Abstract
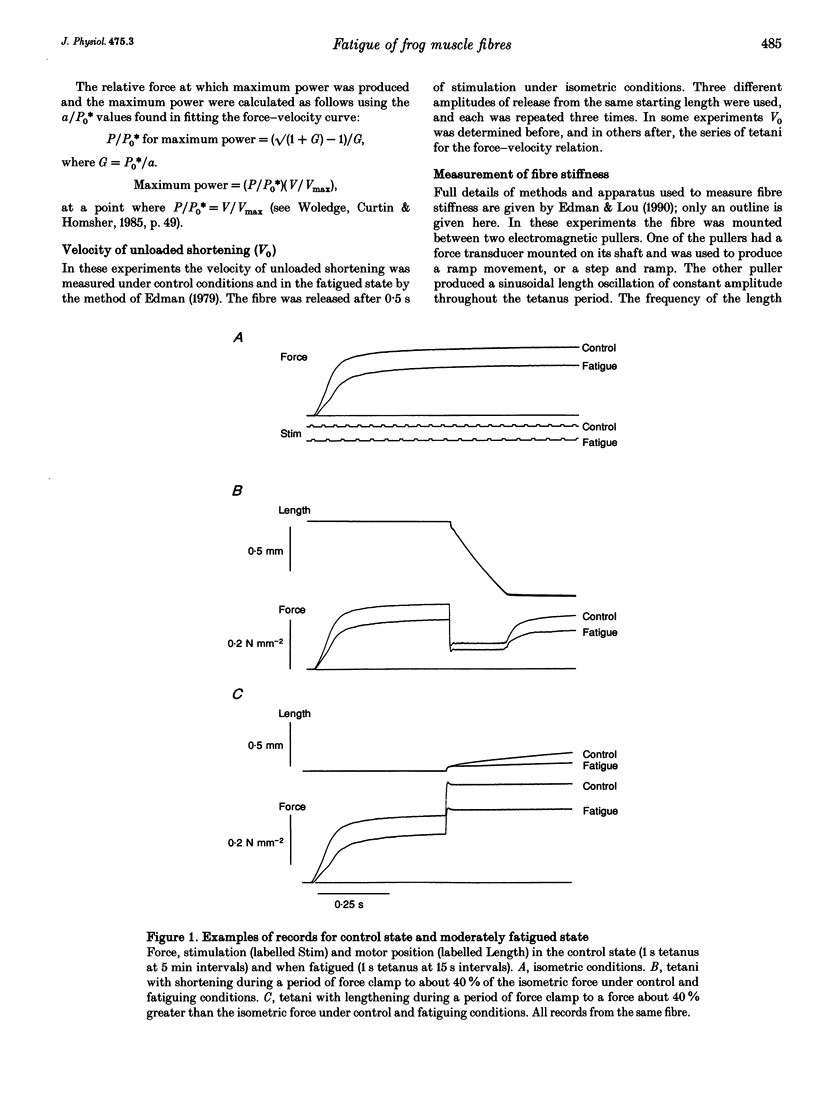
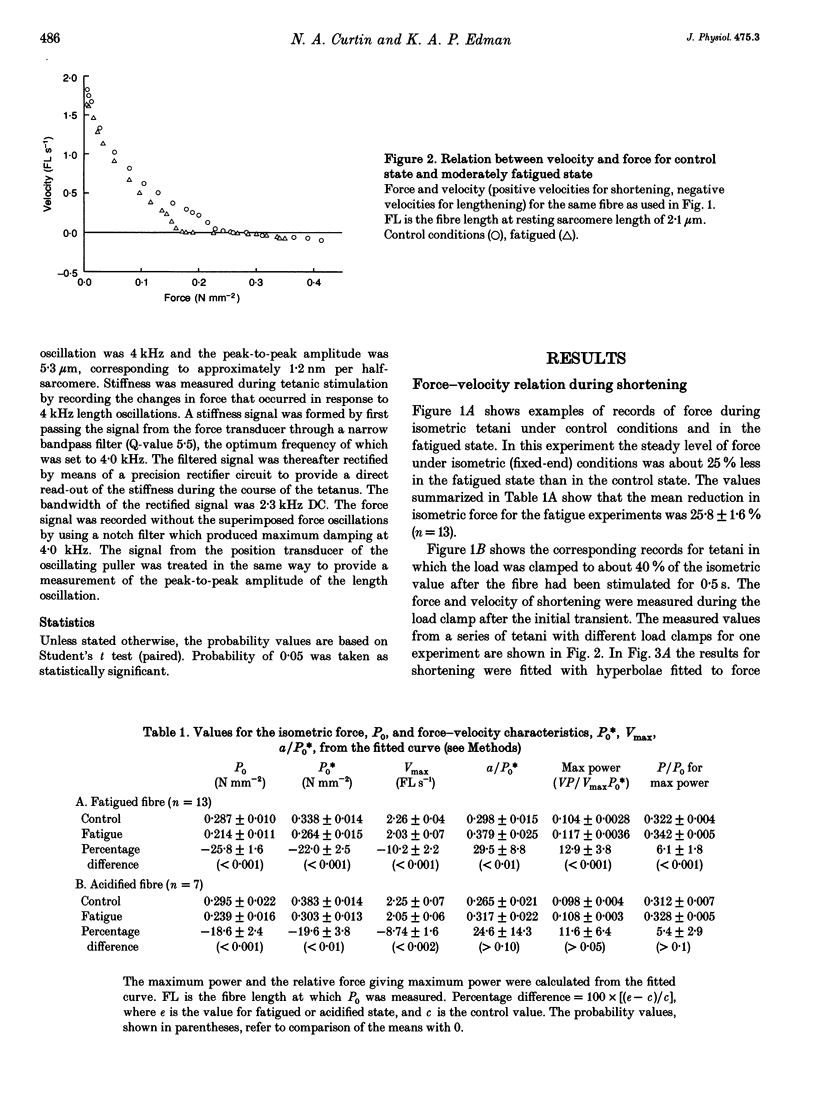
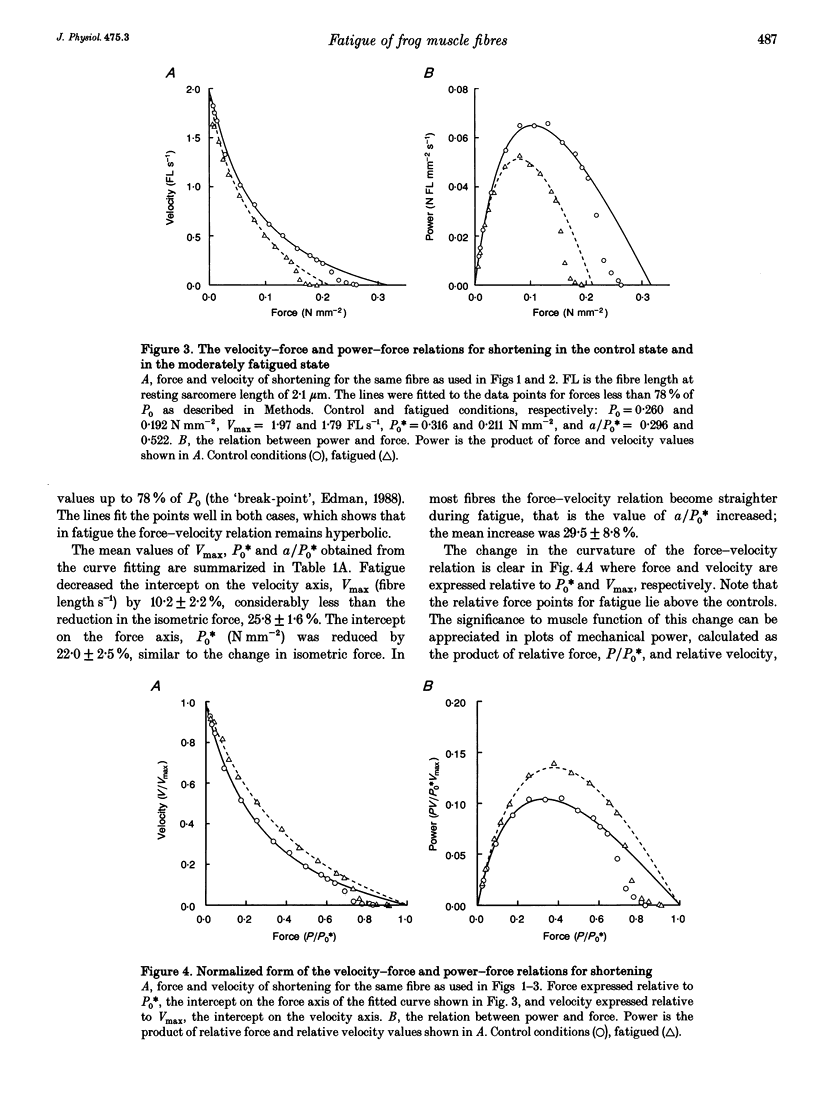
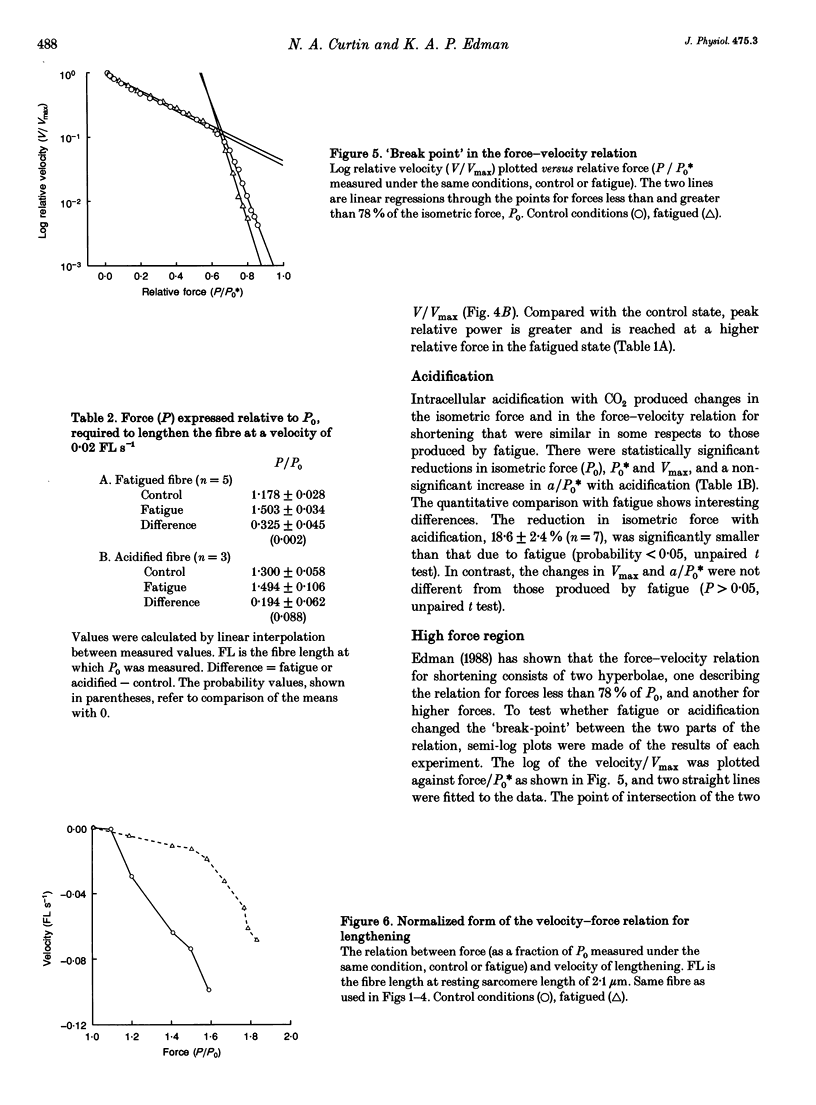
1. Intact frog single fibres were investigated under control conditions (1 s tetanus every 2, 3 or 5 min) and during moderate fatigue (interval between tetani 15 or 30 s). 2. Fatigue reduced isometric force (P0) by 25.8 +/- 1.6% (S.E.M.; n = 13) and depressed the maximum velocity of shortening (Vmax) by 10.2 +/- 2.2% (n = 13). The force-velocity relation became less curved, a/P0* (see Methods) being increased by 29.5 +/- 8.8% (n = 13). Thus, power was less affected than isometric force or Vmax. 3. The velocity of unloaded shortening (V0), from slack test measurements, was reduced proportionally more than Vmax during fatigue. Under control conditions V0 was larger than Vmax, but during fatigue their values were not significantly different. 4. Stiffness during shortening was reduced during fatigue indicating fewer attached cross-bridges in fatigue. Force was reduced more than stiffness indicating that, on average, there is less force per attached cross-bridge. 5. The force-lengthening velocity relation showed that the ability to resist forces greater than isometric was well preserved in fatigue. 6. Compared with fatigue, intracellular acidification with CO2 produced a smaller reduction in isometric force. However, reduction in Vmax was not significantly different from that in fatigue. These results are consistent with both inorganic phosphate and H+ increasing in fatigue, but only H+ increasing during acidification, and isometric force being reduced by both, Vmax being sensitive only to H+.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Lee J. A., Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol. 1989 Aug;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altringham J. D., Johnston I. A. Effects of phosphate on the contractile properties of fast and slow muscle fibres from an Antarctic fish. J Physiol. 1985 Nov;368:491–500. doi: 10.1113/jphysiol.1985.sp015871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P. B., Kushmerick M. J. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988 Jun;53(6):935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K., Luciani G. B., Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988 Jan;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Edman K. A. Effects of fatigue and reduced intracellular pH on segment dynamics in 'isometric' relaxation of frog muscle fibres. J Physiol. 1989 Jun;413:159–174. doi: 10.1113/jphysiol.1989.sp017647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A. Force during stretch and shortening of frog sartorius muscle: effects of intracellular acidification due to increased carbon dioxide. J Muscle Res Cell Motil. 1990 Jun;11(3):251–257. doi: 10.1007/BF01843578. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Kometani K., Woledge R. C. Effect of intracellular pH on force and heat production in isometric contraction of frog muscle fibres. J Physiol. 1988 Feb;396:93–104. doi: 10.1113/jphysiol.1988.sp016952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzig J. A., Goldman Y. E., Millar N. C., Lacktis J., Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. Double-hyperbolic force-velocity relation in frog muscle fibres. J Physiol. 1988 Oct;404:301–321. doi: 10.1113/jphysiol.1988.sp017291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Lou F. Changes in force and stiffness induced by fatigue and intracellular acidification in frog muscle fibres. J Physiol. 1990 May;424:133–149. doi: 10.1113/jphysiol.1990.sp018059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Lou F. Myofibrillar fatigue versus failure of activation during repetitive stimulation of frog muscle fibres. J Physiol. 1992 Nov;457:655–673. doi: 10.1113/jphysiol.1992.sp019400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mattiazzi A. R. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motil. 1981 Sep;2(3):321–334. doi: 10.1007/BF00713270. [DOI] [PubMed] [Google Scholar]
- Edman K. A. Mechanical deactivation induced by active shortening in isolated muscle fibres of the frog. J Physiol. 1975 Mar;246(1):255–275. doi: 10.1113/jphysiol.1975.sp010889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C. Redistribution of sarcomere length during isometric contraction of frog muscle fibres and its relation to tension creep. J Physiol. 1984 Jun;351:169–198. doi: 10.1113/jphysiol.1984.sp015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension transients during steady shortening of frog muscle fibres. J Physiol. 1985 Apr;361:131–150. doi: 10.1113/jphysiol.1985.sp015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Nosek T. M. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989 May;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Jones D. A., Newham D. J., Round J. M., Tolfree S. E. Experimental human muscle damage: morphological changes in relation to other indices of damage. J Physiol. 1986 Jun;375:435–448. doi: 10.1113/jphysiol.1986.sp016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson R. K., Edman K. A. The consequences of fibre heterogeneity on the force-velocity relation of skeletal muscle. Acta Physiol Scand. 1988 Mar;132(3):341–352. doi: 10.1111/j.1748-1716.1988.tb08338.x. [DOI] [PubMed] [Google Scholar]
- Kawano Y., Tanokura M., Yamada K. Phosphorus nuclear magnetic resonance studies on the effect of duration of contraction in bull-frog skeletal muscles. J Physiol. 1988 Dec;407:243–261. doi: 10.1113/jphysiol.1988.sp017413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. A., Westerblad H., Allen D. G. Changes in tetanic and resting [Ca2+]i during fatigue and recovery of single muscle fibres from Xenopus laevis. J Physiol. 1991 Feb;433:307–326. doi: 10.1113/jphysiol.1991.sp018427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi V., Piazzesi G. The contractile response during steady lengthening of stimulated frog muscle fibres. J Physiol. 1990 Dec;431:141–171. doi: 10.1113/jphysiol.1990.sp018324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. Maximum tension and force-velocity properties of fatigued, single Xenopus muscle fibres studied by caffeine and high K+. J Physiol. 1989 Feb;409:473–490. doi: 10.1113/jphysiol.1989.sp017508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newham D. J., Jones D. A., Clarkson P. M. Repeated high-force eccentric exercise: effects on muscle pain and damage. J Appl Physiol (1985) 1987 Oct;63(4):1381–1386. doi: 10.1152/jappl.1987.63.4.1381. [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R. A model of crossbridge action: the effects of ATP, ADP and Pi. J Muscle Res Cell Motil. 1989 Jun;10(3):181–196. doi: 10.1007/BF01739809. [DOI] [PubMed] [Google Scholar]
- Podolin R. A., Ford L. E. Influence of partial activation on force-velocity properties of frog skinned muscle fibers in millimolar magnesium ion. J Gen Physiol. 1986 Apr;87(4):607–631. doi: 10.1085/jgp.87.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen G. J., Roosemalen M. C., Wilson M. G., Elzinga G. Depression of force by phosphate in skinned skeletal muscle fibers of the frog. Am J Physiol. 1990 Aug;259(2 Pt 1):C349–C357. doi: 10.1152/ajpcell.1990.259.2.C349. [DOI] [PubMed] [Google Scholar]
- Stienen G. J., Versteeg P. G., Papp Z., Elzinga G. Mechanical properties of skinned rabbit psoas and soleus muscle fibres during lengthening: effects of phosphate and Ca2+. J Physiol. 1992;451:503–523. doi: 10.1113/jphysiol.1992.sp019176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Lee J. A., Lännergren J., Allen D. G. Cellular mechanisms of fatigue in skeletal muscle. Am J Physiol. 1991 Aug;261(2 Pt 1):C195–C209. doi: 10.1152/ajpcell.1991.261.2.C195. [DOI] [PubMed] [Google Scholar]
- Wohlfart B., Edman K. A. Rectangular hyperbola fitted to muscle force-velocity data using three-dimensional regression analysis. Exp Physiol. 1994 Mar;79(2):235–239. doi: 10.1113/expphysiol.1994.sp003756. [DOI] [PubMed] [Google Scholar]


