Abstract
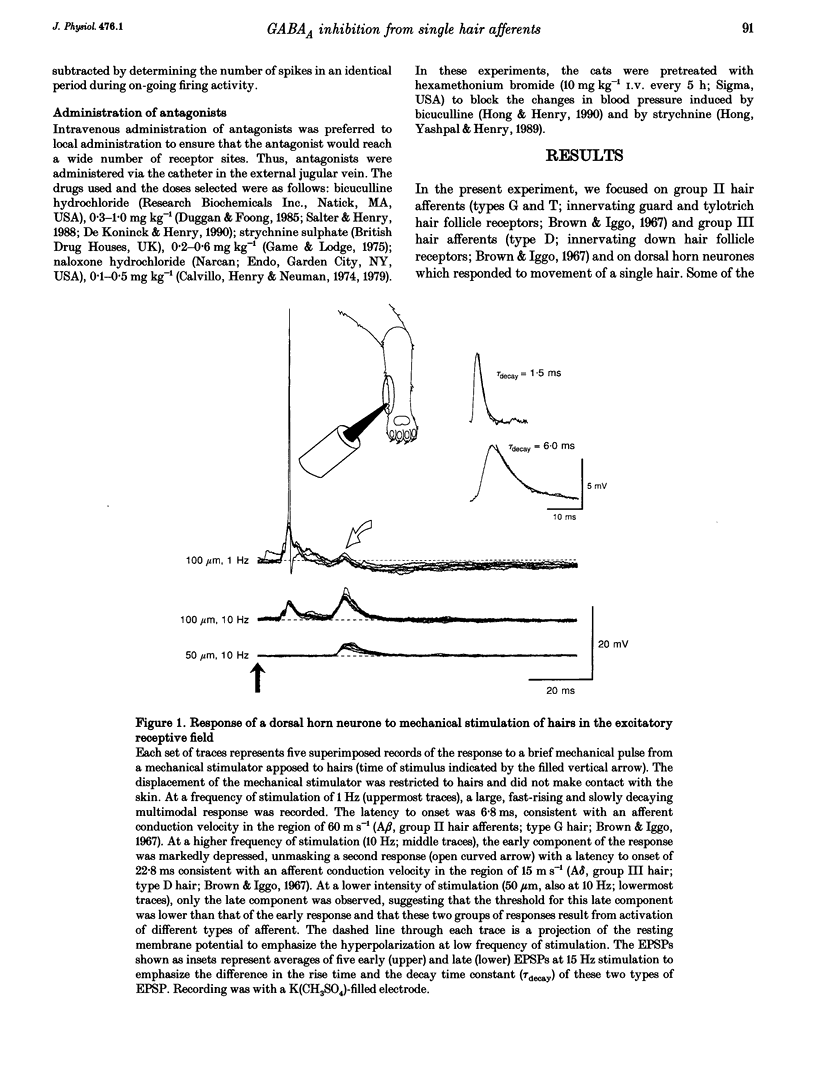
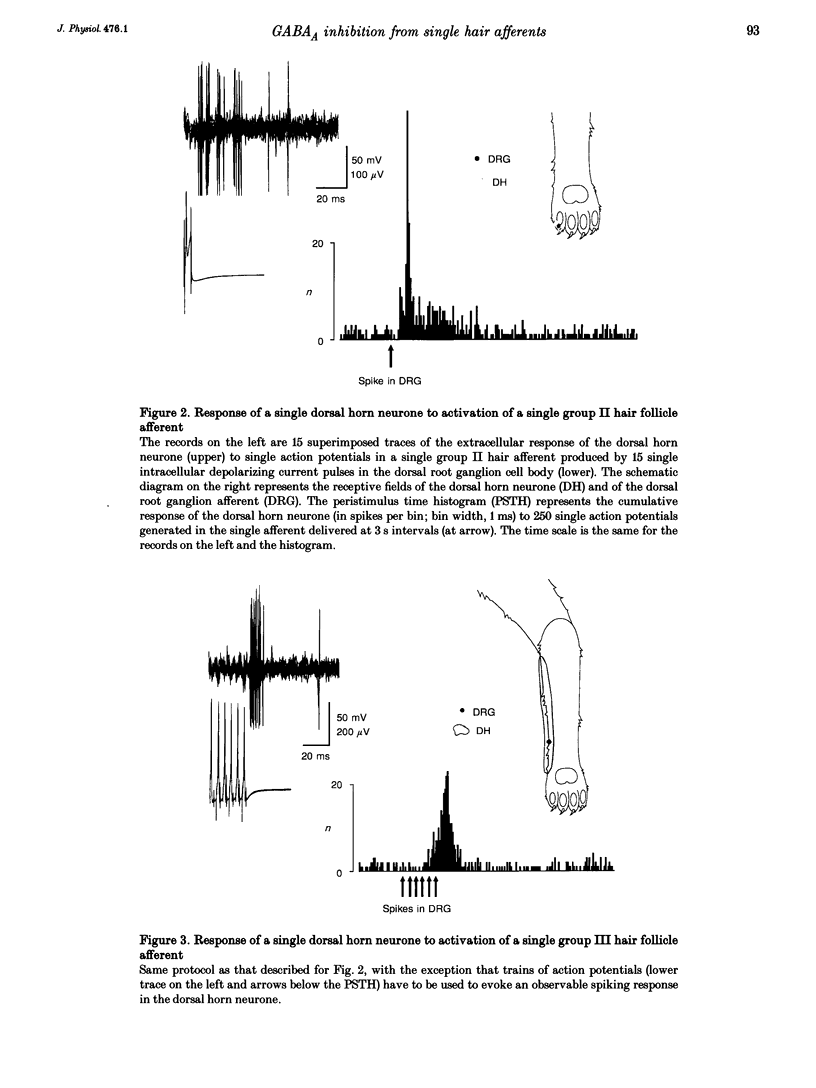
To study the central processing mechanisms of sensory input from low threshold afferents to the spinal cord, we examined the excitatory response of single lumbar dorsal horn neurones to stimulation of hairs in the receptive field using a mechanically driven probe, and to activation of single hair follicle afferents using an intracellular current pulse to the cell bodies in the dorsal root ganglion. Experiments were done on anaesthetized, paralysed cats, spinalized at the L1 lumbar level. Responses of spinal neurones to two types of hair afferent input were characteristically different. The excitatory response to input from a single group II hair afferent (A beta; innervating guard hair follicle receptors) was multimodal, characterized by a small early depolarization followed by a sharp, large component with a slow, prolonged decay phase, whereas the response to input from a single group III hair afferent (A delta; innervating down hair follicle receptors) was unimodal. The unitary EPSPs in response to activation of group III hair afferents had a slower rise time and longer decay time constant than those in response to activation of group II hair afferents. When the receptive field of the afferent was located in the centre of the receptive field of the dorsal horn neurone, the gain of the central response was greater for the input from a single group II afferent (> 1) than that for the input from a single group III afferent (< 1). In the case of single group II hair afferents, when pairs of single action potentials or pairs of trains of action potentials were generated at intervals of 20 ms to 3 s, the response in the dorsal horn neurone to the second volley was markedly depressed at intervals of less than 2 s, without any apparent inhibition of the on-going rate of firing. The response to the second volley in single group III afferents was less depressed. This inhibition of the response to the second of a paired volley in single group II hair afferents was attenuated by administration of bicuculline, but not strychnine or naloxone. This indicates that the inhibition involves a GABAA-receptor-mediated mechanism. Bicuculline did not affect the late component of the response to single group II hair afferent input, but unmasked a late component of the response to mechanical stimulation of hairs.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. Actions of trains and pairs of impulses from single primary afferent fibres on single spinocervical tract cells in cat. J Physiol. 1987 Jan;382:313–329. doi: 10.1113/jphysiol.1987.sp016369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. An intracellular study of spinocervical tract cell responses to natural stimuli and single hair afferent fibres in cats. J Physiol. 1987 Jan;382:331–354. doi: 10.1113/jphysiol.1987.sp016370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. Excitatory actions of single impulses in single hair follicle afferent fibres on spinocervical tract neurones in the cat. J Physiol. 1987 Jan;382:291–312. doi: 10.1113/jphysiol.1987.sp016368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvillo O., Henry J. L., Neuman R. S. Action of narcotic analgesics and antagonists on spinal units responding to natural stimulation in the cat. Can J Physiol Pharmacol. 1979 Jun;57(6):652–663. doi: 10.1139/y79-099. [DOI] [PubMed] [Google Scholar]
- Calvillo O., Henry J. L., Neuman R. S. Effects of morphine and naloxone on dorsal horn neurones in the cat. Can J Physiol Pharmacol. 1974 Dec;52(6):1207–1211. doi: 10.1139/y74-158. [DOI] [PubMed] [Google Scholar]
- De Koninck Y., Henry J. L. Bombesin, neuromedin B and neuromedin C selectively depress superficial dorsal horn neurones in the cat spinal cord. Brain Res. 1989 Sep 25;498(1):105–117. doi: 10.1016/0006-8993(89)90404-6. [DOI] [PubMed] [Google Scholar]
- De Koninck Y., Henry J. L. Peripheral vibration causes an adenosine-mediated postsynaptic inhibitory potential in dorsal horn neurons of the cat spinal cord. Neuroscience. 1992 Sep;50(2):435–443. doi: 10.1016/0306-4522(92)90435-5. [DOI] [PubMed] [Google Scholar]
- De Koninck Y., Henry J. L. Substance P-mediated slow excitatory postsynaptic potential elicited in dorsal horn neurons in vivo by noxious stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11344–11348. doi: 10.1073/pnas.88.24.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y., Ribeiro-da-Silva A., Henry J. L., Cuello A. C. Spinal neurons exhibiting a specific nociceptive response receive abundant substance P-containing synaptic contacts. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5073–5077. doi: 10.1073/pnas.89.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A. W., Foong F. W. Bicuculline and spinal inhibition produced by dorsal column stimulation in the cat. Pain. 1985 Jul;22(3):249–259. doi: 10.1016/0304-3959(85)90025-9. [DOI] [PubMed] [Google Scholar]
- Game C. J., Lodge D. The pharmacology of the inhibition of dorsal horn neurones by impulses in myelinated cutaneous afferents in the cat. Exp Brain Res. 1975 Jul 11;23(1):75–84. doi: 10.1007/BF00238730. [DOI] [PubMed] [Google Scholar]
- Gmelin G., Zimmermann M. Effects of gamma-aminobutyrate and bicuculline on primary afferent depolarization of cutaneous fibres in the cat spinal cord. Neuroscience. 1983 Nov;10(3):869–874. doi: 10.1016/0306-4522(83)90224-5. [DOI] [PubMed] [Google Scholar]
- Henry J. L. Effects of substance P on functionally identified units in cat spinal cord. Brain Res. 1976 Sep 24;114(3):439–451. doi: 10.1016/0006-8993(76)90965-3. [DOI] [PubMed] [Google Scholar]
- Hill R. G., Salt T. E. An ionophoretic study of the responses of rat caudal trigeminal nucleus neurones to non-noxious mechanical sensory stimuli. J Physiol. 1982 Jun;327:65–78. doi: 10.1113/jphysiol.1982.sp014220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y. G., Yashpal K., Henry J. L. Cardiovascular responses to intrathecal administration of strychnine in the rat. Brain Res. 1989 Oct 9;499(1):169–173. doi: 10.1016/0006-8993(89)91148-7. [DOI] [PubMed] [Google Scholar]
- Hong Y., Henry J. L. Spinal mediation of the increases in arterial pressure and heart rate in response to intrathecal administration of bicuculline. Brain Res. 1990 Apr 9;513(1):86–93. doi: 10.1016/0006-8993(90)91092-u. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Convergence of excitatory and inhibitory action on interneurones in the lumbosacral cord. Exp Brain Res. 1966;1(4):338–358. doi: 10.1007/BF00237706. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber H. R., Druzinsky R. E., Mendell L. M. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol. 1988 Nov;60(5):1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- Koerber H. R., Mendell L. M. Functional specialization of central projections from identified primary afferent fibers. J Neurophysiol. 1988 Nov;60(5):1597–1614. doi: 10.1152/jn.1988.60.5.1597. [DOI] [PubMed] [Google Scholar]
- Koerber H. R., Seymour A. W., Mendell L. M. Tuning of spinal networks to frequency components of spike trains in individual afferents. J Neurosci. 1991 Oct;11(10):3178–3187. doi: 10.1523/JNEUROSCI.11-10-03178.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. A. The role of GABA in primary afferent depolarization. Prog Neurobiol. 1977;9(4):211–267. doi: 10.1016/0301-0082(77)90002-8. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. Presynaptic inhibition: transmitter and ionic mechanisms. Int Rev Neurobiol. 1979;21:217–258. doi: 10.1016/s0074-7742(08)60639-x. [DOI] [PubMed] [Google Scholar]
- Noble R., Short A. D. Spatial spread of in-field afferent inhibition in the cat's spinocervical tract. J Physiol. 1989 Jun;413:107–118. doi: 10.1113/jphysiol.1989.sp017644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. D., Koerber H. R., Sedivec M. J., Mendell L. M. Somal action potential duration differs in identified primary afferents. Neurosci Lett. 1986 Jan 30;63(3):259–264. doi: 10.1016/0304-3940(86)90366-6. [DOI] [PubMed] [Google Scholar]
- Salt T. E., Hill R. G. Excitatory amino acids as transmitter candidates of vibrissae afferent fibres to the rat trigeminal nucleus caudalis. Neurosci Lett. 1981 Mar 10;22(2):183–187. doi: 10.1016/0304-3940(81)90085-9. [DOI] [PubMed] [Google Scholar]
- Salter M. W., De Koninck Y., Henry J. L. Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn. Prog Neurobiol. 1993 Aug;41(2):125–156. doi: 10.1016/0301-0082(93)90006-e. [DOI] [PubMed] [Google Scholar]
- Salter M. W., Henry J. L. Evidence that adenosine mediates the depression of spinal dorsal horn neurons induced by peripheral vibration in the cat. Neuroscience. 1987 Aug;22(2):631–650. doi: 10.1016/0306-4522(87)90359-9. [DOI] [PubMed] [Google Scholar]
- Short A. D., Brown A. G., Maxwell D. J. Afferent inhibition and facilitation of transmission through the spinocervical tract in the anaesthetized cat. J Physiol. 1990 Oct;429:511–528. doi: 10.1113/jphysiol.1990.sp018270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper D. N., Wiesenfeld Z., Craig A. D., Jr A dorsal spinal neural network in cat. II. Changes in responsiveness initiated by single conditioning impulses in single type 1 cutaneous input fibers. J Neurophysiol. 1983 Feb;49(2):534–547. doi: 10.1152/jn.1983.49.2.534. [DOI] [PubMed] [Google Scholar]