce excitation or inhibition of motoneurones via other interneurones. 5. Comparison of the properties of group II interneurones in the sacral segments with those of previously studied group II interneurones in the midlumbar segments leads to the conclusion that these two populations of neurones are specialized for the processing of information from different muscles and skin areas. In addition, equivalents of only one of the two subpopulations of midlumbar interneurones have been found at the level of the pudendal nucleus: neurones with input from group II but not from group I muscle afferents. Neurones integrating information from group I and II muscle afferents and in direct contact with motoneurones thus seem to be scarce in the sacral segments.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

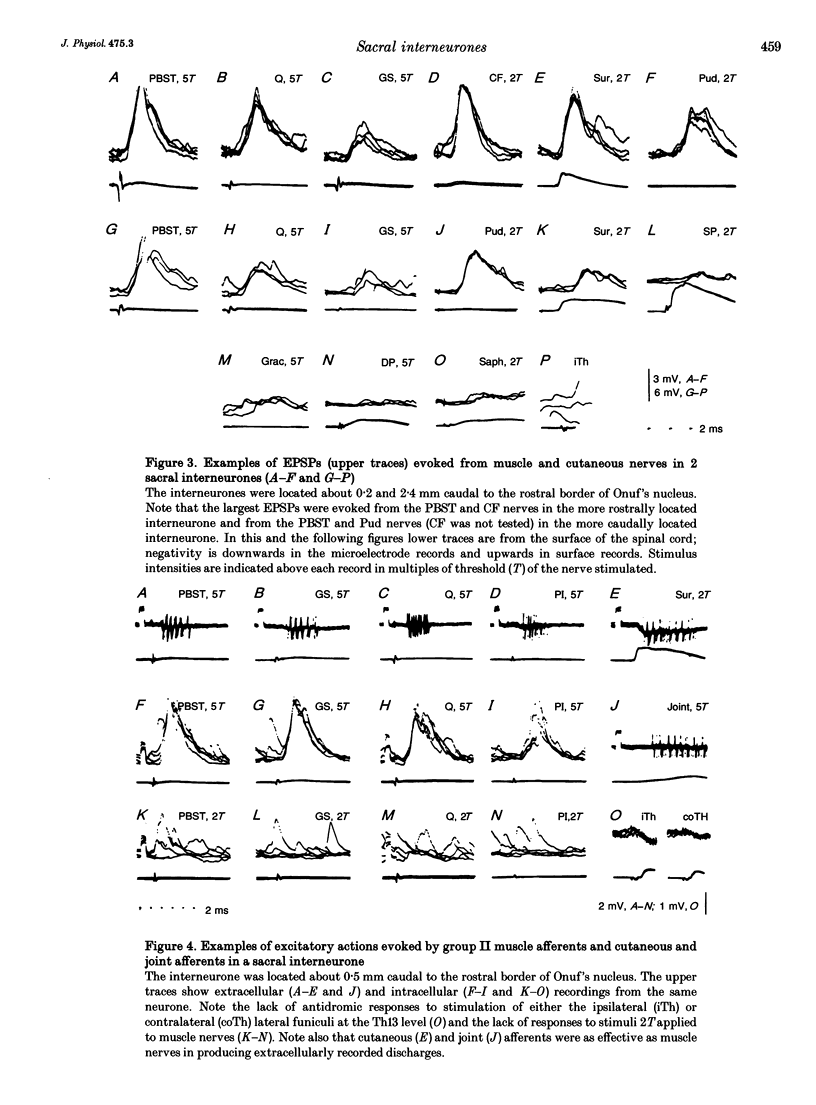
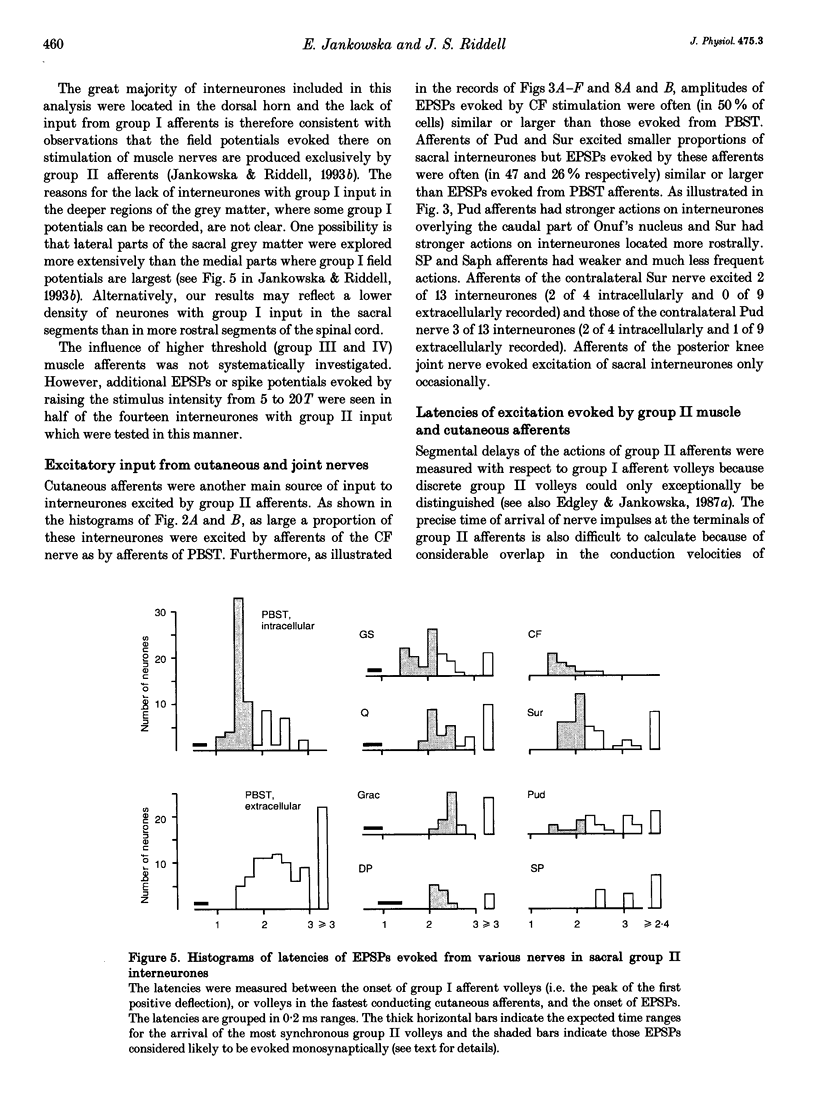

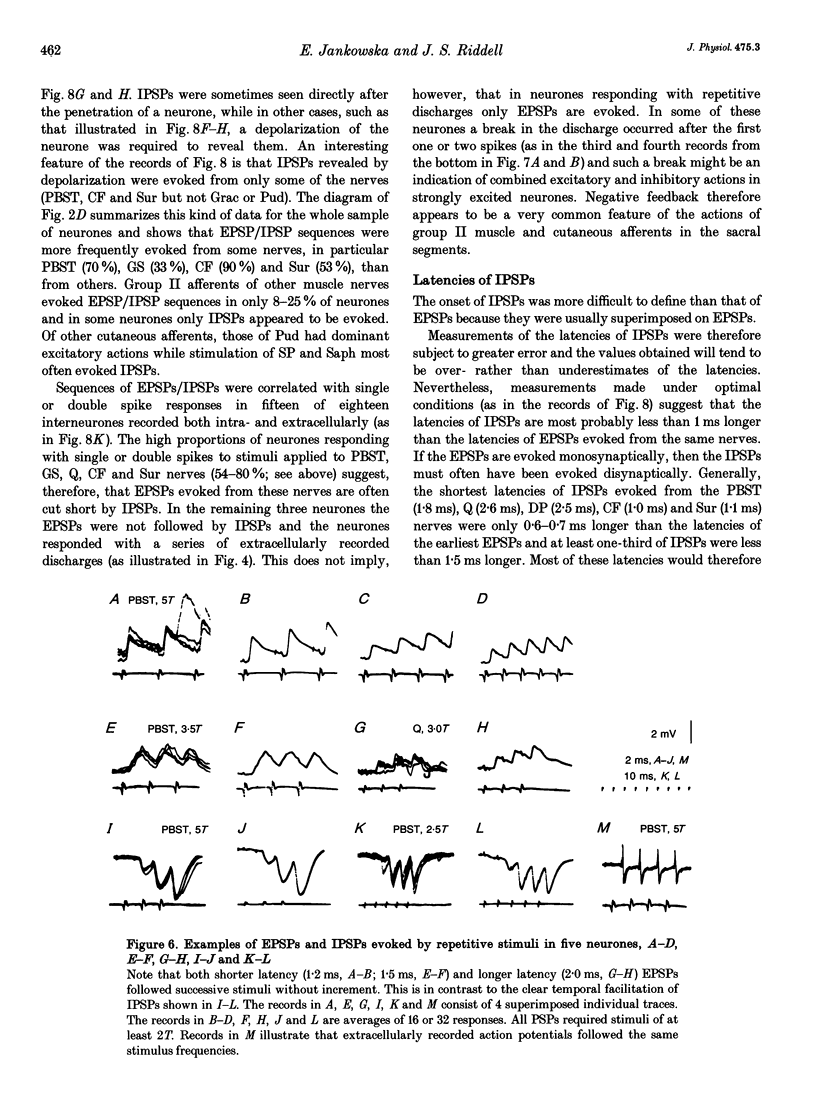
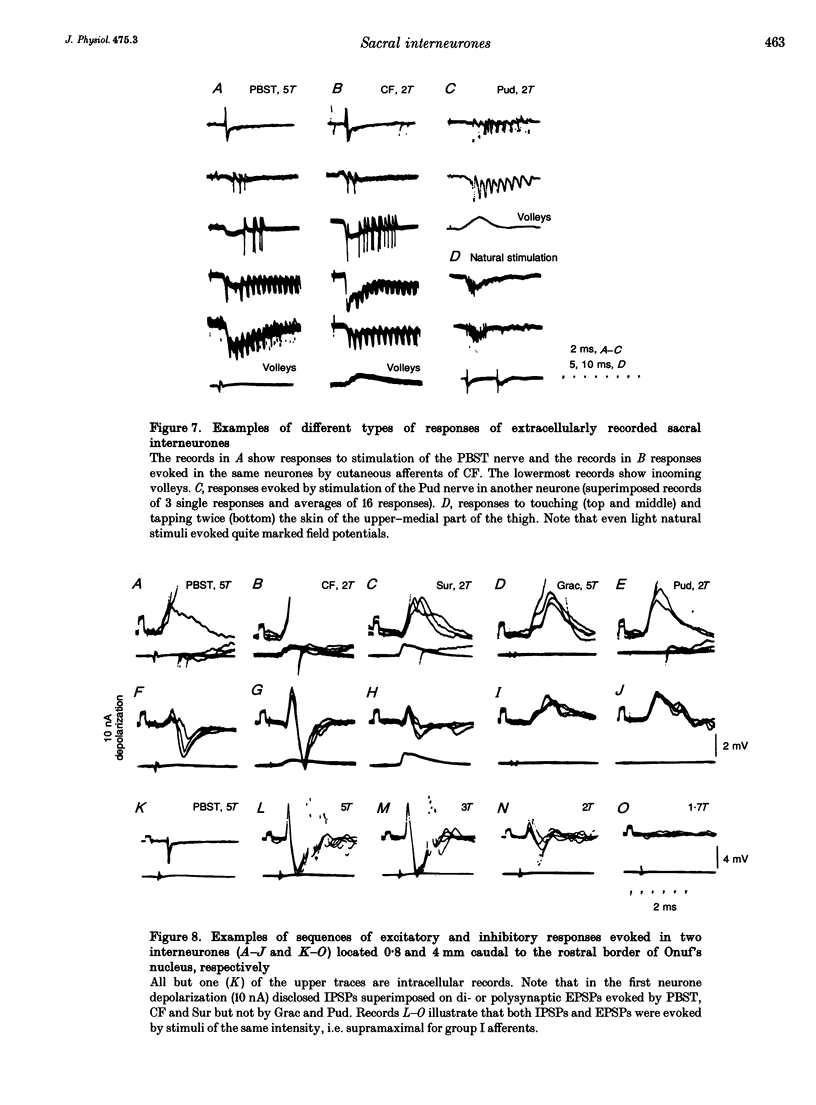




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bras H., Cavallari P., Jankowska E., Kubin L. Morphology of midlumbar interneurones relaying information from group II muscle afferents in the cat spinal cord. J Comp Neurol. 1989 Dec 1;290(1):1–15. doi: 10.1002/cne.902900102. [DOI] [PubMed] [Google Scholar]
- Cavallari P., Edgley S. A., Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol. 1987 Aug;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari P., Edgley S. A., Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol. 1987 Aug;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V., Quintern J., Sillem M. Stumbling reactions in man: significance of proprioceptive and pre-programmed mechanisms. J Physiol. 1987 May;386:149–163. doi: 10.1113/jphysiol.1987.sp016527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987 Apr;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Information processed by dorsal horn spinocerebellar tract neurones in the cat. J Physiol. 1988 Mar;397:81–97. doi: 10.1113/jphysiol.1988.sp016989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E., Shefchyk S. Evidence that mid-lumbar neurones in reflex pathways from group II afferents are involved in locomotion in the cat. J Physiol. 1988 Sep;403:57–71. doi: 10.1113/jphysiol.1988.sp017238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B., Hochman S., Shefchyk S. J. An intracellular study of perineal and hindlimb afferent inputs onto sphincter motoneurons in the decerebrate cat. Exp Brain Res. 1992;89(3):511–516. doi: 10.1007/BF00229875. [DOI] [PubMed] [Google Scholar]
- Fern R., Harrison P. J., Riddell J. S. The dorsal column projection of muscle afferent fibres from the cat hindlimb. J Physiol. 1988 Jul;401:97–113. doi: 10.1113/jphysiol.1988.sp017153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Santini M., Schomburg E. D. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiol Scand. 1974 Jul;91(3):298–313. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Fu T. C., Schomburg E. D. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiol Scand. 1974 Jul;91(3):314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Grottel K., Huber J., Kowalski K. Functional properties of crossed spinocerebellar tract neurones with cell bodies in the S1 segment. Neurosci Res. 1991 Sep;11(4):286–291. doi: 10.1016/0168-0102(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985 Apr;361:379–401. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Riddell J. S. Group II-activated lumbosacral interneurones with an ascending projection to midlumbar segments of the cat spinal cord. J Physiol. 1989 Jan;408:561–570. doi: 10.1113/jphysiol.1989.sp017476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles J. F., Pisini J. V. Vestibular-evoked postural reactions in man and modulation of transmission in spinal reflex pathways. J Physiol. 1992 Sep;455:407–424. doi: 10.1113/jphysiol.1992.sp019308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38(4):335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Riddell J. S. A relay for input from group II muscle afferents in sacral segments of the cat spinal cord. J Physiol. 1993 Jun;465:561–580. doi: 10.1113/jphysiol.1993.sp019693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBella L. A., Kehler J. P., McCrea D. A. A differential synaptic input to the motor nuclei of triceps surae from the caudal and lateral cutaneous sural nerves. J Neurophysiol. 1989 Feb;61(2):291–301. doi: 10.1152/jn.1989.61.2.291. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Exp Brain Res. 1987;65(2):271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Reflex pathways from group II muscle afferents. 2. Functional characteristics of reflex pathways to alpha-motoneurones. Exp Brain Res. 1987;65(2):282–293. doi: 10.1007/BF00236300. [DOI] [PubMed] [Google Scholar]
- Macpherson J. M. Strategies that simplify the control of quadrupedal stance. II. Electromyographic activity. J Neurophysiol. 1988 Jul;60(1):218–231. doi: 10.1152/jn.1988.60.1.218. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Fleshman J. W., Sypert G. W. Properties of single-fiber spindle group II EPSPs in triceps surae motoneurons. J Neurophysiol. 1980 Oct;44(4):713–725. doi: 10.1152/jn.1980.44.4.713. [DOI] [PubMed] [Google Scholar]
- Nashner L. M. Adapting reflexes controlling the human posture. Exp Brain Res. 1976 Aug 27;26(1):59–72. doi: 10.1007/BF00235249. [DOI] [PubMed] [Google Scholar]
- Pinter M. J., Burke R. E., O'Donovan M. J., Dum R. P. Supraspinal facilitation of cutaneous polysynaptic EPSPs in cat medical gastrocnemius motoneurons. Exp Brain Res. 1982;45(1-2):133–143. doi: 10.1007/BF00235772. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Riddell J. S., Jankowska E., Hammar I., Szabo-Läckberg Z. Ascending tract neurones processing information from group II muscle afferents in sacral segments of the feline spinal cord. J Physiol. 1994 Mar 15;475(3):469–481. doi: 10.1113/jphysiol.1994.sp020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypert G. W., Munson J. B., Fleshman J. W. Effect of presynaptic inhibition on axonal potentials, terminal potentials, focal synaptic potentials, and EPSPs in cat spinal cord. J Neurophysiol. 1980 Oct;44(4):792–803. doi: 10.1152/jn.1980.44.4.792. [DOI] [PubMed] [Google Scholar]
- Yates B. J., Kasper J., Wilson V. J. Effects of muscle and cutaneous hindlimb afferents on L4 neurons whose activity is modulated by neck rotation. Exp Brain Res. 1989;77(1):48–56. doi: 10.1007/BF00250566. [DOI] [PubMed] [Google Scholar]