Abstract
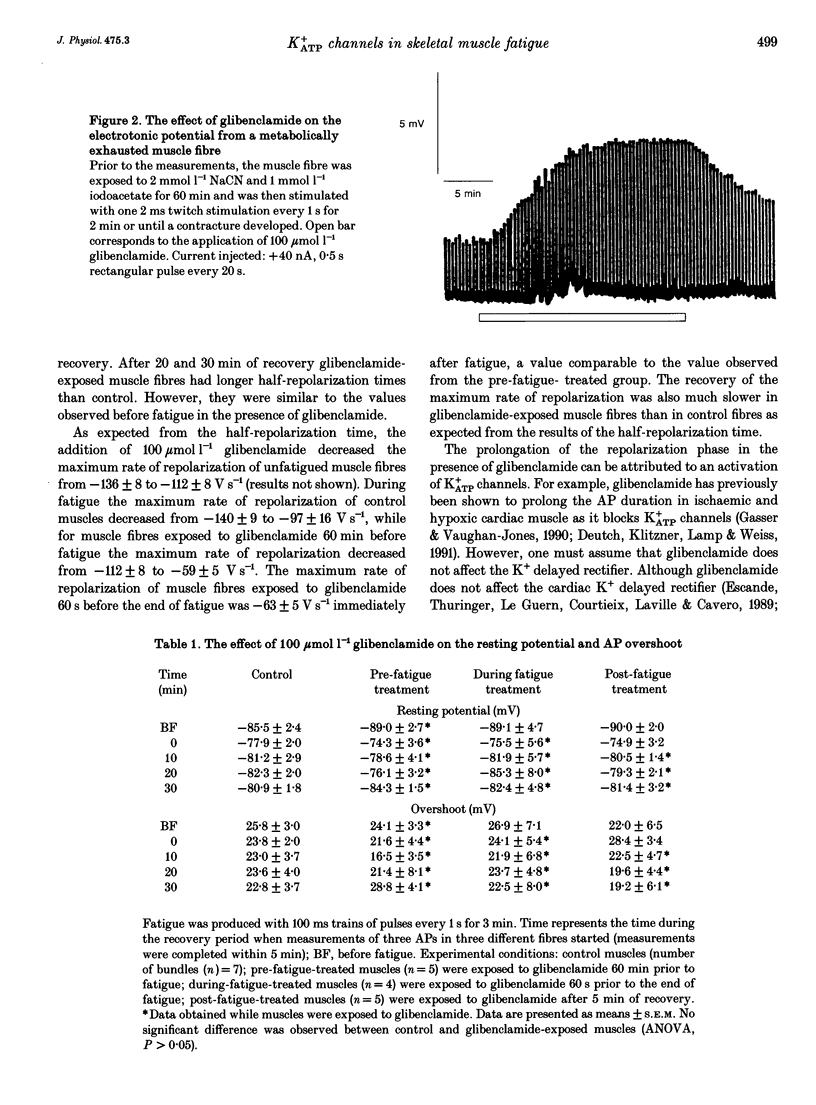
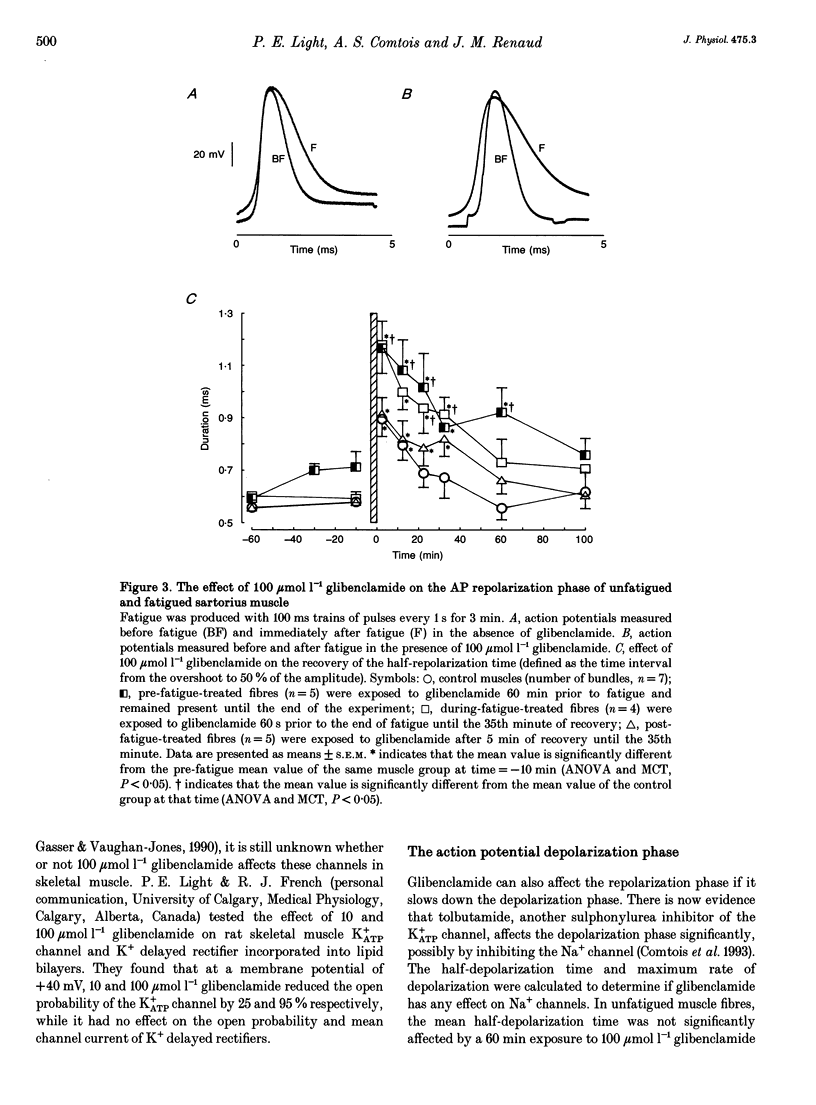
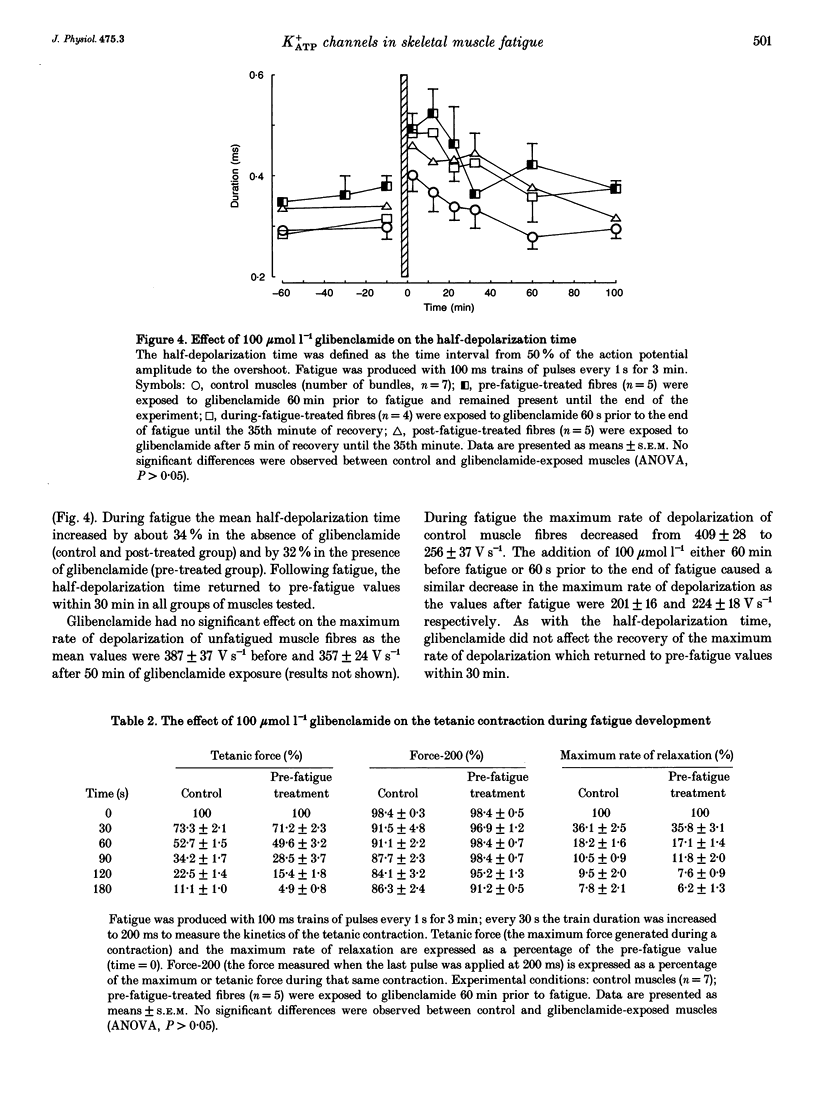
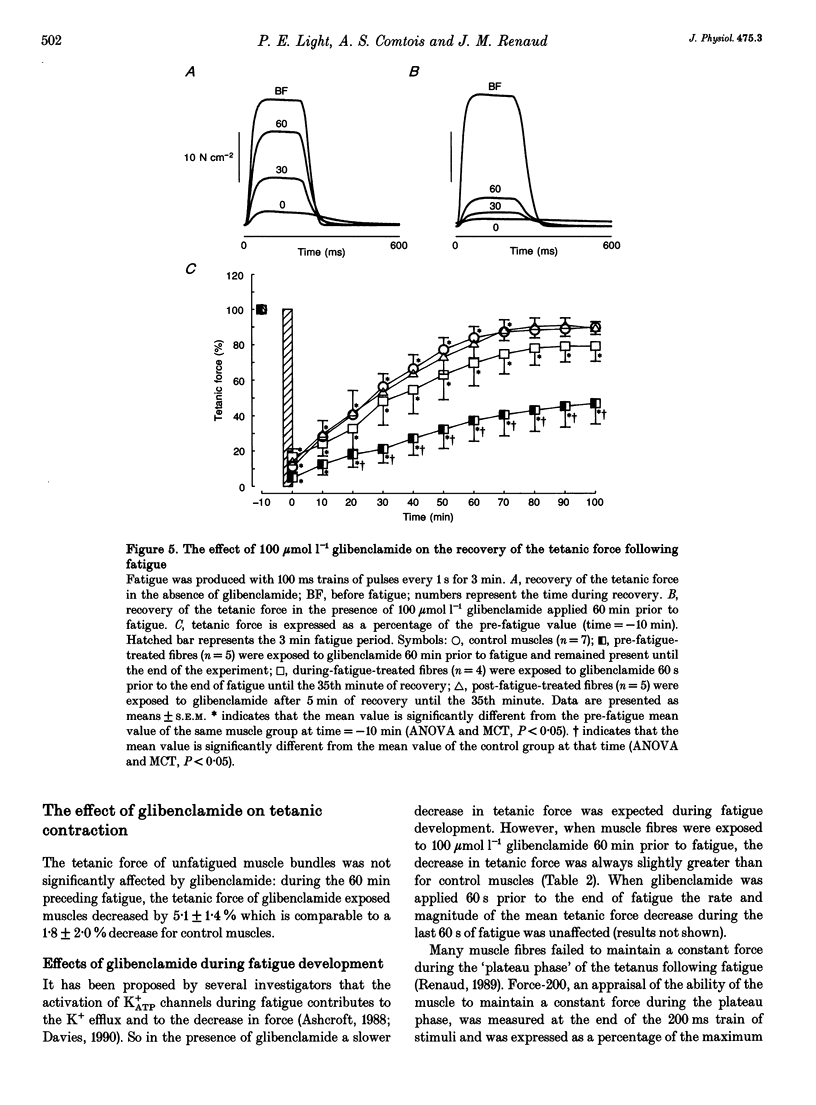
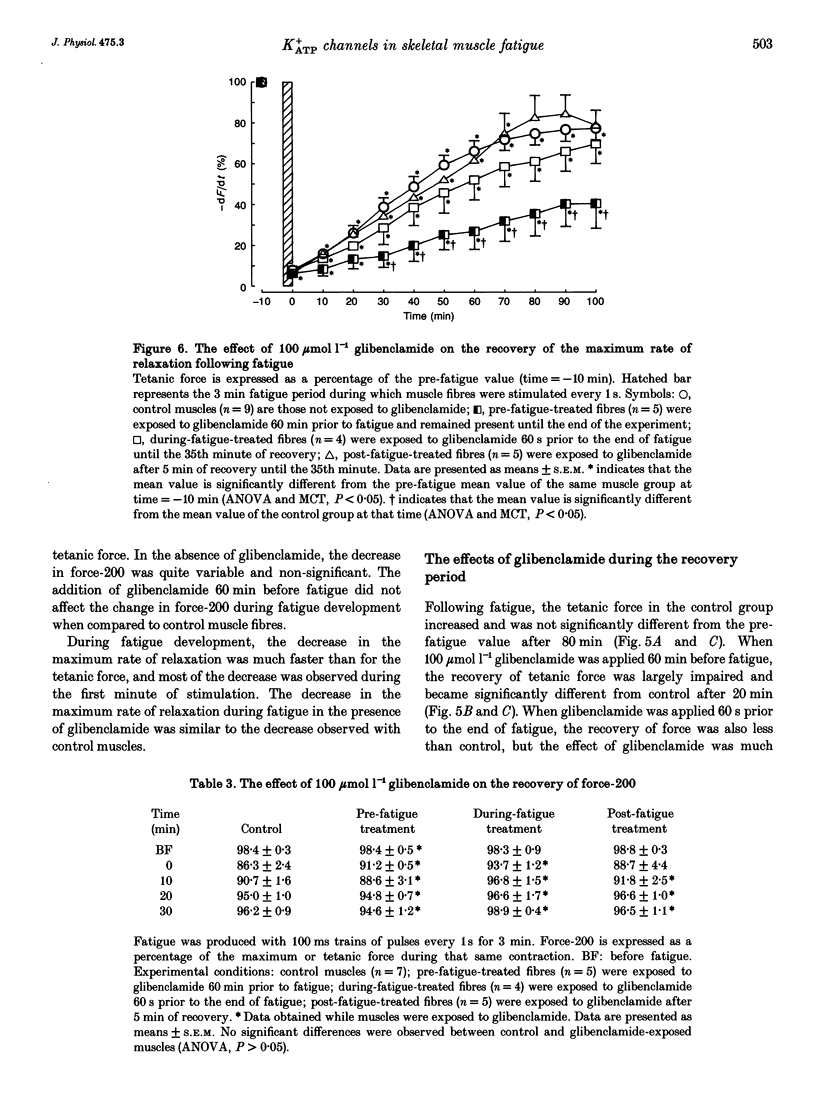
1. The purpose of this study was to determine whether ATP-sensitive K+ (K+ATP) channels are activated and contribute to the decrease in force during fatigue development in the sartorius muscle of the frog, Rana pipiens. Tetanic force (elicited by field stimulation), action potential and membrane conductance (using conventional microelectrodes), were measured in the presence and absence of glibenclamide, a K+ATP channel antagonist. Experiments were performed in bicarbonate-buffered solutions at pH 7.2. 2. In unfatigued muscle 100 mumol l-1 glibenclamide had no effect on the resting potential, the overshoot, the half-depolarization time or the maximum rate of depolarization of action potentials, while the mean half-repolarization time increased by 19 +/- 4% (+/- S.E.M.) and the maximum rate of repolarization decreased by 17 +/- 5%. 3. Fatigue was elicited using 100 ms tetanic contractions every 1 s for 3 min. In the absence of glibenclamide the mean half-repolarization time increased from 0.57 +/- 0.05 to 0.89 +/- 0.05 ms during fatigue. The mean half-repolarization times after fatigue, when muscle fibres were exposed to 100 mumol l-1 glibenclamide either 60 min prior to fatigue or 60 s before the end of fatigue, were 1.16 +/- 0.08 and 1.17 +/- 0.07 ms respectively. Application of 100 mumol l-1 glibenclamide after 5 min of recovery did not increase the half-repolarization time, but decreased the rate of recovery compared to control values. 4. In unfatigued muscles, 100 mumol l-1 glibenclamide did not affect the tetanic contraction. In the absence of glibenclamide, the mean tetanic force after fatigue was 11.0 +/- 0.9% of prefatigue values. Application of 100 mumol l-1 glibenclamide 60 min before fatigue increased the rate of fatigue development as the mean tetanic force was 4.8 +/- 0.8% after 3 min of stimulation. The addition of 100 mumol l-1 glibenclamide 60 s before the end of fatigue had no effect on tetanic force during this time compared to control. 5. In the absence of glibenclamide, muscles recovered 90.1 +/- 1.6% of their tetanic force after 100 min. Addition of 100 mumol l-1 glibenclamide 60 min prior to fatigue significantly reduced the capacity of muscles to recover their tetanic force: after 100 min of recovery tetanic force was only 47.3 +/- 9.4% of the pre-fatigue value.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Sturgess N. C., Trout N. J., Gardner N. J., Hales C. N. Adenosine-5'-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. Pflugers Arch. 1988 Aug;412(3):297–304. doi: 10.1007/BF00582512. [DOI] [PubMed] [Google Scholar]
- Benton D. C., Haylett D. G. Effects of cromakalim on the membrane potassium permeability of frog skeletal muscle in vitro. Br J Pharmacol. 1992 Sep;107(1):152–155. doi: 10.1111/j.1476-5381.1992.tb14478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSON F. D., SIGER A. The mechanochemistry of muscular contraction. I. The isometric twitch. J Gen Physiol. 1960 Sep;44:33–60. doi: 10.1085/jgp.44.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G. Effect of channel blockers on potassium efflux from metabolically exhausted frog skeletal muscle. J Physiol. 1987 Feb;383:31–43. doi: 10.1113/jphysiol.1987.sp016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comtois A., Light P., Renaud J. M., Kong M. Tolbutamide, but not glyburide, affects the excitability and contractility of unfatigued frog sartorius muscle. Eur J Pharmacol. 1993 Sep 21;242(1):65–73. doi: 10.1016/0014-2999(93)90011-6. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Davies N. W. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature. 1990 Jan 25;343(6256):375–377. doi: 10.1038/343375a0. [DOI] [PubMed] [Google Scholar]
- Davies N. W., Standen N. B., Stanfield P. R. The effect of intracellular pH on ATP-dependent potassium channels of frog skeletal muscle. J Physiol. 1992 Jan;445:549–568. doi: 10.1113/jphysiol.1992.sp018939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. W., Standen N. B., Stanfield P. R. ATP-dependent potassium channels of muscle cells: their properties, regulation, and possible functions. J Bioenerg Biomembr. 1991 Aug;23(4):509–535. doi: 10.1007/BF00785809. [DOI] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978 Aug 31;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Deutsch N., Klitzner T. S., Lamp S. T., Weiss J. N. Activation of cardiac ATP-sensitive K+ current during hypoxia: correlation with tissue ATP levels. Am J Physiol. 1991 Sep;261(3 Pt 2):H671–H676. doi: 10.1152/ajpheart.1991.261.3.H671. [DOI] [PubMed] [Google Scholar]
- Escande D., Thuringer D., Le Guern S., Courteix J., Laville M., Cavero I. Potassium channel openers act through an activation of ATP-sensitive K+ channels in guinea-pig cardiac myocytes. Pflugers Arch. 1989 Sep;414(6):669–675. doi: 10.1007/BF00582134. [DOI] [PubMed] [Google Scholar]
- Faivre J. F., Findlay I. Effects of tolbutamide, glibenclamide and diazoxide upon action potentials recorded from rat ventricular muscle. Biochim Biophys Acta. 1989 Aug 21;984(1):1–5. doi: 10.1016/0005-2736(89)90334-9. [DOI] [PubMed] [Google Scholar]
- Findlay I. ATP4- and ATP.Mg inhibit the ATP-sensitive K+ channel of rat ventricular myocytes. Pflugers Arch. 1988 Jul;412(1-2):37–41. doi: 10.1007/BF00583729. [DOI] [PubMed] [Google Scholar]
- Findlay I. Effects of ADP upon the ATP-sensitive K+ channel in rat ventricular myocytes. J Membr Biol. 1988;101(1):83–92. doi: 10.1007/BF01872823. [DOI] [PubMed] [Google Scholar]
- Fink R., Lüttgau H. C. An evaluation of the membrane constants and the potassium conductance in metabolically exhausted muscle fibres. J Physiol. 1976 Dec;263(2):215–238. doi: 10.1113/jphysiol.1976.sp011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser R. N., Vaughan-Jones R. D. Mechanism of potassium efflux and action potential shortening during ischaemia in isolated mammalian cardiac muscle. J Physiol. 1990 Dec;431:713–741. doi: 10.1113/jphysiol.1990.sp018356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirche H., Schumacher E., Hagemann H. Extracellular K+ concentration and K+ balance of the gastrocnemius muscle of the dog during exercise. Pflugers Arch. 1980 Sep;387(3):231–237. doi: 10.1007/BF00580975. [DOI] [PubMed] [Google Scholar]
- Hník P., Holas M., Krekule I., Kŭriz N., Mejsnar J., Smiesko V., Ujec E., Vyskocil F. Work-induced potassium changes in skeletal muscle and effluent venous blood assessed by liquid ion-exchanger microelectrodes. Pflugers Arch. 1976 Mar 11;362(1):85–94. doi: 10.1007/BF00588685. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. Analysis of the membrane capacity in frog muscle. J Physiol. 1972 Feb;221(1):121–136. doi: 10.1113/jphysiol.1972.sp009743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucier G. E., Mainwood G. W. The measurement of potassium efflux in superfused frog sartorius muscles. Can J Physiol Pharmacol. 1972 Feb;50(2):123–131. doi: 10.1139/y72-019. [DOI] [PubMed] [Google Scholar]
- Mainwood G. W., Renaud J. M. The effect of acid-base balance on fatigue of skeletal muscle. Can J Physiol Pharmacol. 1985 May;63(5):403–416. doi: 10.1139/y85-072. [DOI] [PubMed] [Google Scholar]
- Mainwood G. W., Worsley-Brown P., Paterson R. A. The metabolic changes in frog sartorius muscles during recovery from fatigue at different external bicarbonate concentrations. Can J Physiol Pharmacol. 1972 Feb;50(2):143–155. doi: 10.1139/y72-021. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Renaud J. M., Light P. Effects of K+ on the twitch and tetanic contraction in the sartorius muscle of the frog, Rana pipiens. Implication for fatigue in vivo. Can J Physiol Pharmacol. 1992 Sep;70(9):1236–1246. doi: 10.1139/y92-172. [DOI] [PubMed] [Google Scholar]
- Renaud J. M., Mainwood G. W. The effects of pH on the kinetics of fatigue and recovery in frog sartorius muscle. Can J Physiol Pharmacol. 1985 Nov;63(11):1435–1443. doi: 10.1139/y85-236. [DOI] [PubMed] [Google Scholar]
- Renaud J. M., Mainwood G. W. The interactive effects of fatigue and pH on the ionic conductance of frog sartorius muscle fibers. Can J Physiol Pharmacol. 1985 Nov;63(11):1444–1453. doi: 10.1139/y85-237. [DOI] [PubMed] [Google Scholar]
- Renaud J. M. The effect of lactate on intracellular pH and force recovery of fatigued sartorius muscles of the frog, Rana pipiens. J Physiol. 1989 Sep;416:31–47. doi: 10.1113/jphysiol.1989.sp017747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauviat M. P., Ecault E., Faivre J. F., Findlay I. Activation of ATP-sensitive K channels by a K channel opener (SR 44866) and the effect upon electrical and mechanical activity of frog skeletal muscle. Pflugers Arch. 1991 Apr;418(3):261–265. doi: 10.1007/BF00370524. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G., Adams R. P., Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol. 1985 Feb;248(2 Pt 2):R190–R196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G. Role of exercise-induced potassium fluxes underlying muscle fatigue: a brief review. Can J Physiol Pharmacol. 1991 Feb;69(2):238–245. doi: 10.1139/y91-037. [DOI] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Studies of the unitary properties of adenosine-5'-triphosphate-regulated potassium channels of frog skeletal muscle. J Physiol. 1987 Jan;382:213–236. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985 Aug 22;316(6030):736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Pettit A. I., Davies N. W., Stanfield P. R. Activation of ATP-dependent K+ currents in intact skeletal muscle fibres by reduced intracellular pH. Proc Biol Sci. 1992 Mar 23;247(1320):195–198. doi: 10.1098/rspb.1992.0028. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Ashford M. L., Cook D. L., Hales C. N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet. 1985 Aug 31;2(8453):474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- Vivaudou M. B., Arnoult C., Villaz M. Skeletal muscle ATP-sensitive K+ channels recorded from sarcolemmal blebs of split fibers: ATP inhibition is reduced by magnesium and ADP. J Membr Biol. 1991 Jun;122(2):165–175. doi: 10.1007/BF01872639. [DOI] [PubMed] [Google Scholar]
- Woll K. H., Lönnendonker U., Neumcke B. ATP-sensitive potassium channels in adult mouse skeletal muscle: different modes of blockage by internal cations, ATP and tolbutamide. Pflugers Arch. 1989 Sep;414(6):622–628. doi: 10.1007/BF00582126. [DOI] [PubMed] [Google Scholar]


