Abstract
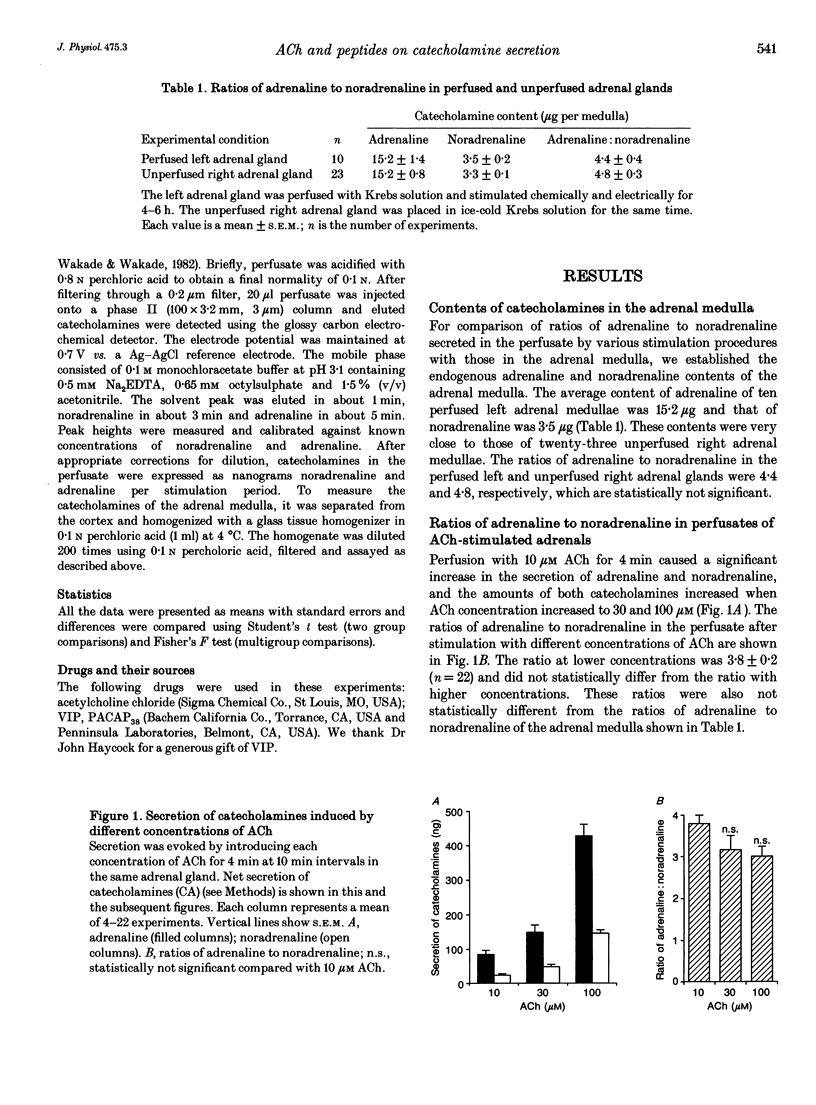
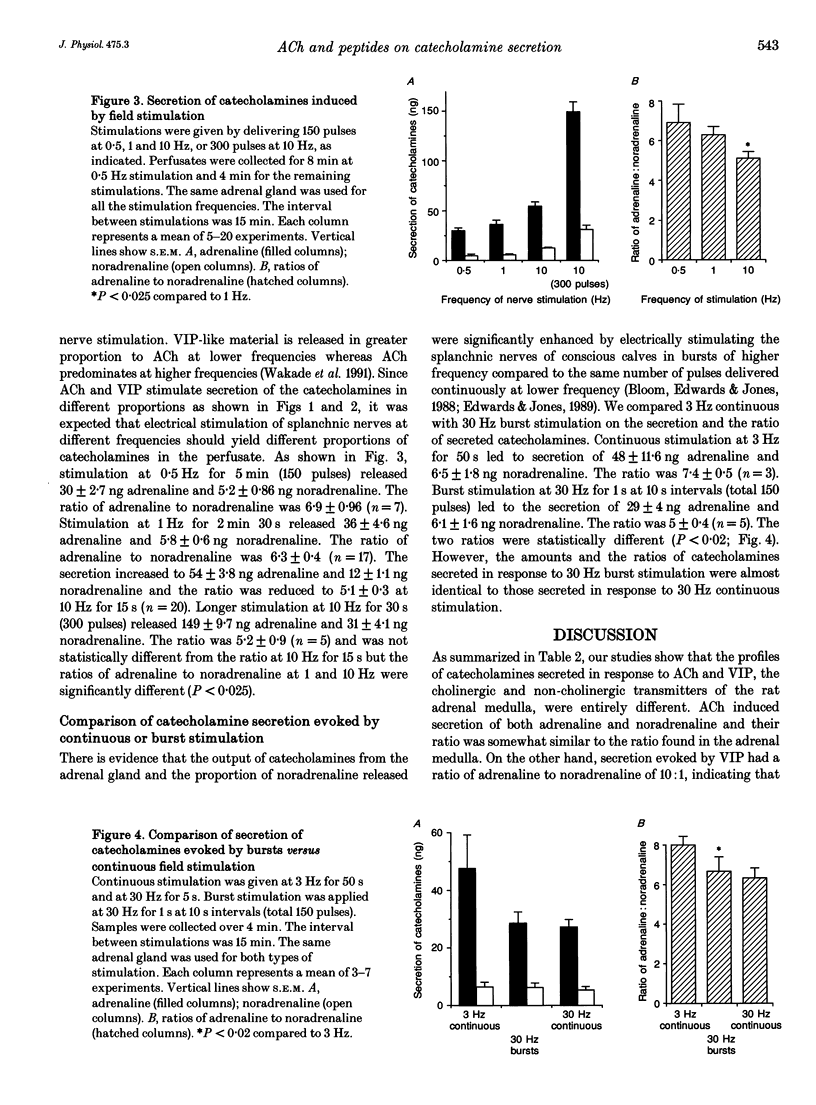
1. Rat adrenal medulla is stimulated by cholinergic and peptidergic transmitters released from splanchnic nerves. The peptidergic transmitter has been identified as vasoactive intestinal polypeptide (VIP) and its contribution in comparison to that of acetylcholine (ACh) is more prominent at low neuronal activity. The purpose of this study is to determine if ACh and VIP cause differential secretion of adrenaline and noradrenaline and whether the differential secretion also occurs when splanchnic nerves are stimulated at different frequencies. 2. Perfusion of the left adrenal gland with Krebs solution for several hours did not change adrenaline and noradrenaline contents (15.2 micrograms and 3.5 micrograms, respectively) and their ratio (4.4) from those of the unperfused right adrenal medulla (15.2 micrograms, 3.3 micrograms and 4.8, respectively). 3. Perfusion with ACh (10 microM for 4 min) resulted in the secretion of 109 ng of catecholamines and the ratio of adrenaline to noradrenaline was 3.8. Although the secretion increased with increased concentrations of ACh (30 and 100 microM), the ratios remained between 3 and 4. 4. Perfusion with VIP (10 microM for 4 min) resulted in the secretion of 27 ng of catecholamines and the ratio of adrenaline to noradrenaline was 9.7. A higher concentration of VIP (20 microM for 4 min) resulted in the secretion of greater amounts of catecholamines (102 ng) without significantly altering the ratio of adrenaline to noradrenaline (10.9). 5. Perfusion with as low as 0.01 microM pituitary adenylate cyclase-activating polypeptide (PACAP) increased the secretion of catecholamines to 31 ng and the secretion increased in a dose-dependent manner up to 0.3 microM.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

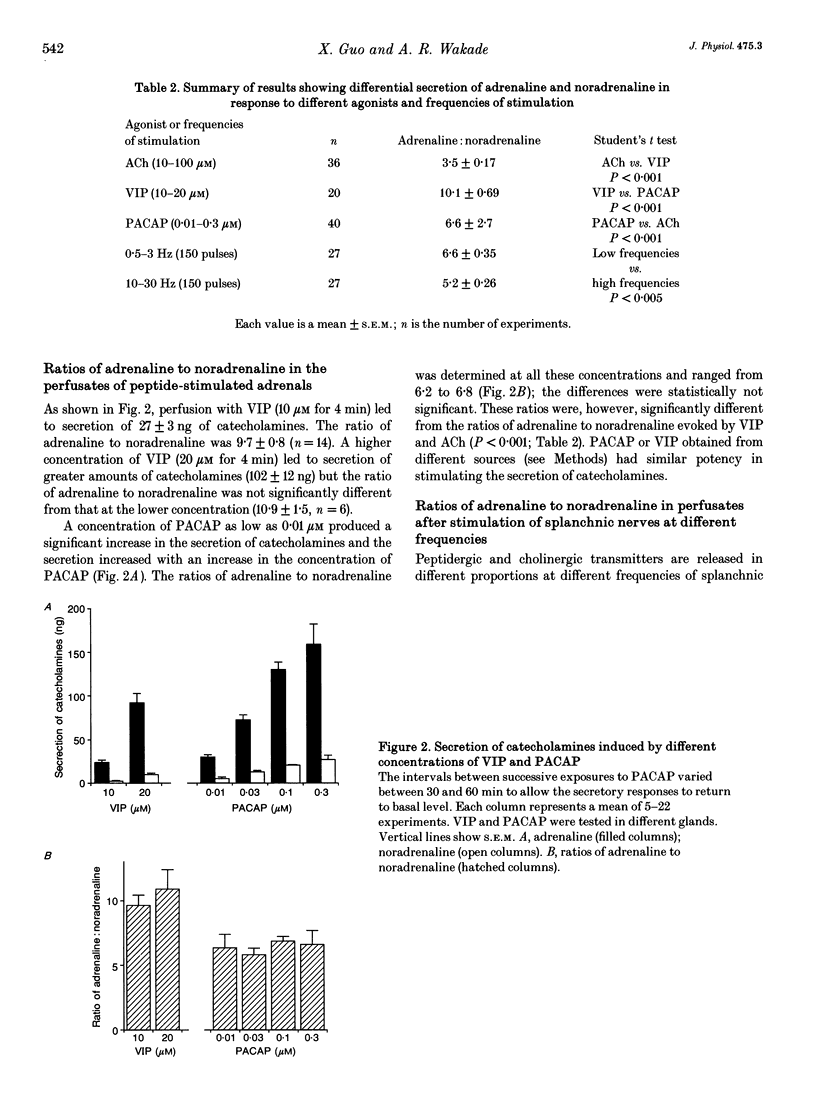


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimura A., Somogyvári-Vigh A., Miyata A., Mizuno K., Coy D. H., Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991 Nov;129(5):2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Jones C. T. The adrenal contribution to the neuroendocrine responses to splanchnic nerve stimulation in conscious calves. J Physiol. 1988 Mar;397:513–526. doi: 10.1113/jphysiol.1988.sp017016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUPLAND R. E., PYPER A. S., HOPWOOD D. A METHOD FOR DIFFERENTIATING BETWEEN NORADRENALINE- AND ADRENALINE-STORING CELLS IN THE LIGHT AND ELECTRON MICROSCOPE. Nature. 1964 Mar 21;201:1240–1242. doi: 10.1038/2011240b0. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Preferential release of adrenaline from the adrenal medulla by muscarine and pilocarpine. Nature. 1965 Dec 11;208(5015):1102–1103. doi: 10.1038/2081102a0. [DOI] [PubMed] [Google Scholar]
- ERANKO O., RAISANEN L. Adrenaline and noradrenaline in the adrenal medulla during postnatal development of the rat. Endocrinology. 1957 Jun;60(6):753–760. doi: 10.1210/endo-60-6-753. [DOI] [PubMed] [Google Scholar]
- Edwards A. V., Jones C. T. Adrenal responses to splanchnic nerve stimulation in conscious calves given naloxone. J Physiol. 1989 Nov;418:339–351. doi: 10.1113/jphysiol.1989.sp017844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Minz B., Tsudzimura H. The mechanism of the nervous discharge of adrenaline. J Physiol. 1934 Jun 9;81(3):286–304. doi: 10.1113/jphysiol.1934.sp003136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein G., Gutman Y. Preferential secretion of adrenaline or noradrenaline by the cat adrenal in vivo in response to different stimuli. Br J Pharmacol. 1971 Dec;43(4):764–775. doi: 10.1111/j.1476-5381.1971.tb07212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M., Fuxe K., Hökfelt T., Joh T. H. Immunohistochemical studies on phenylethanolamine-N-methyltransferase, dopa-decarboxylase and dopamine- -hydroxylase. Experientia. 1971 Aug;27(8):951–952. doi: 10.1007/BF02135767. [DOI] [PubMed] [Google Scholar]
- Khalil Z., Livett B. G., Marley P. D. The role of sensory fibres in the rat splanchnic nerve in the regulation of adrenal medullary secretion during stress. J Physiol. 1986 Jan;370:201–215. doi: 10.1113/jphysiol.1986.sp015930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R. K., Wakade A. R. Non-cholinergic component of rat splanchnic nerves predominates at low neuronal activity and is eliminated by naloxone. J Physiol. 1987 Feb;383:639–652. doi: 10.1113/jphysiol.1987.sp016434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R. K., Wakade T. D., Wakade A. R. Cross-communication between acetylcholine and VIP in controlling catecholamine secretion by affecting cAMP, inositol triphosphate, protein kinase C, and calcium in rat adrenal medulla. J Neurosci. 1989 Dec;9(12):4150–4157. doi: 10.1523/JNEUROSCI.09-12-04150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley P. D., Bunn S. J., Wan D. C., Allen A. M., Mendelsohn F. A. Localization of angiotensin II binding sites in the bovine adrenal medulla using a labelled specific antagonist. Neuroscience. 1989;28(3):777–787. doi: 10.1016/0306-4522(89)90022-5. [DOI] [PubMed] [Google Scholar]
- Marley P. D., Livett B. G. Differences between the mechanisms of adrenaline and noradrenaline secretion from isolated, bovine, adrenal chromaffin cells. Neurosci Lett. 1987 Jun 1;77(1):81–86. doi: 10.1016/0304-3940(87)90611-2. [DOI] [PubMed] [Google Scholar]
- Miyata A., Arimura A., Dahl R. R., Minamino N., Uehara A., Jiang L., Culler M. D., Coy D. H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989 Oct 16;164(1):567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Rubin R. P., Miele E. A study of the differential secretion of epinephrine and norepinephrine from the perfused cat adrenal gland. J Pharmacol Exp Ther. 1968 Nov;164(1):115–121. [PubMed] [Google Scholar]
- Schultzberg M., Andersson C., Undén A., Troye-Blomberg M., Svenson S. B., Bartfai T. Interleukin-1 in adrenal chromaffin cells. Neuroscience. 1989;30(3):805–810. doi: 10.1016/0306-4522(89)90171-1. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Görcs T. J., Gottschall P. E., Arimura A. Two high affinity binding sites for pituitary adenylate cyclase-activating polypeptide have different tissue distributions. Endocrinology. 1991 Jun;128(6):3055–3065. doi: 10.1210/endo-128-6-3055. [DOI] [PubMed] [Google Scholar]
- Tischler A. S., Perlman R. L., Nunnemacher G., Morse G. M., DeLellis R. A., Wolfe H. J., Sheard B. E. Long-term effects of dexamethasone and nerve growth factor on adrenal medullary cells cultured from young adult rats. Cell Tissue Res. 1982;225(3):525–542. doi: 10.1007/BF00214802. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Durbin J., Coupland R. E. A quantitative analysis of rat adrenal chromaffin tissue: morphometric analysis at tissue and cellular level correlated with catecholamine content. Neuroscience. 1987 Mar;20(3):895–904. doi: 10.1016/0306-4522(87)90250-8. [DOI] [PubMed] [Google Scholar]
- Wakade A. R. Facilitation of secretion of catecholamines from rat and guinea-pig adrenal glands in potassium-free medium or after ouabain. J Physiol. 1981;313:481–498. doi: 10.1113/jphysiol.1981.sp013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade A. R. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981;313:463–480. doi: 10.1113/jphysiol.1981.sp013676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Biochemical evidence for re-use of noradrenergic storage vesicles in the guinea-pig heart. J Physiol. 1982 Jun;327:337–362. doi: 10.1113/jphysiol.1982.sp014235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience. 1983 Nov;10(3):973–978. doi: 10.1016/0306-4522(83)90235-x. [DOI] [PubMed] [Google Scholar]
- Wakade T. D., Blank M. A., Malhotra R. K., Pourcho R., Wakade A. R. The peptide VIP is a neurotransmitter in rat adrenal medulla: physiological role in controlling catecholamine secretion. J Physiol. 1991 Dec;444:349–362. doi: 10.1113/jphysiol.1991.sp018882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Masuo Y., Matsumoto H., Suzuki N., Ohtaki T., Masuda Y., Kitada C., Tsuda M., Fujino M. Pituitary adenylate cyclase activating polypeptide provokes cultured rat chromaffin cells to secrete adrenaline. Biochem Biophys Res Commun. 1992 Jan 15;182(1):403–411. doi: 10.1016/s0006-291x(05)80159-7. [DOI] [PubMed] [Google Scholar]


