Abstract
Background
Staphylococcus hyicus causes porcine exudative epidermitis, predominantly affecting suckling and weaned piglets. This bacterium produces various exfoliative toxins (ExhA, ExhB, ExhC, ExhD, SHETA, and SHETB), which are responsible for the clinical manifestations of exudative epidermitis. However, treatment failure is common due to frequent antimicrobial resistance in porcine strains. Therefore, this study aimed to identify the genes encoding exfoliative toxins and assess the antimicrobial resistance profiles of S. hyicus. A total of 17 S. hyicus isolates were collected from piglets with skin lesions from 2014 to 2021. All strains were subjected to species-specific polymerase chain reaction targeting sodA to confirm the presence of S. hyicus, and polymerase chain reaction amplification of exfoliative toxin genes (exhA, exhB, exhC, exhD, sheta, and shetb) was performed to differentiate toxigenic strains. Pulsed-field gel electrophoresis analysis and minimum inhibitory concentration tests using broth microdilution were conducted to further analyze the strains.
Results
Exfoliative toxin genes were detected in 52.9% (n = 9) of the S. hyicus isolates, with notable detection of exhB (17.6%), exhC (17.6%), exhD (11.8%), exhA (5.9%), sheta (0%), and shetb (0%). Pulsed-field gel electrophoresis analysis categorized the isolates into 11 pulsotypes with 70% similarity. Among 18 tested antimicrobials, all isolates exhibited 100% susceptibility to ceftiofur and sulfonamides and high susceptibility rates to neomycin, tilmicosin, and tetracyclines. Whereas the susceptibility rate of spectinomycin was 0% in all isolates, multidrug resistance was observed in 82.4% of the isolates, and in all toxigenic strains.
Conclusions
These findings provide crucial insights for monitoring and devising effective treatment strategies for managing exudative epidermitis in pigs caused by S. hyicus.
Keywords: Antimicrobial resistance, Exfoliative toxin, Exudative epidermitis, Staphylococcus hyicus, Piglets
Background
Staphylococcus hyicus is an important pathogen on swine farms worldwide because it causes exudative epidermitis (EE), also known as greasy pig disease, in young pigs [1]. EE is characterized by skin exfoliation with crusts, vesicles, and pustules, leading to considerable economic losses due to high morbidity and moderate mortality rates in pig-producing countries. It also causes significant discomfort and distress to the affected animals [2, 3]. Furthermore, S. hyicus is known to infect various animal species, including horses, goats, and cattle, and poses a zoonotic risk to humans [4–6].
S. hyicus can be classified into toxigenic and non-toxigenic strains based on their ability to cause EE in pigs, with specific virulence genes identified [7]. Exfoliative toxins (ExhA, ExhB, ExhC, ExhD, SHETA, and SHETB) are critical virulence factors of S. hyicus, inducing the characteristic symptoms of EE by cleaving the cell-to-cell adhesion of keratinocytes in the stratum granulosum of the superficial epidermis [3, 8]. While toxigenic strains of S. hyicus have been extensively examined due to their direct association with EE, non-toxigenic strains also play a significant role in the epidermitis that EE causes in piglets and should be considered in EE research [7, 9].
Research on the characteristics and antimicrobial resistance of S. hyicus isolated from EE is limited in Korea, as most studies are conducted locally [10–12]. S. hyicus infections are a major concern for pig breeders, and antimicrobial therapy is commonly employed during acute disease outbreaks due to the absence of a vaccine [13]. However, effective treatment is frequently hindered by the emergence of antimicrobial resistance among S. hyicus strains. Moreover, comprehensive data on resistance patterns in S. hyicus are lacking. Therefore, this study aimed to evaluate the pathological and molecular characteristics and antimicrobial resistance profiles of toxigenic and non-toxigenic strains of S. hyicus isolated from piglets exhibiting clinical signs of EE in South Korea.
Methods
Samples and bacterial isolation
Piglets with skin lesions were presented to the Animal and Plant Quarantine Agency of Korea for differential diagnosis between 2014 and 2021. The piglets’ bodies were covered with a moist, greasy exudate, and some exhibited thick, crusty lesions either covering the entire body or appearing as discrete, circumscribed lesions that did not coalesce. After necropsy, bacterial cultures were performed on selected skin samples corresponding to the gross lesions in each case. The samples were inoculated on 5% sheep blood agar plates (Asan Pharm Co., Seoul, Republic of Korea), and incubated aerobically at 37 °C. The suspected Staphylococcus colonies were isolated and identified as S. hyicus by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; VITEK® MS; bioMérieux, Marcy I’Etoile, France).
Polymerase chain reaction (PCR) assay for exfoliative toxin genes
Genomic DNA was extracted using the Maxwell® RSC PureFood GMO Kit (REF AS1600; Promega, Madison, WI, USA) following the manufacturer’s instructions. Extracted DNA from S. hyicus isolates was subjected to species-specific PCR targeting the sodA (superoxide dismutase A encoding gene) to confirm S. hyicus identity [14]. The presence of exfoliative toxin genes (exhA, exhB, exhC, exhD, sheta, and shetb) in the genomes of the isolates was confirmed using previously published primers and protocols [15, 16].
Pulsed-field gel electrophoresis (PFGE)
Following the CDC PulseNet protocol [17], DNA from S. hyicus isolates was digested using the SmaI enzyme (Takara Bio Inc., Shiga, Japan). Electrophoresis was conducted using the CHEF-DR® III PFGE system (Bio-Rad Laboratories, Hercules, CA, USA), and PFGE banding profiles were analyzed using BioNumerics software version 8.0 (Applied Maths, Sint-Martens-Latem, Belgium). The Dice coefficient and unweighted pair group method with arithmetic mean were employed for analysis. Isolates exhibiting a coefficient of similarity of ≥ 70% were considered genetically closely related [18].
Antimicrobial susceptibility test
Minimum inhibitory concentrations (MICs) were determined using the standard micro broth dilution method, as recommended by the Clinical and Laboratory Standards Institute [19]. The Sensititre Standard Susceptibility MIC Plates BOPO6F panel (Trek Diagnostic Systems, Cleveland, OH, USA), which contains 18 antimicrobials, was used according to the manufacturer’s instructions. Staphylococcus aureus (ATCC 25923) served as the quality control strain. Multidrug resistance (MDR) was defined as resistance to three or more antimicrobial subclasses.
Results
Detection of exfoliative toxin genes from Staphylococcus hyicus
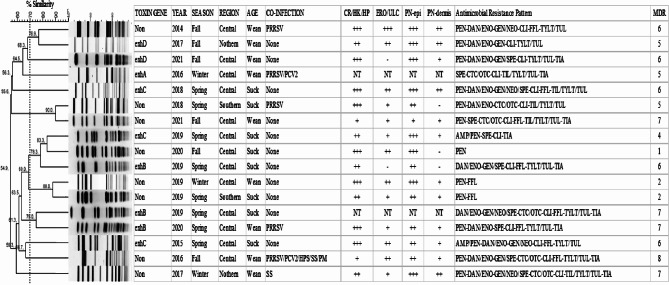
A total of 17 S. hyicus isolates were obtained from different farms, with their clinical descriptions summarized in Fig. 1. All piglets were suckling (n = 8) or weaned (n = 9) and exhibited systemic atrophy and skin lesions, including erythema, skin thickening, and crust formation. Most cases demonstrated the following histological lesions, except for two cases with no records: hyperkeratosis and/or epithelial hyperplasia (n = 15), erosion and/or ulceration in the epidermis (n = 13), pyonecrotic epidermitis (n = 15), and/or dermatitis (n = 12). Additionally, one suckling piglet (12.5%) and five weaned piglets (55.6%) were co-infected with other viral or bacterial pathogens, including porcine reproductive and respiratory syndrome virus, porcine circovirus 2, Streptococcus suis, Glaesserella parasuis, and Pasteurella multocida. Overall, 9 of the 17 isolates (52.9%) were identified as toxigenic strains. The highest frequencies were for exhB (n = 3) and exhC (n = 3) at 17.6% each, followed by for exhD (11.8%, n = 2) and exhA (5.9%, n = 1), with neither sheta nor shetb detected. Toxigenic strains were predominantly found in the central region (n = 8/9) and during spring (n = 6/9), with no difference between suckling (n = 5) and weaned (n = 4) piglets (Fig. 1).
Fig. 1.
Dendrogram showing the relationship among 17 Staphylococcus hyicus pulsotypes, exfoliative toxin gene detection, pathologic features, and antimicrobial resistance profiles
PFGE analysis of Staphylococcus hyicus
All isolates were categorized into 11 pulsotypes with 70% similarity. The dendrogram revealed no cluster formation based on toxin gene presence, years of isolation, season, region, age, or antimicrobial resistance patterns (Fig. 1).
Antimicrobial susceptibility of Staphylococcus hyicus
Table 1 describes the antimicrobial resistance and cumulative percentages of S. hyicus isolates, including nine toxigenic and eight non-toxigenic strains. All isolates were 100% susceptible to ceftiofur and sulfonamides, while neomycin (70.6%), tilmicosin (70.6%), and tetracyclines (64.7%) showed relatively high susceptibility rates. Conversely, susceptibility rates for spectinomycin (0%), clindamycin (17.6%), penicillin (17.6%), ampicillin (23.5%), florfenicol (23.5%), tylosin (23.5%), tulathromycin (23.5%), and fluoroquinolones (29.4%) were relatively low. Resistance to ampicillin, fluoroquinolones, aminoglycosides, clindamycin, tylosin tartrate, tulathromycin, and tiamulin was higher in toxigenic strains than in non-toxigenic strains, whereas resistance to penicillin, tetracyclines, and tilmicosin was higher in non-toxigenic strains. The prevalence of MDR was very high at 82.4%, excluding three non-toxigenic strains (Fig. 1). Additionally, toxigenic strains were all resistant to clindamycin and exhibited 100% MDR, whereas nontoxigenic strains were all resistant to penicillin.
Table 1.
Antimicrobial resistance and cumulative percentage of Staphylococcus hyicus isolates in piglets with exudative epidermitis
| Antimicrobial class or subclass | Antimicrobials | Cumulative percentage of strains inhibited at antimicrobial concentration (µg/ml) | MIC50 (µg/mL)a | MIC90 (µg/mL)a | S (%)b | I (%) b | R (%) b | MIC breakpoint (µg/mL)c |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | ||||||||
| Penicillinase-labile penicillins | Ampicillin | 23.5 | 88.2 | 100 | 0.5 | 1 | 23.5 | 64.7 | 11.8 | ≥ 1 | |||||||||
| Penicillin | 17.6 | 23.5 | 35.3 | 64.7 | 94.1 | 100 | 1 | 2 | 17.6 | ND d | 82.4 | ≥ 0.25 | |||||||
| Cephalosporin III | Ceftiofur | 94.1 | 100 | 1 | 1 | 100 | ND d | 0 | ≥ 8 | ||||||||||
| Fluoroquinolone | Danofloxacin | 17.6 | 29.4 | 35.3 | 100 | 1 | 1 | 29.4 | 5.9 | 64.7 | ≥ 1 | ||||||||
| Enrofloxacin | 29.4 | 35.3 | 100 | 4 | 4 | 29.4 | 5.9 | 64.7 | ≥ 4 | ||||||||||
| Aminoglycosides | Gentamicin | 41.2 | 47.1 | 64.7 | 100 | 8 | 16 | 47.1 | 17.6 | 35.3 | ≥ 16 | ||||||||
| Neomycin | 35.3 | 70.6 | 88.2 | 100 | 8 | 32 | 70.6 | ND d | 29.4 | ≥ 16 | |||||||||
| Spectinomycin | 41.2 | 100 | 128 | 128 | 0 | 41.2 | 58.8 | ≥ 128 | |||||||||||
| Tetracyclines | Oxytetracycline | 64.7 | 100 | 0.5 | 8 | 64.7 | ND d | 35.3 | ≥ 2 | ||||||||||
| Chlortetracycline | 64.7 | 100 | 0.5 | 8 | 64.7 | ND d | 35.3 | ≥ 2 | |||||||||||
| Lincosamides | Clindamycin | 17.6 | 23.5 | 100 | 16 | 16 | 17.6 | ND d | 82.4 | ≥ 4 | |||||||||
| Phenicols | Florfenicol | 23.5 | 41.2 | 100 | 8 | 8 | 23.5 | 17.6 | 58.8 | ≥ 8 | |||||||||
| Macrolides | Tilmicosin | 35.3 | 64.7 | 70.6 | 100 | 8 | 64 | 70.6 | ND d | 29.4 | ≥ 32 | ||||||||
| Tylosin | 23.5 | 100 | 32 | 32 | 23.5 | ND d | 76.5 | ≥ 4 | |||||||||||
| Tulathromycin | 23.5 | 100 | 64 | 64 | 23.5 | ND d | 76.5 | ≥ 64 | |||||||||||
| Sulfonamides | Sulfadimethoxine | 100 | 256 | 256 | 100 | ND d | 0 | ≥ 512 | |||||||||||
| Trimethoprim/Sulfamethoxazole | 100 | 2 | 2 | 100 | ND d | 0 | ≥ 4/76 | ||||||||||||
| Pleuromutilins | Tiamulin | 41.2 | 47.1 | 100 | 32 | 32 | 47.1 | ND d | 52.9 | ≥ 32 | |||||||||
n = 17 (piglets with exudative epidermitis)
The gray zone represents the tested concentration range for each antimicrobial on the BOPO6F plate
a. MIC50 and MIC90 concentrations at which isolate growth was inhibited by 50% and 90%, respectively
b. S, susceptible; I, intermediate; R, resistant
c. MIC breakpoints of 18 antimicrobials are indicated as vertical double lines according to CLSI guidelines (2018), except for neomycin, which follows the recommendations from a previous study (Moreno et al., 2023)
d. ND, not determined
Susceptibility and resistance are indicated by vertical double (sensitive) lines based on the reference guidelines for each antimicrobial
Discussion
S. hyicus has been globally recognized as the causative pathogen of EE in pigs for over 180 years, establishing it as a significant staphylococcal skin disease. Clinical manifestations are most severe in piglets aged 3–32 days, often leading to dehydration and potential mortality [20, 21]. While extensive research has been conducted on staphylococcal-induced EE [22–26], studies specifically targeting S. hyicus remains sparse, both nationally and globally.
In this study, we investigated the exfoliative toxins produced by S. hyicus isolated from pigs with EE. Prior studies in South Korea have documented swine EE and associated mortality caused by S. hyicus on farms in the Gyeongsang [10], Chungcheong [27], and Jeolla provinces [12], with exacerbation of some cases due to concurrent viral infections. However, in-depth studies of the exfoliative toxins produced by S. hyicus are limited. These toxins are key virulence factors of S. hyicus, and ExhA, ExhB, ExhC, ExhD, SHETA, and SHETB toxins facilitate skin exfoliation in pigs [3, 15, 16]. All variants of these exfoliative toxins induce blister formation in porcine skin by cleaving desmoglein-1, though human desmoglein-1 is resistant to these toxins [3, 8]. Although toxigenic strains of S. hyicus have been isolated from both healthy and diseased pigs, the isolation rate is higher in pigs affected by EE than in healthy pigs [16, 22, 28]. In this study, 52.9% of the isolates were identified as toxigenic, consistent with the findings of Andresen et al. [22], who reported that 47.1–66.7% of S. hyicus strains isolated from pigs with EE appeared toxigenic. However, these findings contrast with those of Russian [29] and Brazilian [18] studies which reported that approximately 90% of isolates are toxigenic. The highest detection rates in this study were for exhB and exhC (17.6% each), followed by for exhD (11.8%) and exhA (5.9%). Although the number of isolates was insufficient for a robust comparison with other studies, previous research has shown variable detection rates of exfoliative toxins; for instance, 18–22% with the highest rate for exhB was observed in Denmark [30], 0.7–48.9% with the highest rate for exhA in Japan [28], 24.4–76.1% with the highest rate for exhC in Brazil [18], and 89.5% with the highest detection rate for exhD in Russia [29]. Additionally, studies within the same country have shown temporal changes in the distribution of toxin genes, such as a decrease in the prevalence of exhB [15, 18]. Therefore, distribution of exfoliative toxins and prevalence of toxigenic strains reported in the literature vary according to different countries and study periods.
Both toxigenic and nontoxigenic strains of S. hyicus have been reported to induce hyperkeratosis and inflammatory cell hyperplasia of the epidermis in pigs [7, 9, 31]. Consistent with this, our findings showed no correlation between the presence or type of exfoliative toxins and clinicopathological presentation of skin lesions. For instance, 47.1% of the S. hyicus isolates were identified as non-toxigenic strains, yet al.l were isolated from skin displaying mild-to-severe pathological lesions of EE. The absence of toxins in the isolates even in cases with severe skin lesions suggests the involvement of other virulence factors in EE, necessitating further research utilizing whole-genome sequencing to identify potential virulence determinants beyond exfoliative toxins involved in EE in pigs. Additional predisposing factors that may contribute to S. hyicus colonization and virulence in pigs include viral diseases, nutritional deficiencies, dermatophytosis, pityriasis rosea, parasitism, poor hygiene, inadequate ventilation, high humidity, trauma, and genetic predisposition [20].
The current study demonstrated a high diversity of both toxigenic and non-toxigenic S. hyicus strains in South Korea, irrespective of the year of isolation, season, region, age, or antimicrobial resistance pattern. Consistent with our finding, previous studies have reported significant diversity in the PFGE patterns of S. hyicus strains isolated from pigs [18, 31–33], with no clustering based on toxigenic strains or resistance profiles. Furthermore, various PFGE patterns have been identified on the same farm [32]. PFGE analysis of S. hyicus strains isolated from other animal species has shown diverse patterns and high variability in chickens and bovine milk [32, 34]. Given these studies, the high diversity observed in the PFGE results of this study appears to be inherent to the characteristics of S. hyicus. Therefore, PFGE results have limitations in cross-national comparative analyses for epidemiological research, necessitating the application of other molecular analysis techniques.
Antimicrobial susceptibility testing revealed that all S. hyicus isolates were 100% susceptible to ceftiofur and sulfonamides. However, the isolates demonstrated low susceptibility to penicillins (17.6–23.5%) and fluoroquinolones (29.4%). Among the macrolides, resistance to tilmicosin was 29.4%, while resistance to tylosin and tulathromycin was 76.5%. Consistent with our findings, other studies have shown low resistance rates to ceftiofur (0–0.97%) and sulfadimethoxine (1.9–5.2%) in Brazil and trimethoprim/sulfamethoxazole (9.7–25.8%) in Brazil and Japan among S. hyicus isolates from porcine EE [18, 28]. Despite the low resistance to ceftiofur, third-generation cephalosporins are classified as highest priority critically important antimicrobials (HPCIA) for humans and veterinary critically important antimicrobials (VCIA) for animals, according to the WHO (in 2024) [35] and WOAH (in 2021), respectively [36]. Fluoroquinolones are also classified into the HPCIA and VCIA categories. However, our results indicated a higher fluoroquinolone resistance rate at 64.7% compared to 0–13.2% in European countries, except for that reported by one Brazilian study [13, 18, 37]. Thus, there is an urgent need to address the high rate of fluoroquinolone resistance. Penicillin resistance rates vary widely across different countries and study periods, including 25.0% in Germany and 76.8% in Japan, and even fluctuate within the same country over time [13, 18, 28, 37]. However, a direct comparison of the MDR results obtained in this study with those reported by previous Korean studies poses challenges due to differing antimicrobials and testing methods used, even though a previous Korean report indicated a 12.6% MDR rate [10]. Furthermore, MDR has been observed to increase over time [18] and is predominant in toxigenic strains [28]. Consistent with this, 82.4% of isolates were MDR, with 76.5% (n = 13) resistant to five or more antimicrobial subclasses, and all toxigenic strains were 100% MDR. These findings showed that S. hyicus isolates from EE exhibited increased resistance to most antimicrobials, which was unlike the findings of previous studies. Ensuring that bacteria do not develop resistance to antimicrobials is crucial for both animal and human health. Therefore, it is essential to confirm diagnoses using susceptibility tests rather than base diagnoses on clinical symptoms alone to select appropriate antimicrobials [20]. Likewise, developing a vaccine against S. hyicus should also be considered, as autogenous vaccines using strains isolated from affected herds have reduced metaphylactic antimicrobial treatment and lowered morbidity and mortality rates in weaned pigs [20, 38].
Despite pigs developing disease resistance with age, S. hyicus can still be recovered from older pigs’ skin, and these asymptomatic carriers can contaminate naïve herds [39]. Research has shown that suckling piglets are primarily infected by dams, some of whom are vaginally infected at birth [20]. Moreover, S. hyicus has been isolated from healthy pigs. However, this study included limited samples for the differential diagnosis of piglets with skin lesions. Therefore, further studies are warranted to determine the overall distribution of S. hyicus based on clinical manifestations, age groups, and pig farm environments in South Korea.
Conclusion
This study analyzed the pathological findings, toxin types, and antimicrobial resistance of S. hyicus isolated from EE lesions from affected pigs in the Republic of Korea. All exfoliative toxins (ExhA, ExhB, ExhC, and ExhD) were detected, except for sheta and shetb. Ceftiofur and sulfonamides exhibited 100% antimicrobial susceptibility. Additionally, most S. hyicus isolates were found to be MDR. Thus, our study showed that selecting effective antimicrobials is crucial for enhancing treatment efficiency and preventing antimicrobial resistance. Owing to the limited number of samples available for disease diagnosis, further nationwide prevalence studies are necessary, regardless of clinical symptoms.
The results were calculated using the Dice coefficient and the unweighted pair group method with arithmetic averages (UPGMA), shown with a similarity greater than 70%. Seasonal divisions: Spring, March-May; Summer, June-August; Fall, September-November; Winter, December-February. Regional divisions: Northern, Gyeonggi and Gangwon; Central, Chungbuk, Chungnam, Gyeongbuk, and Jeonbuk; Southern, Jeonnam and Gyeongnam. Age categories: Wean: Weaned piglets (25–70 days old); Suck: Suckling piglets (1–24 days old). Co-infections: PRRSV: Porcine reproductive and respiratory syndrome virus; PCV2: Porcine circovirus type2; SS: Streptococcus suis; HPS: Haemophilus parasuis; PM: Pasteurella multocida. Pathological features: CR/HK/HP: Crust, Hyperkeratosis, or Hyperplasia in the epidermis; ERO/ULC: Erosion or Ulceration; PN-epi: Pyonecrotic epidermatitis; PN-dermis: Pyonecrotic dermatitis. Severity indicator: +++, severe; ++, moderate; +, weak; -, no histological lesion; NT, not tested. Anitmicrobial resistance profile: AMP, ampicillin; PEN, penicillin; XNL, ceftiofur; DAN, danofloxacin; ENO, enrofloxacin; GEN, gentamicin; NEO, neomycin; SPE, spectinomycin; CTC, chlortetracycline; OTC, oxytetracycline; CLI, clindamycin; FFL, florfenicol; TIL, tilmicosin; TYLT, tylosin tartrate; TUL, tulathromycin; SDM, sulfadimethoxine; SXT, trimethoprim/sulfamethoxazole; TIA, tiamulin; MDR, multidrug resistance.
Acknowledgements
We thank the farmers of the sampled pigs for their active cooperation.
Author contributions
Conceptualization: Kim HY, Yun CS Data curation: Kim HY, Yun CS Formal analysis: Yun CS, Kang SM Methodology: Kim HY, Hwang MH Software: Yun CS Validation: Kim HY, Byun JW, Ku BK Investigation: Kwon DH, Lee S, Jeon GT, Kang HJ, Kim J Writing - original draft: Yun CS, Kang SM, Kwon DH, Lee S, Jeon GT, Kang HJ, Kim J Writing - review & editing: Kim HY, Hwang MH, Byun JW, Ku BK.
Funding
This research was supported by a grant from the Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs of the Republic of Korea (B-1543069-2024-25-01). The funding body had no role in the design of the study, collection, analysis, interpretation of data, and in writing the manuscript.
Data availability
Upon reasonable request, the datasets of this study can be made available by the corresponding author.
Declarations
Ethics approval and consent to participate
This study did not require ethics approval because the piglets’ bodies were submitted to the Animal and Plant Quarantine Agency for diagnosis by veterinarians and animal owners with their consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Foster AP. Staphylococcal skin disease in livestock. Vet Dermatol. 2012;23. 10.1111/j.1365-3164.2012.01093.x. 342 – 51, e63. [DOI] [PubMed]
- 2.Andresen LO, Wegener HC, Bille-Hansen V. Staphylococcus hyicus-skin reactions in piglets caused by crude extracellular products and by partially purified exfoliative toxins. Microb Pathog. 1993;15:217–25. 10.1006/mpat.1993.1072. [DOI] [PubMed] [Google Scholar]
- 3.Fudaba Y, Nishifuji K, Andresen LO, Yamaguchi T, Komatsuzawa H, Amagai M, et al. Staphylococcus hyicus exfoliative toxins selectively digest porcine desmoglein 1. Microb Pathog. 2005;39:171–6. 10.1016/j.micpath.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Österlund A, Nordlund E. Wound infection caused by Staphylococcus hyicus subspecies hyicus after a donkey bite. Scand J Infect Dis. 1997;29:95. 10.3109/00365549709008674. [DOI] [PubMed] [Google Scholar]
- 5.Casanova C, Iselin L, von Steiger N, Droz S, Sendi P. Staphylococcus hyicus bacteremia in a farmer. J Clin Microbiol. 2011;49:4377–8. 10.1128/JCM.05645-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirk F, Mashicharan M, Braddick M, Saxena P. Staphylococcus hyicus, a novel pathogen causing destructive infective endocarditis requiring mitral annular reconstruction. JTCVS Tech. 2022;13:70–3. 10.1016/j.xjtc.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leekitcharoenphon P, Pamp SJ, Andresen LO, Aarestrup FM. Comparative genomics of toxigenic and non-toxigenic Staphylococcus hyicus. Vet Microbiol. 2016;185:34–40. 10.1016/j.vetmic.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Nishifuji K, Sugai M, Amagai M. Staphylococcal exfoliative toxins: molecular scissors of bacteria that attack the cutaneous defense barrier in mammals. J Dermatol Sci. 2008;49:21–31. 10.1016/j.jdermsci.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Gou H, Chu P, Zhang K, Jiang Z, Cai R, et al. Comparison of host cytokine response in piglets infected with toxigenic and non-toxigenic Staphylococcus hyicus. Front Vet Sci. 2021;8:639141. 10.3389/fvets.2021.639141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee D, Yeo S. Prevalence of Staphylococcus hyicus subsp. hyicus in pigs with reference to antibiotic susceptibility of isolates. Korean J Vet Res. 1990;30.
- 11.Park JE, Shin HJ, Easwaran M, Park JW. Antimicrobial susceptibility of Staphylococcus hyicus isolated from Korean pigs with exudative epidermitis. J Prev Vet Med. 2018;42:41–5. 10.13041/jpvm.2018.42.1.41. [Google Scholar]
- 12.Kang SC, Kim JH, Kim B, Song JK, Lee HY, Shin S, et al. Congenital swinepox of neonatal pigs in a Korean domestic farm. Korean J Vet Res. 2020;60:241–4. 10.14405/kjvr.2020.60.4.241. [Google Scholar]
- 13.Werckenthin C, Cardoso M, Martel JL, Schwarz S. Antimicrobial resistance in staphylococci from animals with particular reference to bovine Staphylococcus aureus, porcine Staphylococcus hyicus, and canine Staphylococcus intermedius. Vet Res. 2001;32:341–62. 10.1051/vetres:2001129. [DOI] [PubMed] [Google Scholar]
- 14.Voytenko AV, Kanbar T, Alber J, Lämmler C, Weiss R, Prenger-Berninghoff E, et al. Identification of Staphylococcus hyicus by polymerase chain reaction mediated amplification of species specific sequences of superoxide dismutase A encoding gene sodA. Vet Microbiol. 2006;116:211–6. 10.1016/j.vetmic.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Onuma K, Uoya Y, Koide T, Shibata A, Tanabe T, Sato H. Detection of Staphylococcus hyicus exfoliative toxin genes by dot blot hybridization and multiplex polymerase chain reaction. Microbiol Immunol. 2011;55:168–73. 10.1111/j.1348-0421.2011.00308.x. [DOI] [PubMed] [Google Scholar]
- 16.Kanbar T, Voytenko AV, Alber J, Lämmler C, Weiss R, Skvortzov VN. Distribution of the putative virulence factor encoding gene sheta in Staphylococcus hyicus strains of various origins. J Vet Sci. 2008;9:327–9. 10.4142/jvs.2008.9.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Center for Disease Control and Prevention (CDC). Pulsed-field gel electrophoresis (PFGE) [Internet]. 2016. https://www.cdc.gov/pulsenet/pathogens/pfge.html. Accessed 2023 Jan 18.
- 18.Moreno AM, Moreno LZ, Poor AP, Matajira CEC, Moreno M, Gomes VTM, et al. Antimicrobial resistance profile of Staphylococcus hyicus strains isolated from Brazilian swine herds. Antibiot (Basel). 2022;11:205. 10.3390/antibiotics11020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CLSI Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial disks and dilution susceptibility tests for bacteria isolated from animals. CLSI supplement VET08. CLSI Document VET08. 4th ed. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 20.Frana TS, Hau SJ. Staphylococcosis. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J, editors. Diseases of swine. John Wiley & Sons, Inc.; 2019. pp. 926–33.
- 21.Wang M, Hu J, Zhu L, Guo C, Lu H, Guo C, et al. A fatal suppurative pneumonia in piglets caused by a pathogenic coagulase-positive strain of Staphylococcus hyicus. Vet Res Commun. 2017;41:139–46. 10.1007/s11259-017-9682-0. [DOI] [PubMed] [Google Scholar]
- 22.Andresen LO, Ahrens P, Daugaard L, Bille-Hansen V. Exudative epidermitis in pigs caused by toxigenic Staphylococcus chromogenes. Vet Microbiol. 2005;105:291–300. 10.1016/j.vetmic.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 23.van Duijkeren E, Jansen MD, Flemming SC, de Neeling H, Wagenaar JA, Schoormans AHW, et al. Methicillin-resistant Staphylococcus aureus in pigs with exudative epidermitis. Emerg Infect Dis. 2007;13:1408–10. 10.3201/eid1309.061268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L, He K, Ni Y, Yu Z, Mao A. Exudative epidermitis of piglets caused by non-toxigenic Staphylococcus sciuri. Vet Microbiol. 2017;199:79–84. 10.1016/j.vetmic.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Moon DC, Jeong SK, Hyun BH, Lim SK. Prevalence and characteristics of methicillin-resistant Staphylococcus aureus isolates in pigs and pig farmers in Korea. Foodborne Pathog Dis. 2019;16:256–61. 10.1089/fpd.2018.2509. [DOI] [PubMed] [Google Scholar]
- 26.Lee GY, Lee HH, Yang SJ. Antimicrobial resistance profiles and clonal diversity of Staphylococcus epidermidis isolates from pig farms, slaughterhouses, and retail pork. Vet Microbiol. 2023;282:109753. 10.1016/j.vetmic.2023.109753. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Jung JY, Kim SH, Kim JW, Park JW, Kang DY, et al. Porcine ear necrosis syndrome by coinfection of porcine reproductive and respiratory syndrome virus and Staphylococcus hyicus. Korean J Vet Res. 2017;57:143–46. 10.14405/kjvr.2017.57.2.143. [Google Scholar]
- 28.Futagawa-Saito K, Ba-Thein W, Fukuyasu T. Antimicrobial susceptibilities of exfoliative toxigenic and non-toxigenic Staphylococcus hyicus strains in Japan. J Vet Med Sci. 2009;71:681–4. 10.1292/jvms.71.681. [DOI] [PubMed] [Google Scholar]
- 29.Kanbar T, Voytenko AV, Alber J, Lämmler C, Weiss R, Zschöck M, et al. Prevalence of genes encoding exfoliative toxins among Staphylococcus hyicus isolated in Russia and Germany. J Vet Med B Infect Dis Vet Public Health. 2006;53:429–33. 10.1111/j.1439-0450.2006.00988.x. [DOI] [PubMed] [Google Scholar]
- 30.Andresen LO, Ahrens P. A multiplex PCR for detection of genes encoding exfoliative toxins from Staphylococcus hyicus. J Appl Microbiol. 2004;96:1265–70. 10.1111/j.1365-2672.2004.02258.x. [DOI] [PubMed] [Google Scholar]
- 31.Hassler C, Nitzsche S, Iversen C, Zweifel C, Stephan R. Characteristics of Staphylococcus hyicus strains isolated from pig carcasses in two different slaughterhouses. Meat Sci. 2008;80:505–10. 10.1016/j.meatsci.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu A, Kloos WE, Berkhoff HA, George CG, Ballard DN. Pulsed-field gel electrophoresis of Staphylococcus hyicus and Staphylococcus chromogenes genomic DNA and its taxonomic, epidemiological, and ecologic applications in veterinary medicine. J Vet Med Sci. 1997;59:443–50. 10.1292/jvms.59.443. [DOI] [PubMed] [Google Scholar]
- 33.Adkins PRF, Middleton JR, Calcutt MJ, Stewart GC, Fox LK. Species identification and strain typing of Staphylococcus agnetis and Staphylococcus hyicus isolates from bovine milk by use of a novel multiplex PCR assay and pulsed-field gel electrophoresis. J Clin Microbiol. 2017;55:1778–88. 10.1128/JCM.02239-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillespie BE, Headrick SI, Boonyayatra S, Oliver SP. Prevalence and persistence of coagulase-negative Staphylococcus species in three dairy research herds. Vet Microbiol. 2009;134:65–72. 10.1016/j.vetmic.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization (WHO). WHO list of medically important antimicrobials [Internet]. 2024. gcp > who-mia-list-2024-lv. https://cdn.who.int. Accessed 2024 May 18.
- 36.World Organization for Animal Health (WOAH). List of Antimicrobial Agents of Veterinary Importance [Internet]. 2021. https://www.woah.org. Accessed 2024 May 18 app > uploads > 2021/06.
- 37.Aarestrup FM, Jensen LB. Trends in antimicrobial susceptibility in relation to antimicrobial usage and presence of resistance genes in Staphylococcus hyicus isolated from exudative epidermitis in pigs. Vet Microbiol. 2002;89:83–94. 10.1016/s0378-1135(02)00177-3. [DOI] [PubMed] [Google Scholar]
- 38.Arsenakis I, Boyen F, Haesebrouck F, Maes DGD. Autogenous vaccination reduces antimicrobial usage and mortality rates in a herd facing severe exudative epidermitis outbreaks in weaned pigs. Vet Rec. 2018;182:744. 10.1136/vr.104720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaillancourt K, LeBel G, Yi L, Grenier D. In vitro antibacterial activity of plant essential oils against Staphylococcus hyicus and Staphylococcus aureus, the causative agents of exudative epidermitis in pigs. Arch Microbiol. 2018;200:1001–7. 10.1007/s00203-018-1512-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon reasonable request, the datasets of this study can be made available by the corresponding author.