Abstract
Background and Objectives
Biologics that target pathogenic antibodies (Abs) and their effector functions such as the complement inhibitor ravulizumab and the neonatal Fc receptor agonist efgartigimod have recently been approved for the treatment of acetylcholine receptor (AChR)-Ab–positive myasthenia gravis (MG), but comparative studies are lacking.
Methods
In a prospective, exploratory real-world study, we assessed clinical efficacy, safety, and biological effects of ravulizumab and efgartigimod treatment initiation. Myasthenia Gravis-Activities of Daily Living and Quantitative Myasthenia Gravis scores were used as clinical endpoints. Ab effector functions were determined by AChR-Ab–dependent complement activation and phagocytosis assays and systemic complement activation profiling.
Results
We observed similar moderate short-term efficacy of ravulizumab and efgartigimod in achieving clinical improvement. Ravulizumab reduced systemic terminal complement activation, but neither treatment showed significant effects on complement pathways proximal to C5 or functional capacities of AChR-Abs. Both treatment modalities were well tolerated with no serious adverse events reported.
Discussion
Clinical benefits obtained with ravulizumab and efgartigimod can be remarkably heterogeneous in daily clinical practice. Neither treatment relevantly changed effector functions of pathogenic AChR-Abs, supporting the concept that durable disease control in MG requires continuous administration of both fast-acting agents.
Classification of Evidence
This study provides Class III evidence that in AChR-Ab–positive patients with generalized MG, ravulizumab and efgartigimod provide comparable modest improvement in MG functional scales.
Introduction
Myasthenia gravis (MG) is an autoimmune disease characterized by localized or general muscle weakness, which is caused by immunoglobulin G (IgG) antibodies (Abs) binding to acetylcholine receptors or functionally related molecules in the postsynaptic membrane at the neuromuscular junction.1 Recently approved treatment options specifically targeting Ab-mediated disease mechanisms include ravulizumab, targeting the terminal complement component C5,2,3 and the neonatal Fc receptor (FcRn) modulator efgartigimod, which enhances degradation of endogenous IgG including AChR-specific Abs.4 Both therapies led to sustained meaningful improvement in the MG-specific Activities of Daily Living scale (Myasthenia Gravis-Activities of Daily Living [MG-ADL]), the primary end point in pivotal placebo-controlled phase III clinical trials.3,4
Although diagnosis in patients with typical symptoms and a positive Ab test is usually unambiguous, patients present remarkably heterogeneous in terms of disease phenotype, clinical course, and response to immunotherapy. This study aimed to estimate clinical efficacy of ravulizumab and efgartigimod and to profile their biological effects on auto-Ab features in a real-world cohort of patients with AChR-Ab+ generalized MG (gMG).
Methods
Patients and Study Design
Patients with AChR-Ab–positive gMG were recruited at the Departments of Neurology at the Charité- Universitätsmedizin Berlin and the Universitätsklinikum Münster. Both are certified German “Integrated MG Centers of Excellence”, accredited by the German MG Society. Diagnosis of MG was based on international guidelines.5,6 All patients were treatment-naïve for either ravulizumab or efgartigimod at study entry. Both sites used identical protocols for the collection of demographic and clinical data and for sample processing and storing at −20°C. MG-related clinical scores were prospectively assessed at baseline and at follow-up using the MG-ADL scale7 and quantitative MG (QMG) score8 for disease severity. Patients receiving ravulizumab were followed up at weeks 2 and 10, and patients receiving efgartigimod were followed up at weeks 4 and 8 after treatment initiation to assess immediate treatment benefits of these fast-acting therapies.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was conducted according to the Declaration of Helsinki and approved by the Ethics Committees of the University of Münster (registration nos. 2010-262-f-S, 2011-665-f-S, 2013-350-f-S, 2014-068-f-S and 2016-053-f-S) and Charité—Universitätsmedizin Berlin (EA1/281/10); written informed consent was given by all participants.
Complement Factor Profiling
We used a previously described, established multiplex ELISA based on chemiluminescence according to the manufacturer's recommendations Quidel, San Diego, cat. Number: A910.9,10 Data were obtained with Imager L from Quansys, using Q-View Software 3.11 for analysis.
AChR-specific Ab-dependent Complement Deposition and Ab-Dependent Cellular Phagocytosis
Both functional assays were performed as reported previsouly11 (please, see supplemental material for details) using purified recombinant human AChR extracellular domain α-subunit (rhAChRECD).12
Determination of Total IgG Levels
Quantitative detection of total IgG was measured by ELISA according to the manufacturer's recommendations (Fisher Scientific—Invitrogen, Schwerte, Germany, cat. Number: 88-50550).
Statistics
Continuous data are presented as median and interquartile range (IQR) and categorical variables as absolute frequencies and percentages. Differences regarding patient-reported clinical improvement measured with MG-ADL and QMG are displayed as median and median change with IQR and as mean along with 95% CI. Adjusted effect estimates are derived from linear regression adjusted for sex and age. Analyses of antibody features' changes over time were performed with the Wilcoxon signed-rank test. Owing to the exploratory nature of this study, p values were not corrected for multiple testing. Safety was assessed through incidence of adverse events in a descriptive manner. All statistical analyses were performed using R13 and additional R packages.14,15 The study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.
Data Availability
The data that support the findings of this study are available from the corresponding author, on reasonable request.
Results
A total of 41 patients with AChR-Ab–positive gMG starting with ravulizumab and 21 patients starting with efgartigimod were prospectively included between January 2023 and December 2023 (Table 1). At study entry, all patients were treatment-naïve for either ravulizumab or efgartigimod. Clinical severity as assessed by MG-ADL and QMG scores and Myasthenia Gravis Foundation of America (MGFA) status at baseline was comparable between both groups. Most had moderate disease severity (MGFA II 29% [n = 18], MGFA III 63% [n = 39], MGFA IV 8% [n = 5]) and most patients received steroids and/or standard immunosuppressive therapy at baseline. Most patients (63% [n = 38]) met the criteria for treatment refractory status according to the randomized controlled trial of eculizumab in refractory, AChR-Ab–positive, generalized myasthenia gravis (REGAIN)16: MG-ADL score at baseline of ≥6 and a history of 2 or more immunosuppressive therapies or MG-ADL score at baseline of ≥6 and at least one immunosuppressive therapy with IVIg or plasma exchange given at least 4 times per year, for 12 months without symptom control.
Table 1.
Baseline Demographic and Clinical Characteristics
| Efgartigimod (n = 21) | Ravulizumab (n = 41) | Total (N = 62) | |
| Age (y), median (IQR) | 61.00 (43.00–73.00) | 69.00 (47.00–77.00) | 64.00 (44.00–75.50) |
| Early-onset myasthenia gravis, n (%) | 7 (33.3) | 16 (39.0) | 23 (37.1) |
| Sex, n (%) | |||
| Female | 12 (57.1) | 27 (65.9) | 39 (62.9) |
| Male | 9 (42.9) | 14 (34.1) | 23 (37.1) |
| Weight (kg), median (IQR) | 77.00 (65.00–87.00) | 77.00 (60.00–88.00) | 77.00 (62.00–87.75) |
| Disease duration (y), median (IQR) | 4.00 (2.00–6.00) | 7.00 (3.00–10.00) | 5.00 (2.00–9.75) |
| MGFA class at screening, n (%) | |||
| II | 8 (38.1) | 10 (24.4) | 18 (29.0) |
| III | 12 (57.1) | 27 (65.9) | 39 (62.9) |
| IV | 1 (4.8) | 4 (9.8) | 5 (8.1) |
| Previous thymectomy, n (%) | 13 (61.9) | 25 (61.0) | 38 (61.3) |
| Thymoma, n (%) | 2 (11.8) | 7 (19.4) | 9 (17.0) |
| n missing | 4 | 5 | 9 |
| History of myasthenic crisis, n (%) | 12 (57.1) | 24 (58.5) | 36 (58.1) |
| Total MG-ADL score, median (IQR) | 10.00 (8.00–11.00) | 9.00 (7.00–12.00) | 10.00 (7.00–12.00) |
| n missing | 0 | 2 | 2 |
| Total QMG score, median (IQR) | 14.00 (10.00–17.00) | 14.50 (11.00–19.25) | 14.00 (11.00–18.00) |
| n missing | 0 | 1 | 1 |
| Total MG-QOL15r score, median (IQR) | 37.00 (31.00–40.00) | 22.50 (19.00–26.50) | 25.00 (19.50–36.50) |
| n missing | 8 | 11 | 19 |
| At least one previous IST, n (%) | 21 (100) | 41 (100) | 62 (100) |
| Myasthenia gravis therapy at baseline, n (%) | |||
| Any steroid | 12 (63.2) | 26 (63.4) | 38 (63.3) |
| n missing | 2 | 0 | 2 |
| Any immunosuppressive therapy | 18 (85.7) | 27 (65.9) | 45 (72.6) |
| Rituximab | 0 (0.0) | 5 (12.2) | 5 (8.1) |
| Steroid and any immunosuppressive therapy | 10 (52.6) | 20 (48.8) | 30 (50.0) |
| No steroid and no immunosuppressive therapy | 1 (4.8) | 8 (19.5) | 9 (14.5) |
| Refractory MG at baselinea, n (%) | 15 (71.4) | 23 (59.0) | 38 (63.3) |
| n missing | 0 | 2 |
Abbreviations: AChR = acetylcholine receptor; IQR = interquartile range; IST = immunosuppressive therapy; MG-ADL = MG specific activity of daily life score; MGFA = Myasthenia Gravis Foundation of America classification; MG-QoL15 = MG-Specific Quality of Life Score; QMG = Quantitative MG Score.
Reported are median (IQR) for continuous variables and n (%) for categorical variables. Disease duration is the time from diagnosis until baseline. The data on the thymoma findings refer to the number of patients in the respective group, regardless of whether a thymectomy was performed. We cannot exclude the possibility that a thymoma was present in the non-thymectomized patients, but there was no evidence of a thymoma on the CT scan of the thorax.
Immunotherapy refractory status was defined according to the randomized controlled phase 3 trial of eculizumab in refractory, AChR-Ab-positive, generalized MG (REGAIN): diagnosis of a generalized AChR-Ab–positive MG with a MG-ADL score at baseline of >5 and a history of either 1 2 or more immunosuppressive therapies, or 2 at least one immunosuppressive therapy with IVIg or plasma exchange given at least 4 times per year, for 12 months without symptom control.
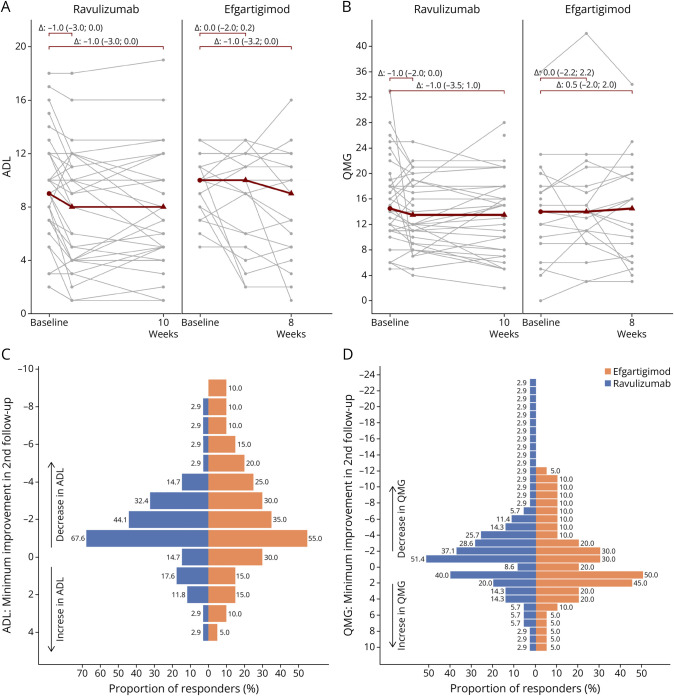
Treatment initiation was associated with moderate, although for all patients not clinically meaningful, improvement in MG-ADL scores assessed at the follow-up visits (week 10 for ravulizumab, mean difference −1.35 [95% CI −2.15 to −0.55]; weeks 4 and 8 for efgartigimod, mean difference −1.10 [95% CI −2.48 to 0.28] and −1.65 [95% CI −3.28 to −0.02], respectively; Figure 1A). QMG scores obtained at the follow-up visits slightly decreased from baseline levels for ravulizumab, but not for efgartigimod (ravulizumab: mean difference −1.06 [95% CI −2.86 to 0.75]; efgartigimod: mean difference 0.45 [95% CI −1.39 to 2.29] at weeks 4 and 0.05 [95% CI −2.28 to 2.38] at week 8) (Figure 1B). Effect estimates adjustment for age and sex did not provide any evidence for a superior efficacy of one of the 2 treatments (eTable 1). A clinically meaningful improvement, defined as a reduction of at least 2 points on the MG-ADL scale or at least 3 points on the QMG scale, was observed in 44% (MG-ADL) and 29% (QMG) of ravulizumab-treated patients compared with 35% (MG-ADL) and 20% (QMG) of efgartigimod-treated patients (Figure 1, C and D). Minimal manifestation status as defined by MG-ADL ≤2 was achieved in 14% in ravulizumab and 15% in efgartigimod patients. Both treatment modalities were well tolerated with no serious adverse events reported.
Figure 1. Clinical Response to Ravulizumab and Efgartigimod in Patients With AChR-Ab–Positive gMG.
(A) Myasthenia gravis activities of daily living (MG-ADL) score in patients treated with ravulizumab (baseline, week 2, week 10) or efgartigimod (baseline, week 4, week 8). Indicated in gray are individual patients. Indicated in red are the median at baseline, median differences for follow-up along with the interquartile range; (B) Quantitative Myasthenia Gravis (QMG) score in individual patients treated with ravulizumab (baseline, week 2, week 10) or efgartigimod (baseline, week 4, week 8). (C) Bar chart for the distribution of minimal improvement in MG-ADL score from baseline to second follow-up (week 8 or week 10), separately for ravulizumab-treated and efgartigimod-treated patients. (D) Bar chart for the distribution of minimal improvement in QMG score from baseline to second follow-up (week 8 or week 10), separately for ravulizumab-treated and efgartigimod-treated patients. AChR-Ab = acetylcholine receptors-Ab; gMG = generalized MG.
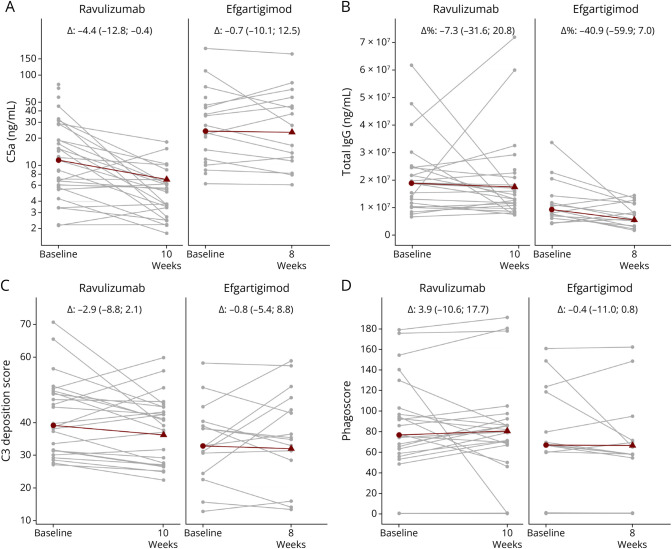
We next assessed the biological efficacy of both treatment modalities in restraining pathogenic Ab functions such as complement activation. Follow-up samples have been available for 24 ravulizumab-treated and 15 efgartigimod-treated patients. Quantification of serum complement factors C3a, C5a, and sC5b9, indicating general activation of the complement system, whether via the classical and lectin pathways (C4a) or the alternative pathway (factors Ba and Bb), revealed a significant decrease in C5a (median change: −4.4, p = 0.0002) (Figure 2A) and sC5b9 (median change: −189, p = 0.009) on treatment initiation with ravulizumab but not in patients receiving efgartigimod (C5a: −0.7, p = 0.762; sC5b9: +162, p = 0.762; eFigure 1). In patients treated with efgartigimod, total serum IgG concentrations were significantly reduced by a median of 41% (mean 22%) at week 8 compared with baseline levels (Figure 2B). The extent of IgG reduction at week 8 corresponds to what was previously observed in the randomized controlled phase 3 clinical trial on efgartigimod in MG.4 IgG levels in ravulizumab-treated patients only decreased to a minor extent by a median of 7% (Figure 2B). Treatment initiation with either ravulizumab or efgartigimod was not associated with statistically significant changes in functional capacities of isolated AChR-specific Abs to induce Ab-dependent complement deposition (ADCD) (Figure 2C) and Ab-dependent and cellular Fc receptor-mediated phagocytosis (ADCP) (Figure 2D). Thus, this study provides Class III evidence that in AChR-Ab-positive with gMG, ravulizumab and efgartigimod provide comparable modest improvement in MG functional scales.
Figure 2. Profiling Activated Complement Protein and IgG Levels Along With AChR-Specific Antibody Effector Functions on Treatment Initiation With Ravulizumab and Efgartigimod.
(A) Serum complement C5a and (B) total igG protein levels were quantified at baseline, at 10 weeks after ravulizumab therapy, and 8 weeks after initiation of efgartigimod therapy. Only ravulizumab-treated patients presented with a significant decrease in terminal complement factors C5a (p = 0.00024). Only efgartigimod-treated patients presented with a significant decrease in total IgG levels (p = 0.03). Mean relative difference for total IgG levels in efgartigimod-treated patients is −22.0 and for ravulizumab-treated patients is 8.2. (C) Antibody-dependent complement deposition and (D) phagocytosis induced by isolated AChR-specific IgG quantified at baseline, at 10 weeks after ravulizumab therapy, and 8 weeks after initiation of efgartigimod therapy. Indicated in gray are individual patients. Indicated in red are the median at baseline and median differences for follow-up along with the interquartile range. AChR = acetylcholine receptor; IgG = immunoglobulin G.
Discussion
Our study indicates similar moderate short-term efficacy of ravulizumab and efgartigimod in achieving clinical improvement in a real-world cohort of patients with AChR-Ab–positive MG. Ravulizumab treatment substantially reduced circulating levels of activated terminal complement components, i.e., C5a and sC5b9, with no statistically significant effects on complement activation proximal to C5 and the functional capacity of AChR-Abs to activate complement. Efgartigimod significantly reduced total serum IgG concentrations, in line with previous observations,4 but did not relevantly affect circulating complement protein levels and was not associated with relevant changes in AChR-Ab effector functions.
Clinically meaningful improvement as defined by a decrease of at least 2 on the MG-ADL scale was achieved for more than 60% of patients in pivotal phase 3 studies after 26 responses in 8 weeks that led to the approval of ravulizumab17 and efgartigimod.4 MG-ADL–based response rates in our real-world cohorts were considerably lower, and clinical efficacy assessed by the physician-reported QMG scale did not reach statistical significance in either treatment group after 4, 8, and 10 weeks. Compared with aforementioned randomized controlled clinical trials, cohort sizes in our exploratory study were smaller, particularly in the efgartigimod-treated cohort, and follow-up time points were different. Moreover, most of the patients included in our study had difficult-to-treat MG, fulfilling the REGAIN criteria for being immunotherapy refractory.16 In the REGAIN study and its open-label extension, although most patients with refractory MG showed a response within the first 12 weeks of treatment, some took longer to respond and the response rate gradually increased from week 12 to after week 26.18 Thus, enrichment for patients with difficult-to-treat MG and a relatively short observation period in our study might have contributed to the fact that the pharmacologic mechanism of actions did not sufficiently translate into clinical efficacies, and we cannot exclude the possibility that more patients included in our study would have shown first responses after longer‐term treatment. Although these factors might limit the overall significance of our findings, data obtained support the notion that the response to one of the 2 therapies can be remarkably heterogeneous in daily clinical practice, highlighting the need for treatment response-predicting biomarkers.
Complement activation by AChR-specific Abs is thought to be an important pathomechanism in MG and circulating levels of activated complement proteins are prominently increased in patients with AChR-Ab MG.9 The marked downregulation of C5a and sC5bC9 levels on ravulizumab initiation is in line with the immediate complete inhibition of serum free C5 observed in the pivotal CHAMPION MG study which led to approval of ravulizumab for the treatment of AChR-Ab–positive MG.19
Neither treatment relevantly changed effector functions of pathogenic AChR-Abs nor exhibited relevant effects on complement activation proximal to C5, supporting the concept that both therapies require continuous administration and that pausing or discontinuation are likely associated with high risk of disease worsening. Whether inhibition of the classical or alternative pathway proximal to C5 can provide additional benefit to what can be achieved with currently approved biologicals requires further investigation.
Limitations of our study include the relatively small sample size and short observation period. Although sustained clinical benefits and discovery of treatment response-predictive biomarkers require further investigations in larger real-world cohorts, our observations suggest that of both fast-acting agents might require combination with more deeply and sustainably intervening immunotherapies to qualitatively affect AChR-Ab effector functions and to improve long-term outcomes for patients with AChR-Ab–positive MG.
Acknowledgment
The authors thank Kerstin Stein for expert technical assistance. Additionally, the authors thank our coworkers of the Neuroscience Clinical Research Center Claudia Heibutzki, Dike Remstedt, Jens Bestrich, Gabriele Nieweiler, Daniela Kodalla, and Sophia Schiemann for patient management of the MG department and Nora Seelig for management of serum samples. The authors acknowledge support by the German Research Foundation Grant Numbers LU 900/3-1, LU 900/4-1 and SFB-TR128/Teilprojekt A11—Collaborative Research Centre TR-128 ‘Initiating/Effector vs Regulatory Mechanisms in Multiple Sclerosis—Progress towards Tackling the Disease' to J.D. Lunemann.; Grant Number ME 1562/6-1 in the Clinical Research Group 5023 “BECAUSE-Y Berlin Center for Diagnosis, Understanding and Treatment of Antibody(Y)-mediated Neurological Diseases” to A. Meisel; funding support by the Einstein Foundation Berlin (EVF-BUA-2022-694) to A. Aigner; the Innovative Medizinische Forschung (IMF) and der Medizinischen Universität Münster (Grant Number SO 112305, to C.S.).
Glossary
- Abs
antibodies
- AChR
acetylcholine receptor
- gMG
generalized MG
- IgG
immunoglobulin G
- IQR
interquartile range
- MG-ADL
Myasthenia Gravis-Activities of Daily Living
- MG
myasthenia gravis
- MGFA
Myasthenia Gravis Foundation of America classification
- QMG
quantitative MG
Appendix. Authors
| Name | Location | Contribution |
| Frauke Stascheit, MD | Department of Neurology with Experimental Neurology, Neuroscience Clinical Research Center, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt Universität zu Berlin, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Carla Daiane Ferreira de Sousa, PhD | Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Annette Aigner, PhD | Institute of Biometry and Clinical Epidemiology, Center for Stroke Research Berlin, Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, Germany | Analysis or interpretation of data |
| Malina Behrens, MSc | Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Christian W. Keller, MD, PhD | Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Luisa Klotz, MD | Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Sophie Lehnerer, MD | Department of Neurology with Experimental Neurology, Neuroscience Clinical Research Center, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt Universität zu Berlin, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Maike Stein, MD | Department of Neurology with Experimental Neurology, Neuroscience Clinical Research Center, Center for Stroke Research Berlin, Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Meret Herdick, MD | Department of Neurology with Experimental Neurology, Neuroscience Clinical Research Center, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt Universität zu Berlin, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Paolo Doksani, MD | Department of Neurology with Experimental Neurology, Neuroscience Clinical Research Center, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt Universität zu Berlin, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Lea M. Gerischer, MD | Department of Neurology with Experimental Neurology, Neuroscience Clinical Research Center, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt Universität zu Berlin, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Sarah Hoffmann, MD | Department of Neurology with Experimental Neurology, Neuroscience Clinical Research Center, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt Universität zu Berlin, Germany | Major role in the acquisition of data; analysis or interpretation of data |
| Konstantinos Lazaridis, PhD | Department of Immunology, Hellenic Pasteur Institute, Athens, Greece | Major role in the acquisition of data |
| John Tzartos, MD | 2nd Neurology Department, School of Medicine, “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece | Major role in the acquisition of data |
| Heinz Wiendl, MD | Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Andreas Meisel, MD | Department of Neurology with Experimental Neurology, Neuroscience Clinical Research Center, Center for Stroke Research Berlin, Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Jan D. Lünemann, MD | Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
DFG - LU 900/3-1, LU 900/4-1 and SFB-TR128/Teilprojekt A11 Grant Number ME 1562/6-1 in the Clinical Research Group 5023 “BECAUSE-Y Berlin Center for Diagnosis, Understanding and Treatment of Antibody(Y)-mediated Neurologic Diseases” Innovative Medizinische Forschung (IMF) and der Medizinischen Universität Münster (Grant Number SO 112305, to C.S.). Einstein Foundation Berlin (EVF-BUA-2022-694).
Disclosure
F. Stascheit received travel/accommodation/meeting expenses from Alexion Pharmaceuticals and argnx and received speaking honoria and honoria for attendance at advisory boards from Alexion Pharmaceuticals, argnx and UCB pharma; A. Aigner and M. Behrens reports no conflict of interests; C.W. Keller received travel, accommodation and meeting expenses from Alexion and UCB; L. Klotz received speaking honoraria and travel/meeting expenses from Argenx, Bayer, Biogen, Bristol-Myers Squibb, Genzyme, Grifols, Merck Serono, Novartis, Roche, Santhera and Teva. She participated in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Genzyme, Horizon, Janssen, Merck Serono, Novartis, Roche, Sandoz and Viatris; S. Lehnerer has received speakers honoraria from Alexion, argenx, Hormosan and UCB, honoraria for attendance at advisory boards from Alexion, argenx, Biogen, UCB and Roche, travel/accommodation/meeting expenses from Alexion Pharmaceuticals and argnx and research funding from Hormosan, Alexion, UCB and argnx; M. Stein has received speaking honoria and honoria for attendance at advisory boards from Argenx and Alexion Pharmaceuticals; M. Herdick has received speaker's honaria from argenx; P. Doksani and L. Gerischer reports no conflict of interest; S. Hoffmann has received speakers' honoraria from Alexion, argenx, UCB, Grifols and Roche, honoraria for attendance at advisory boards from Alexion, argenx and Roche and research funding from argenx and Janssen. S. Hoffmann is member of the medical advisory board of the German Myasthenia Society, DMG. K. Lazaridis reports no conflict of interest; J. Tzartos reports speaking honoria from Astra Zeneca and Medison Pharma; H. Wiendl received speaker honoraria from Alexion, Biogen, Bristol Myers Squibb, Genzyme, Merck, Neurodiem, Novartis, Ology, Roche, TEVA, and WebMD Global. He received honoraria for consulting services from Abbvie, Actelion, Argenx, BD, Bristol Myers Squibb, EMD Serono, Fondazione Cariplo, Gossamer Bio, Idorsia, Immunic, Immunovant, INmune Bio_Syneos Health, Janssen, Merck, NexGen, Novartis, Roche, Sanofi, Swiss MS Society, UCB and Worldwide Clinical Trials. His research is supported by the German Myasthenia Gravis Society; A. Meisel received speaker or consultancy honoraria or financial research support (paid to his institution) from Alexion Pharmaceuticals, argenx, Axunio, Destin, Grifols, Hormosan Pharma, Janssen, Merck, Octapharma, UCB, and Xcenda. He serves as medical advisory board chairman of the German Myasthenia Gravis Society; J.D. Lünemann has received speaker fees, research support, travel support, and/or served on advisory boards by Abbvie, Alexion, Argenx, Biogen, Merck, Moderna, Novartis, Roche, Sanofi and Takeda, and is member of the medical advisory board of the German Myasthenia Gravis Society. Go to Neurology.org/NN for full disclosures.
References
- 1.Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren J. Myasthenia gravis. Nat Rev Dis Primers. 2019;5(1):30. doi: 10.1038/s41572-019-0079-y [DOI] [PubMed] [Google Scholar]
- 2.Dalakas MC. Immunotherapy in myasthenia gravis in the era of biologics. Nat Rev Neurol. 2019;15(2):113-124. doi: 10.1038/s41582-018-0110-z [DOI] [PubMed] [Google Scholar]
- 3.Vu T, Meisel A, Mantegazza R, et al. Summary of research: terminal complement inhibitor ravulizumab in generalized myasthenia gravis. Neurol Ther. 2023;12(5):1435-1438. doi: 10.1007/s40120-023-00514-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard JF Jr., Bril V, Vu T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20(7):526-536. doi: 10.1016/S1474-4422(21)00159-9 [DOI] [PubMed] [Google Scholar]
- 5.Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021;96(3):114-122. doi: 10.1212/WNL.0000000000011124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiendl H, Abicht A, Chan A, et al. Guideline for the management of myasthenic syndromes. Ther Adv Neurol Disord. 2023;16:17562864231213240. doi: 10.1177/17562864231213240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. 1999;52(7):1487-1489. doi: 10.1212/wnl.52.7.1487 [DOI] [PubMed] [Google Scholar]
- 8.Bedlack RS, Simel DL, Bosworth H, Samsa G, Tucker-Lipscomb B, Sanders DB. Quantitative myasthenia gravis score: assessment of responsiveness and longitudinal validity. Neurology. 2005;64(11):1968-1970. doi: 10.1212/01.WNL.0000163988.28892.79 [DOI] [PubMed] [Google Scholar]
- 9.Stascheit F, Chuquisana O, Keller CW, et al. Complement activation profiles in anti-acetylcholine receptor positive myasthenia gravis. Eur J Neurol. 2023;30(5):1409-1416. doi: 10.1111/ene.15730 [DOI] [PubMed] [Google Scholar]
- 10.Spatola M, Chuquisana O, Jung W, et al. Humoral signatures of MOG-antibody-associated disease track with age and disease activity. Cel Rep Med. 2023;4(2):100913. doi: 10.1016/j.xcrm.2022.100913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartsch YC, Wang C, Zohar T, et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat Med. 2021;27(3):454-462. doi: 10.1038/s41591-021-01263-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazaridis K, Zisimopoulou P, Giastas P, et al. Expression of human AChR extracellular domain mutants with improved characteristics. Int J Biol Macromol. 2014;63:210-217. doi: 10.1016/j.ijbiomac.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 13.R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2023. [Google Scholar]
- 14.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1-48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 15.Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. doi: 10.21105/joss.01686 [DOI] [Google Scholar]
- 16.Howard JF Jr., Utsugisawa K, Benatar M, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16(12):976-986. doi: 10.1016/S1474-4422(17)30369-1 [DOI] [PubMed] [Google Scholar]
- 17.Vu T, Meisel A, Mantegazza R, et al. Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. NEJM Evid. 2022;1(5):EVIDoa2100066. doi: 10.1056/EVIDoa2100066 [DOI] [PubMed] [Google Scholar]
- 18.Howard JF Jr., Karam C, Yountz M, O'Brien FL, Mozaffar T, REGAIN Study Group. Long-term efficacy of eculizumab in refractory generalized myasthenia gravis: responder analyses. Ann Clin Transl Neurol. 2021;8(7):1398-1407. doi: 10.1002/acn3.51376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vu T, Ortiz S, Katsuno M, et al. Ravulizumab pharmacokinetics and pharmacodynamics in patients with generalized myasthenia gravis. J Neurol. 2023;270(6):3129-3137. doi: 10.1007/s00415-023-11617-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, on reasonable request.