Abstract
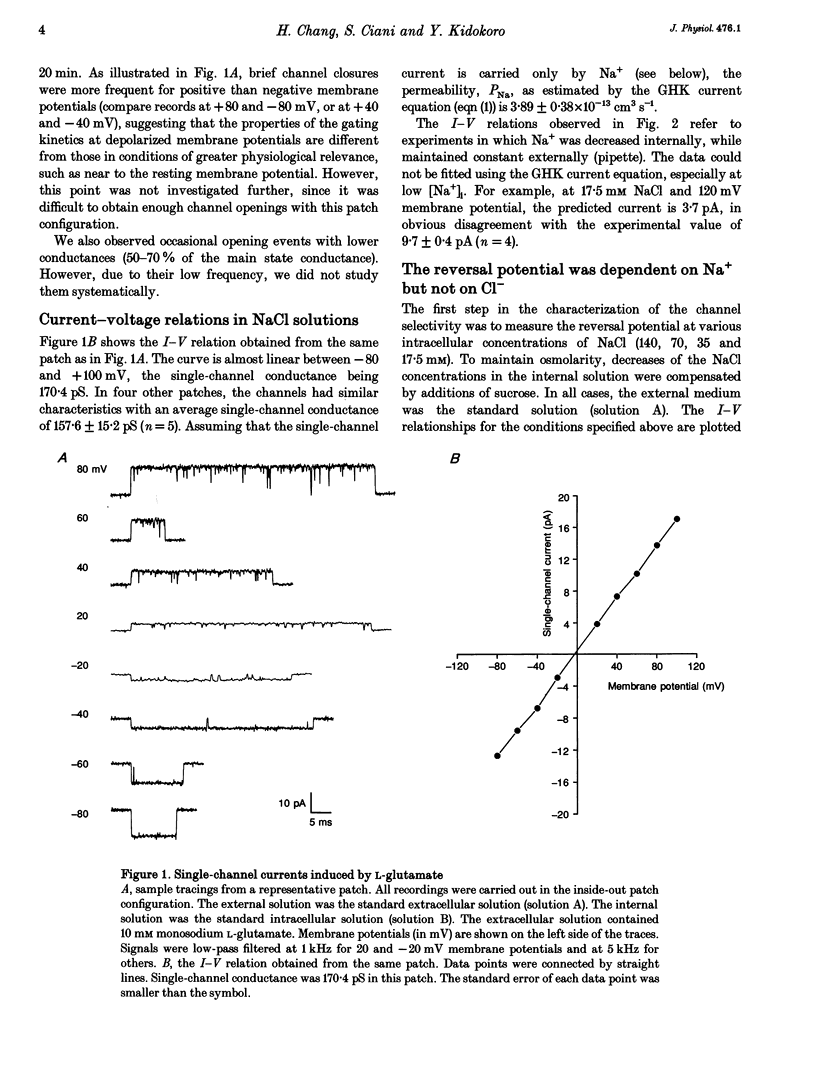
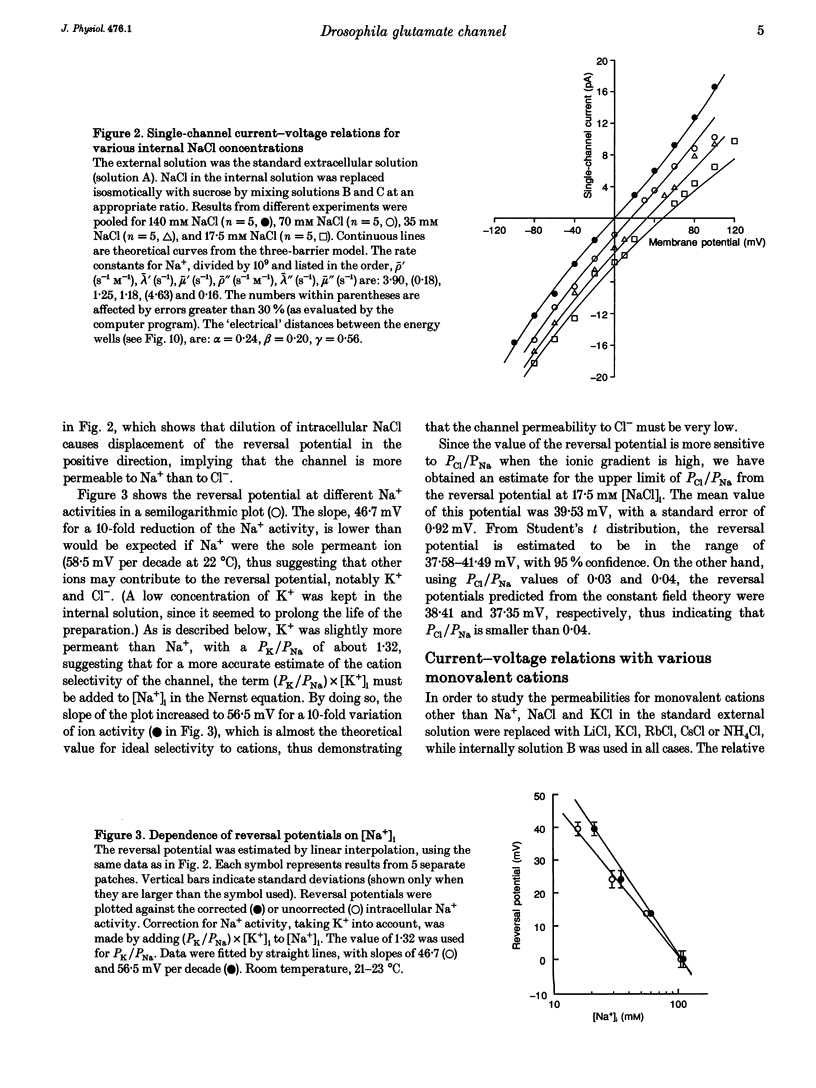
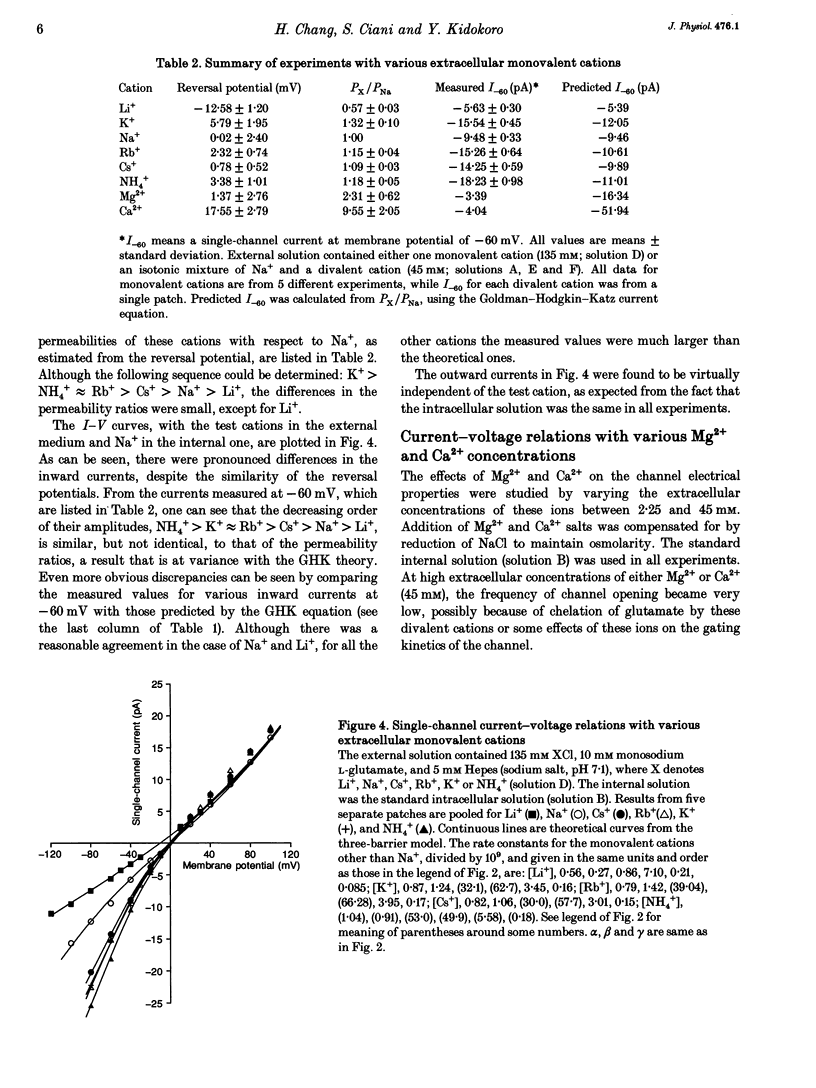
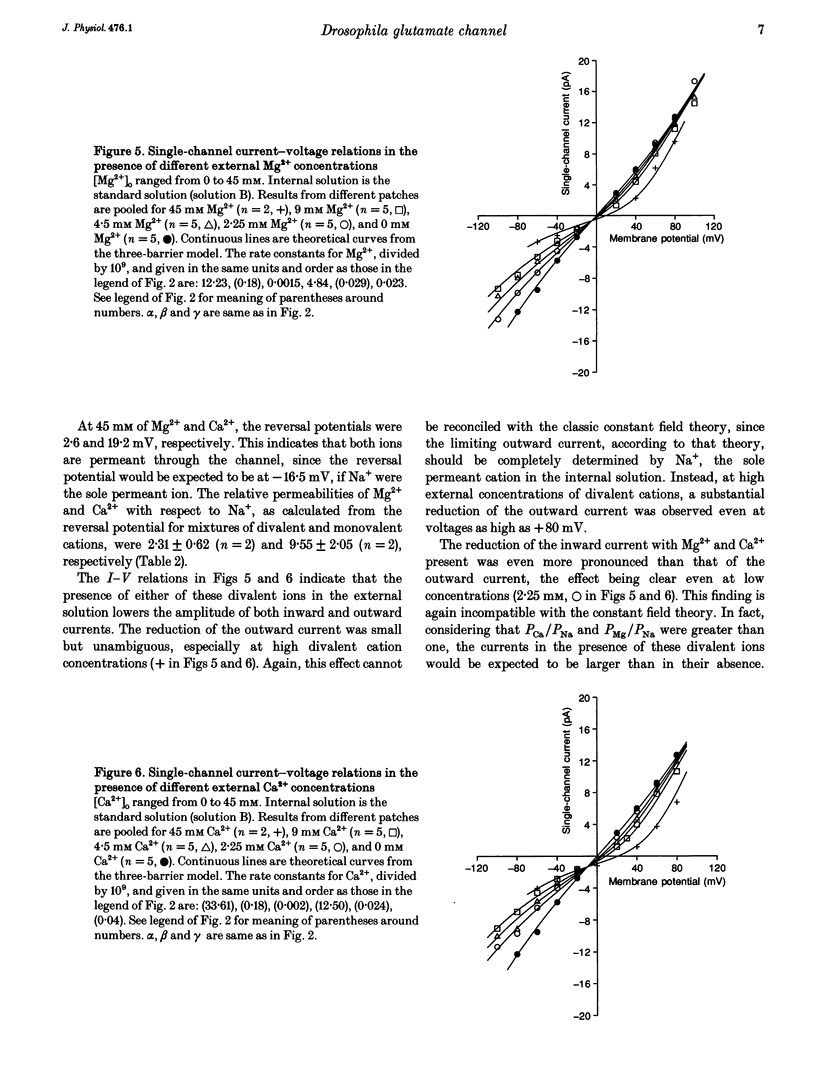
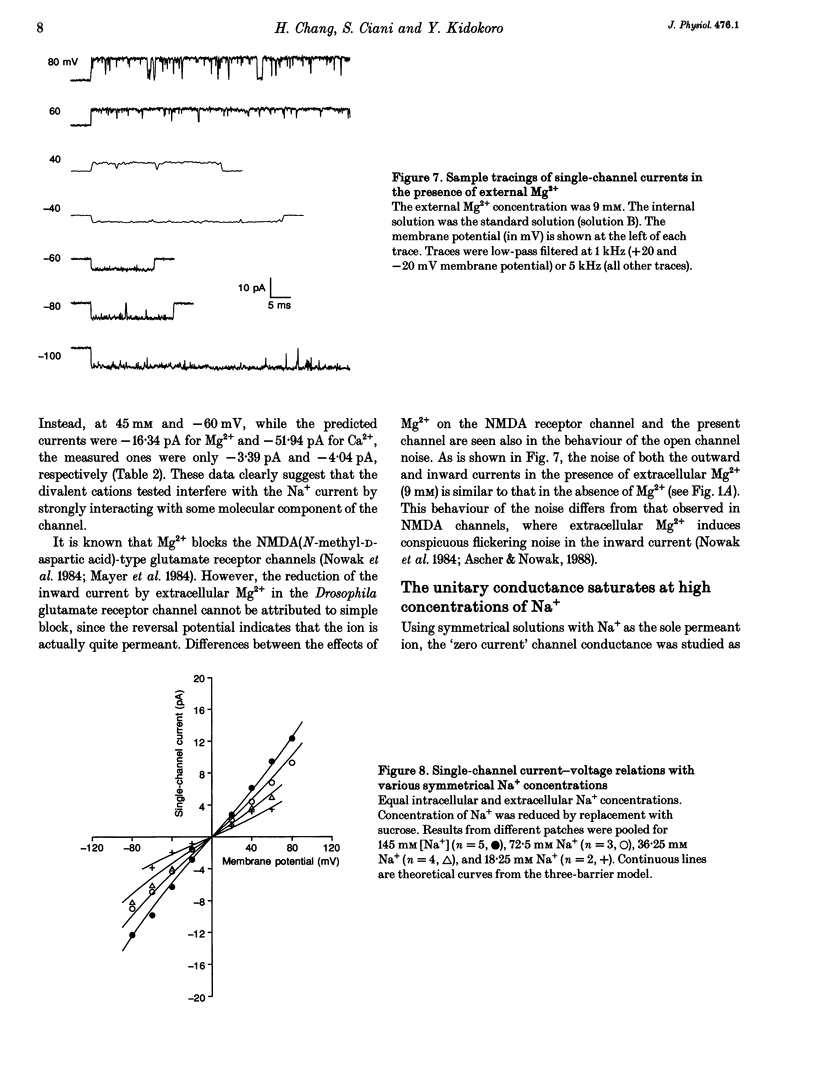
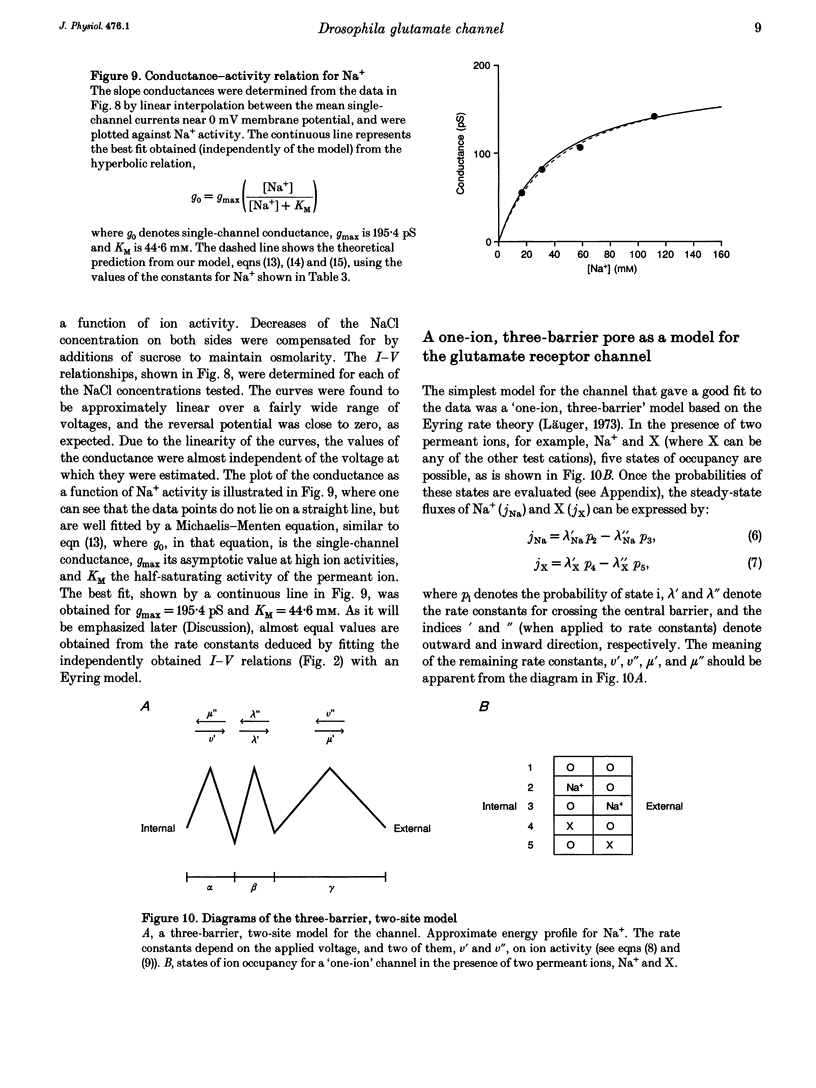
Ion permeation properties of the glutamate receptor channel in cultured myotubes of Drosophila embryos were studied using the inside-out configuration of the patch-clamp technique. Lowering the NaCl concentration in the bath (intracellular solution), while maintaining that of the external solution constant, caused a shift of the reversal potential in the positive direction, thus indicating a higher permeability of the channel to Na+ than to Cl- (PCl/PNa < 0.04), and suggesting that the channel is cation selective. With 145 mM Na+ on both sides of the membrane, the single-channel current-voltage relation was almost linear in the voltage range between -80 and +80 mV, the conductance showing some variability in the range between 140 and 170 pS. All monovalent alkali cations tested, as well as NH4+, permeated the channel effectively. Using the Goldman-Hodgkin-Katz equation for the reversal potential, the permeability ratios with respect to Na+ were estimated to be: 1.32 for K+, 1.18 for NH4+, 1.15 for Rb+, 1.09 for Cs+, and 0.57 for Li+. Divalent cations, i.e. Mg2+ and Ca2+, in the external solution depressed not only the inward but also the outward Na+ currents, although reversal potential measurements indicated that both ions have considerably higher permeabilities than Na+ (PMg/PNa = 2.31; PCa/PNa = 9.55). The conductance-activity relation for Na+ was described by a hyperbolic curve. The maximal conductance was about 195 pS and the half-saturating activity 45 mM. This result suggests that Na+ ions bind to sites in the channel. All data were fitted by a model based on the Eyring's reaction rate theory, in which the receptor channel is a one-ion pore with three energy barriers and two internal sites.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Permeability of the post-synaptic membrane of an excitatory glutamate synapse to sodium and potassium. J Physiol. 1977 Dec;273(2):367–388. doi: 10.1113/jphysiol.1977.sp012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. The effect of foreign cations, pH and pharmacological agents on the ionic permeability of an excitatory glutamate synapse. J Physiol. 1977 Dec;273(2):389–404. doi: 10.1113/jphysiol.1977.sp012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregestovski P. D., Miledi R., Parker I. Calcium conductance of acetylcholine-induced endplate channels. Nature. 1979 Jun 14;279(5714):638–639. doi: 10.1038/279638a0. [DOI] [PubMed] [Google Scholar]
- Butler J. N. The thermodynamic activity of calcium ion in sodium chloride-calcium chloride electrolytes. Biophys J. 1968 Dec;8(12):1426–1433. doi: 10.1016/S0006-3495(68)86564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Cooper K. E., Gates P. Y., Eisenberg R. S. Surmounting barriers in ionic channels. Q Rev Biophys. 1988 Aug;21(3):331–364. doi: 10.1017/s0033583500004480. [DOI] [PubMed] [Google Scholar]
- Dani J. A., Eisenman G. Monovalent and divalent cation permeation in acetylcholine receptor channels. Ion transport related to structure. J Gen Physiol. 1987 Jun;89(6):959–983. doi: 10.1085/jgp.89.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker E. R., Dani J. A. Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci. 1990 Oct;10(10):3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekin M. S. Permeability changes induced by L-glutamate at the crayfish neuromuscular junction. J Physiol. 1983 Aug;341:105–125. doi: 10.1113/jphysiol.1983.sp014795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Gilbertson T. A., Scobey R., Wilson M. Permeation of calcium ions through non-NMDA glutamate channels in retinal bipolar cells. Science. 1991 Mar 29;251(5001):1613–1615. doi: 10.1126/science.1849316. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Huang L. Y., Catterall W. A., Ehrenstein G. Selectivity of cations and nonelectrolytes for acetylcholine-activated channels in cultured muscle cells. J Gen Physiol. 1978 Apr;71(4):397–410. doi: 10.1085/jgp.71.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifune C. K., Steinbach J. H. Voltage-dependent block by magnesium of neuronal nicotinic acetylcholine receptor channels in rat phaeochromocytoma cells. J Physiol. 1991 Nov;443:683–701. doi: 10.1113/jphysiol.1991.sp018858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 1976 Oct;262(1):215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976 Oct;262(1):189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassignal N. L., Martin A. R. Effect of acetylcholine on postjunctional membrane permeability in eel electroplaque. J Gen Physiol. 1977 Jul;70(1):23–36. doi: 10.1085/jgp.70.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder T. M., Quastel D. M. A voltage-clamp study of the permeability change induced by quanta of transmitter at the mouse end-plate. J Physiol. 1978 Aug;281:535–558. doi: 10.1113/jphysiol.1978.sp012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Ion transport through pores: a rate-theory analysis. Biochim Biophys Acta. 1973 Jul 6;311(3):423–441. doi: 10.1016/0005-2736(73)90323-4. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Weinstock M. M. Nickel and calcium ions modify the characteristics of the acetylcholine receptor-channel complex at the frog neuromuscular junction. J Physiol. 1980 Feb;299:203–218. doi: 10.1113/jphysiol.1980.sp013120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchais D., Marty A. Interaction of permeant ions with channels activated by acetylcholine in Aplysia neurones. J Physiol. 1979 Dec;297(0):9–45. doi: 10.1113/jphysiol.1979.sp013025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Neuhaus R., Cachelin A. B. Changes in the conductance of the neuronal nicotinic acetylcholine receptor channel induced by magnesium. Proc Biol Sci. 1990 Aug 22;241(1301):78–84. doi: 10.1098/rspb.1990.0069. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Sanchez J. A., Dani J. A., Siemen D., Hille B. Slow permeation of organic cations in acetylcholine receptor channels. J Gen Physiol. 1986 Jun;87(6):985–1001. doi: 10.1085/jgp.87.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C. M., Ultsch A., Schloss P., Cox J. A., Schmitt B., Betz H. Molecular cloning of an invertebrate glutamate receptor subunit expressed in Drosophila muscle. Science. 1991 Oct 4;254(5028):112–114. doi: 10.1126/science.1681587. [DOI] [PubMed] [Google Scholar]
- Shatkay A. Individual activity of calcium ions in pure solutions of CaCl2 and in mixtures. Biophys J. 1968 Aug;8(8):912–919. doi: 10.1016/S0006-3495(68)86528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]


