Abstract
Background
JAK2 V617F (JAK2) mutation is associated with clonal hemopoiesis in myeloproliferative neoplasms as well as with faster progression of cardiovascular diseases. Little is known about the relationship between allele burden and the degree of atherosclerotic alteration of coronary vasculature. We previously reported that carotid artery stiffness progressed faster in patients with JAK2 positive essential thromocythemia (ET) patients. After a four-year follow-up we investigated whether mutation burden of a JAK2 allele correlates with a higher coronary calcium score.
Patients and methods
Thirty-six patients with JAK2 positive ET and 38 healthy matched control subjects were examined twice within four years. At each visit clinical baseline characteristics and laboratory testing were performed, JAK2 mutation burden was determined, and coronary calcium was measured.
Results
JAK2 allele burden decreased in 19 patients, did not change in 5 patients, and increased in 4 patients. The coronary calcium Agatston score increased slightly in both groups. Overall, there was no correlation between JAK2 allele burden and calcium burden of coronary arteries. However, in patients with the JAK2 mutation burden increase, the coronary calcium score increased as well.
Conclusions
The average JAK2 allele burden decreased in our patients with high-risk ET during the four-year period. However, in the small subgroup whose JAK2 mutation burden increased the Agatston coronary calcium score increased as well. This finding, which should be interpreted with caution and validated in a larger group, is in line with emerging evidence that JAK2 mutation accelerates atherosclerosis and can be regarded as a non-classical risk factor for cardiovascular disease.
Keywords: essential thrombocythemia, JAK2 V617F mutation, JAK2 V617F allele burden, coronary calcium score
Introduction
Essential thrombocythemia (ET) is one of the classic Philadelphia chromosome negative chronic myeloproliferative neoplasms (MPN), along with polycythemia vera and primary myelofibrosis.1 MPNs are characterized by clonal expansion of abnormal hematopoietic stem cells.1 In about 50–60% of patients with ET, JAK2 V617F mutation is identified, followed by CALR and MPL mutations2 The JAK2 V617F mutation causes constitutive activation of the JAK2-STAT tyrosine kinase signal transducers that mediate intracellular signals from different cytokine receptors and affect gene transcription, cell cycle regulation, cell differentiation and apoptosis.3 In about 20% patients no mutation is identified which does not preclude ET diagnosis, the so-called triple-negative patients.4
JAK2 V617F mutation is associated with clonal hemopoiesis in MPN leading to development of the hematologic disease. However, cell clones with JAK2 V617F are associated with multiple cardiovascular diseases: atherosclerosis and aortic thrombosis leading to ischemic stroke, coronary artery disease and heart failure, pulmonary hypertension, venous thrombosis, and aortic aneurysm.5 Furthermore, clonal hematopoiesis of indeterminate potential, defined as the presence of an expanded somatic blood-cell clone in persons without any hematologic abnormalities, is common among older persons and is associated with nearly a doubling in the risk of coronary heart disease in humans and with accelerated atherosclerosis in mice.6
We have previously reported that the increase in carotid artery stiffness and pulse wave velocity over the four-year observation period was much more pronounced in high-risk patients with JAK2 V617F ET than in the control group.7 In the same cohort, we further determined the burden of the JAK2 V617F mutation at the beginning and at the end of the four-year observation period and correlated changes in the mutation burden with the coronary artery calcium score. Our hypothesis was that the JAK2 V617F mutation burden would be correlated with the coronary calcium score.
Patients and methods
Study design
The study design was described previously.7,8 Briefly, among 61 patients with JAK2 V617F positive ET who did not have clinically evident atherosclerotic disease, 40 participated at the first visit and of these 36 at the second visit after four-year time period. The control group consisted of 42 healthy control subjects participated at the first visit and 38 at the second visit. The study was approved by the Committee of Medical Ethics of the Republic of Slovenia (No. 154/05/12 and No. 0120-428/2017/4). The study has been registered at ClinicalTrials.gov PRS: Protocol Section NCT03828422.
Baseline characteristics
At the first visit and at the fourth-year follow-up visit we physically examined the participants, measured their height, weight, waist circumference and blood pressure. The participants completed a structured questionnaire about personal and family medical history, medication and risk factors for cardiovascular disease.
Blood was drawn at the first and at the fourth-year follow-up visit for blood cell count, electrolytes, urea, creatinine, liver function tests and lipid profile. Inflammatory markers, i.e., high sensitivity C-reactive protein, interleukins IL-1, IL-6, IL-8, IL-10, tumor necrosis factor -alpha (TNF-α), P-selectin, vascular adhesion molecule -1 (VCAM-1) and von Willebrand factor (VWF-A2) were measured at the second visit.
JAK2 V617F mutation burden
JAK2 V617F allele burden was determined in DNA extracted from granulocytes in peripheral blood, from samples collected at the Hematology Department, UMC Ljubljana at the time of the first visit, and from samples and the four-year follow-up visit. Real-time quantitative polymerase chain reaction (qPCR) was done with double-dye oligonucleotide hydrolysis, using Ipsogen JAK2 MutaQuant Kit, Qiagen (ZDA).9 Allele burden was calculated from the standard curves and was defined as the percentage of JAK2 V617F mutated alleles in total JAK2.
At the first visit we analyzed 28 blood samples, as eight patients did not have their blood samples collected for allele burden determination. Blood samples were collected from all patients at the second visit. In total, we had 28 pairs of samples taken four years apart.
Coronary calcium
The Biograph M 128-row PET-CT scanner (Siemens, Erlangen, Germany) was used for coronary artery calcium scanning. Scanning was done in sustained breath hold, from the carina to the base of the heart. We used a non-contrast protocol with sequential, prospective ECG triggering. Rotation time was 0.33 sec, tube voltage 120 kV, CARE Dose 4D and slice thickness 3 mm, with no slice overlap. Post-processing was done on a Syngo Leonardo workstation. The coronary calcium burden was expressed as the Agatston score.10 Measurements were done tree times for each visit and the average value was used for analysis.
Statistical analysis
Variables were presented as mean and standard deviation (SD) or as median and interquartile range (IQR) when asymmetrically distributed. Paired versions of statistical tests were used when comparing study group in time. Counts were used for discrete variables, and differences between groups were assessed by Fisher exact test. Spearman correlation coefficient was used to calculate monotonic correlation between different parameters. All p-values were two-sided and p-values of < 0.05 were considered statistically significant.
Results
Patients and baseline characteristics
We included 36 subjects (12 male and 24 female) with ET and 38 control subjects (14 male and 24 female). The patient baseline characteristics are shown in Table 1.
TABLE 1.
Baseline characteristics of patients with JAK2 V617F positive essential thromocythemia (ET) and of control group at the first visit and at the second visit (body mass index, BMI)
| FIRST VISIT | SECOND VISIT | Patient group | Control group | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| First vs. second visit | First vs. second visit | |||||||
| Patient group | Control group | p-value | Patient group | Control group | p-value | p-value | p-value | |
| Age (years) | 55.11 (13.40) | 59.07 (12.02) | 0.186 | 58.36 (13.44) | 62.08 (11.99) | 0.214 | - | - |
| Sex (M/F) | 12/24 | 14/24 | 0.811 | 12/24 | 14/24 | 0.754 | - | - |
| BMI (kg/m2) | 25.22 (3.65) | 27.27 (4.64) | 0.038 | 26.13 (4.66) | 27.54 (4.60) | 0.195 | 0.021 | 0.184 |
| Waist (cm) | ||||||||
| Male | 94.6 (11.1) | 102.2 (10.4) | 0.086 | 98.4 (11.2) | 104.0 (12.7) | 0.260 | 0.014 | 0.201 |
| Female | 89.5 (9.1) | 89.3 (14.2) | 0.965 | 93.1 (11.1) | 93.6 (15.0) | 0.899 | 0.086 | 0.005 |
| Systolic blood pressure (mmHg) | 140 (22) | 134 (14) | 0.219 | 144 (19) | 141 (20) | 0.615 | 0.134 | 0.018 |
| Diastolic blood pressure (mmHg) | 81 (9) | 82 (11) | 0.870 | 86 (12) | 89 (10) | 0.247 | 0.044 | < 0.001 |
| Smoking | 0.267 | 0.403 | 0.892 | 0.924 | ||||
| Current | 5/36 | 3/38 | 4/36 | 3/38 | ||||
| Former | 10/36 | 6/38 | 12/36 | 8/38 | ||||
BMI = body mass index; M/F = male/fimale
Laboratory tests at the second visit are shown in Table 2. No correlation of the laboratory parameters with JAK2 V617F mutation burden was found.
TABLE 2.
Laboratory parameters of patients with JAK2 V617F positive essential thromocythemia (ET) and of the control group at the second visit after 4-year follow-up. Means and standard deviations are given for the normally distributed data, medians and interquartile range are given for non-normally distributed data
| THE SECOND VISIT | ET PATIENTS (n = 36) | CONTROL GROUP (n = 38) | COMPARISON BETWEEN GROUPS (p-value) |
|---|---|---|---|
| 2Glucose (mmol/l) | 5.00 (4.60–5.40) | 5.00 (4.70–5.60) | 0.565 |
| 2Creatinine (μmol/l) | 76.10 (63.85–85.85) | 71.30 (62.45–83.55) | 0.351 |
| 1Total cholesterol (mmol/l) | 5.00 (1.05) | 5.33 (0.93) | 0.163 |
| 1HDL-cholesterol (mmol/l) | 1.45 (0.59) | 1.66 (0.53) | 0.118 |
| 1LDL-cholesterol (mmol/l) | 2.66 (0.89) | 2.94 (0.82) | 0.168 |
| 1Triglycerides (mmol/l) | 1.97 (0.89) | 1.61 (0.80) | 0.850 |
| 1Leukocytes (109/L) | 7.86 (2.83) | 6.54 (1.63) | 0.016 |
| 1Red blood cells (1012/L) | 4.42 (0.69) | 4.84 (0.42) | 0.002 |
| 1Haemoglobinb | 133 (15) | 145 (12) | 0.001 |
| 1Platelets (109/L) | 524.56 (218.67) | 250.38 (60.05) | < 0.001 |
| 1Lymphocytes (109/L) | 1.88 (0.90) | 2.02 (0.72) | 0.195 |
| 1Mixed cells (109/L) | 0.71 (0.29) | 0.59 (0.20) | 0.067 |
| 1Neutrophils (109/L) | 4.81 (1.97) | 3.93 (1.31) | 0.031 |
| 1IL-1 (ng/L) | 43.26 (4.98) | 34.59 (6.48) | < 0.001 |
| 1IL-8 (ng/L) | 28.89 (8.45) | 20.64 (9.51) | < 0.001 |
| 1P-selectin (ug/L) | 76.24 (19.54) | 43.33 (13.36) | < 0.001 |
| 1VCAM-1 (mg/L) | 1.17 (0.52) | 0.72 (0.26) | < 0.001 |
| 2IL-6 (ng/L) | 8.70 (7.70–9.38) | 6.80 (6.28–7.40) | < 0.001 |
| 2IL-10 (ng/L) | 5.65 (0.33–9.35) | 5.25 (0.00–7.90) | 0.417 |
| 1TNFa (ng/L) | 43.90 (7.34) | 37.60 (9.15) | 0.002 |
| 2VWF-A2 (ng/L) | 231.50 (199.25–256.25) | 195.00 (167.50–213.00) | < 0.001 |
| 2hs-CRP (mg/L) | 0.87 (0.50–2.16) | 0.91 (0.55–4.43) | 0.314 |
Comparisons between groups were tested by Student's t-test or the Mann-Whitney test2
HDL = high-density lipoprotein; IL = interkeucin; ldl = low-density lipoprotein; TNF-α = tumor necrosis factor –alpha; VCAM-1 = vascular adhesion molecule -1; VWF-A2 = von Willebrand factor -A2
JAK2 V617F allele burden
The average JAK2 V617F allele burden at the first visit (n = 28) was 28.57% (SD 20.45%) and at the four-year follow-up visit (n = 36) 15.92% (SD 15.42%); p = 0.001. Over the four-year observation period JAK2 V617F allele burden decreased in 19 patients, did not change in five patients and increased in four patients. Overall, the allele burden decreased significantly.
In the subgroup of patients where the allele burden decreased, the average JAK2 V617F allele burden at the first visit was 37.93% (SD 15.57%) and at the fourth-year follow-up visit 19.65% (SD 14.86%), p < 0.001. In the subgroup of patients where allele burden increased or stayed the same, the median JAK2 V617F allele burden at the first visit was 0.00% (0.00 – 15.93) and at the four-year follow-up visit 0.00% (0.0 – 31.55), p = 0.068.
In the control group JAK2 V617F allele burden was measured only at the four-year follow-up visit (median 0.00% (IQR 0.00–0.00)).
Coronary calcium
Table 3 presents coronary calcium burden of patents and control subjects at the first and at the four-year follow-up visit. The ET and control group did not differ in the Agatston score at both visits (p = 0.252 at the first visit and p = 0.954 at the four-year follow-up visit). The coronary calcium Agatston score increased slightly, but significantly in both groups: in the ET group from 0 (IQR 0–8.6) to 0.6 (IQR 0–40.3), p = 0.009 and in the control group from 0 (0–8.6) to 2.6 (0–30.1), p < 0.001. Overall, there was no correlation between the JAK2 V617F allele burden and the calcium burden of coronary arteries (at the first visit rs = 0.182, p = 0.355 and at the fourth-year follow-up visit rs = 0.161, p = 0.355).
TABLE 3.
Coronary calcium burden expressed as Agatston score of patients with JAK2 V617F positive essential thromocythemia (ET) and control subjects at the first visit and at the fourth-year follow-up visit and the correlation with JAK2 V617F allele burden for the ET patient group
| FIRST VISIT | SECOND VISIT | Patient group First vs. second visit | Control group First vs. second visit | Correlation with JAK2 V617F allele burden, p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Patient group | Control group | p-value | Patient group | Control group | p-value | p-value | p-value | First visit | Second visit | |
| Agatston score | 0 (0–8.6) | 0 (0–8.6) | 0.525 | 0.6 (0–40.3) | 2.6 (0–30.1) | 0.954 | 0.009 | < 0.001 | 0.191 | 0.069 |
| Calcium burden LM | 0 (0–0) | 0 (0–0) | 0.359 | 0 (0–1.2) | 0 (0–0) | 0.274 | 0.014 | 0.953 | 0.700 | 0.657 |
| Calcium burden LAD | 0 (0–2.7) | 0 (0–2.68) | 0.581 | 0 (0–32.5) | 0 (0–21.3) | 0.861 | 0.435 | < 0.001 | 0.243 | 0.069 |
| Calcium burden LCX | 0 (0–0) | 0 (0–0) | 0.630 | 0 (0–0) | 0 (0–0) | 0.825 | 0.208 | 0.204 | 0.433 | 0.074 |
| Calcium burden RCA | 0 (0–0) | 0 (0–0) | 0.245 | 0 (0–0.8) | 0 (0–0) | 0.676 | 0.074 | 0.012 | 0.700 | 0.045 |
LAD = left anterior descending artery; LCX = left circumflex artery; LM = the left main coronary artery; RCA = right coronary artery
In the subgroup of patients with ET, in which the JAK2 V617F mutation burden decreased, the coronary calcium score remained low without a change. However, in the patients in whom the JAK2 V617F mutation burden increased, the coronary calcium score also increased (Figure 1).
FIGURE 1.
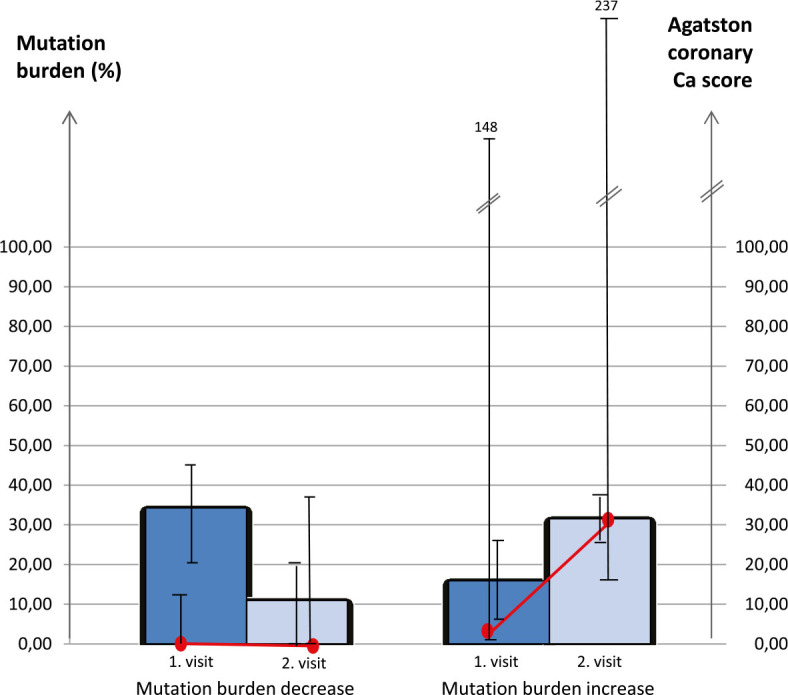
Change in JAK2 V617F mutation burden and Agatston coronary calcium score over the 4-year observation period in patients whose JAK2 V617F mutation burden increased (left-side columns) and in patients whose mutation burden decreased (right-side columns). Bar charts represent the mean mutation burden (%), and line graphs represent the median Agatston coronary calcium score.
No differences were found in inflammatory parameters between the subgroup with increased and the one with decreased JAK2 V617F mutation burden. All measured inflammatory parameters (IL-1, IL-8, P-selectin, VCAM-1, IL-6, TNFα and VWF-A2) were elevated in both subgroups of patients with ET. On the other hand, IL-10 which is an anti-inflammatory parameter, was in normal range in both subgroups.
Discussion
JAK2 V617F mutation is the predominant mutation in MPNs and also the mutation most strongly associated with cardiovascular disease risk.11 It causes constitutive activation of JAK/STAT signaling which promotes expression of inflammatory cytokines, reactive oxygen species, production of oxidative low-density lipoproteins and formation of foam cells. This creates a chronic inflammatory state as a driving force for atherosclerosis.12,13
It is known that patients with JAK2 V617F positive MPNs have accelerated atherosclerosis, higher incidence of acute coronary syndrome and other cardiovascular events.14 Experimental studies on animal models elucidated the pathophysiologic mechanisms that underlie increased cardiovascular disease risk in MPNs. Wang et al., experimenting on mice, showed that JAK2 V617F mutation promotes neutrophil infiltration and leukocytes attachment to the vascular wall, impairs macrophage function, accelerates atherosclerotic lesion formation with larger plaques which have unstable necrotic cores.15,16 In rabbits, Yang et al. found that JAK2 inhibitor blocks upregulation of inflammatory mediators, decreases plasma triglycerides, total cholesterol, and LDL, enhances HDL-C and reduces formation of atheromatous plaque.
Association between JAK2 V617F mutation and atherosclerosis is being more thoroughly investigated in the last decade, however, little is known about correlation between allele burden and CVS risk. Our study examined the JAK2 V617F allele burden in correlation with coronary artery calcium burden and inflammatory mediators in patients with high-risk ET. We found no overall correlation between the JAK2 V617F mutation burden and the coronary artery calcium score. On the contrary, the recent publication by Nordheim Solli et al. showed that there is a significant association between the variant allele fraction in the upper quartile (≥ 52%) and severe coronary calcifications in patients with MPNs. The study had limited statistical power to focus on MPN subgroups, though, and it did not specifically address patients with ET.17 However, in our study with high-risk ET patients coronary calcium burden increased during the four-year follow-up in the small subgroup of patients whose mutation burden increased as well. This, though not statistically significant, was to our knowledge observed for the first time in the developing field of research in MPN, atherosclerosis and coronary calcium burden. Conversely, in the subgroup of our patients where mutation burden decreased during the observation time, there was no progression in coronary artery calcium burden.
In the subgroup of patients where mutation burden increased, the average JAK2 allele burden at the first visit was lower in comparison to the subgroup where allele burden decreased However, they reached comparable levels at the second visit (Figure 1). Six out of 19 patients (32%) whose allele burden decreased and three out of nine patients (33%) whose allele burden increased or stayed the same had undergone a change in hematologic therapy. Changes in therapy were all different and overall did not seem to have any influence on allele burden. An uneven change in kinetics of the JAK2 V617F allele burden over time was observed also by Antonioli et al.19
The mutation burden was independent of patients' age which was previously observed by Kittur et.al.20 Also, consistent with previous data19,20,21, we found no correlation of JAK2 V617F mutation burden with levels of erythrocytes, leukocytes or platelets. Previous research found an association of JAK2 V617F allele burden with increased CRP levels in patients with ET.22 In contrast, we did not find any correlation between allele burden, CRP and other inflammatory mediators. Yet, there was a negative correlation between JAK2 V617F allele burden and IL-10 (r = −0.333, p = 0.047), which is an anti-inflammatory factor.
Among chronic myeloproliferative disorders, ET is characterized by the greatest heterogeneity in clinical profile, as well as in cellular and molecular levels.19 Autonomous activation of the JAK-STAT pathway in ET patients is progressively increased with the amount of mutant allele.19 Splenomegaly was significantly more frequent when the mutation burden was over 50%, and symptoms due to microvascular disease were present when the mutant allele level was over 25%. Also, a 3-fold greater risk of arterial thrombosis was found in those patients.19 In our study group, the overall JAK2 V617F mutation burden was relatively low. Therefore, a lack of correlation between overall allele burden and coronary artery calcium burden or inflammatory mediators might be accounted for by confounding factors overshadowing the relatively low burden of mutant alleles.
JAK2 V617F associated abnormalities are more common in patients with polycythemia vera or primary myelofibrosis where allele burden is much higher than in ET.23,24 Correlations between JAK2 V617F allele burden and clinical features in ET are not as definite. Available data about the clinical and prognostic importance of the JAK2 V617F mutation in patients with ET are still incomplete and sometimes even controversial.21,25
In some reports, higher mutated JAK2 allele burden was associated with increased blood counts and hemoglobin26,27 but this was not confirmed by others.28,29,30 Thrombotic risk was elevated in patients with ET.26,31 Also, higher mutant allele burden together with histology classification was associated with disease progression to primary myelofibrosis.32
All ET patients in our study were identified as high risk for thrombotic complications and were treated accordingly. Acetylsalicylic acid (ASA) was started in all except if they had an indication for anticoagulation therapy. Low-dose ASA significantly reduces thrombotic complications in ET patients33 and may have some anti-inflammatory effect in the setting of atherosclerosis.34 Anagrelide was the most common choice of drug for platelet reduction, followed by hydroxyurea. Anagrelide successfully achieves hematologic response in ET35,36, however, it does not have any impact on JAK2 allele burden.37 Hydroxyurea is a preferable choice to anagrelide in older patient population with similar effectiveness as anagrelide but with less cardiovascular side effects33,36, also not affecting the JAK2 allele burden.38,39 Interferon is a second line treatment choice, that can prolong the time to disease progression, may prolong survival in MPNs and ET and often significantly reduces the JAK2 allele burden.40,41,42 However, we used interferon for a short period in only two patients, in whom it did not lead to a significant change in JAK2 burden. Ruxolitinib, a JAK1/2 inhibitor, though not a standard of care in ET, was used in two patients. Ruxolitinib was shown to affect JAK2 burden in patients with ET and could lead to molecular remissions.43,44 However, again as in patients on interferon, we could not draw any conclusions due to the low patient numbers. Thus, the treatment landscape of patients in our study was very heterogenous and primarily focused on hematologic responses with most probably no impact on JAK2 allele burden.
Study limitations
The main limitation of our study is the small number of participants. As we decided to determine JAK2 V617F allele burden after our initial study, blood samples from eight participants were not collected at the first visit and we were unable to determine their initial JAK2 V617F allele burden.
A minor limitation is that all participants were not examined at the exact time interval between both visits, however, the time difference varied at most for a few weeks.
In conclusion, our study, contrary to expectation, showed a decrease of the average JAK2 V617F allele burden in patients with high-risk ET during four-year observation period. However, in the small subgroup of four patients whose JAK2 V617F mutation burden increased the Agatston coronary calcium score increased as well but the significance of this finding cannot be calculated due to the small sample. This preliminary finding, which should be interpreted with caution and validated in a larger study, is in line with the emerging evidence that the JAK2 V617F mutation is a non-classical risk factor for cardiovascular disease.
Acknowledgement
This study was funded by the Research Program P3-0308 of the Slovenian Research Agency, and the Tertiary Research Project TP 20180038 of the University Medical Centre Ljubljana.
References
- 1.Tefferi A, Pardanani A. Myeloproliferative neoplasms: a contemporary review. JAMA Oncol. 2015;1:97–105. doi: 10.1001/jamaoncol.2015.89. [DOI] [PubMed] [Google Scholar]
- 2.Chuzi S, Stein BL. Essential thrombocythemia: a review of the clinical features, diagnostic challenges, and treatment modalities in the era of molecular discovery. Leuk Lymphoma. 2017;58:2786–2798. doi: 10.1080/10428194.2017.1312371. [DOI] [PubMed] [Google Scholar]
- 3.Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? A closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287. doi: 10.3389/fonc.2018.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babakhanlou R, Masarova L, Verstovsek S. A review of essential thrombocythemia and its complications. Clin Adv Hematol Oncol. 2023;21:76–84. PMID: [PubMed] [Google Scholar]
- 5.Misaka T, Kimishima Y, Yokokawa T, Ikeda K, Takeishi Y. Clonal hematopoiesis and cardiovascular diseases: role of JAK2V617F. J Cardiol. 2023;81:3–9. doi: 10.1016/j.jjcc.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–21. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anžič Drofenik A, Vrtovec M, Božič Mijovski M, Sever M, Preložnik Zupan I, Kejžar N. et al. Progression of coronary calcium burden and carotid stiffness in patients with essential thrombocythemia associated with JAK2 V617F mutation. Atherosclerosis. 2020;296:25–31. doi: 10.1016/j.atherosclerosis.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Vrtovec M, Anzic A, Preložnik Zupan I, Zaletel K, Blinc A. Carotid artery stiffness, digital endothelial function, and coronary calcium in patients with essential thrombocytosis, free of overt atherosclerotic disease. Radiol Oncol. 2017;51:203–10. doi: 10.1515/raon-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ipsogen JAK2 MutaQuant Kit Handbook. Hilden, Germany: QIAGEN GmbH; 2013. pp. 1–48. Version 1(Catalog no. 673523). [Google Scholar]
- 10.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 11.Leiva O, Gabriela Hobbs G, Ravid K, Libby P. Cardiovascular disease in myeloproliferative neoplasms. JACC CardioOncology state-of-the-art review. JACC CardioOncol. 2022;4:166–82. doi: 10.1016/j.jaccao.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lussana F, Rambaldi A. Inflammation and myeloproliferative neoplasms. J Autoimmun. 2017;85:58–63. doi: 10.1016/j.jaut.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Genovese E, Mirabile M, Rontauroli S, Sartini S, Fantini S, Tavernari L. et al. The response to oxidative damage correlates with driver mutations and clinical outcome in patients with myelofibrosis. Antioxidants. 2022;11:113. doi: 10.3390/antiox11010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malak S, Labopin M, Saint-Martin C, Bellanne-Chantelot C, Najman A. Long term follow up of 93 families with myeloproliferative neoplasms: life expectancy and implications of JAK2V617F in the occurrence of complications. Blood Cells Mol Dis. 2012;49:170–6. doi: 10.1016/j.bcmd.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Liu W, Fidler T, Wang Y, Tang Y, Woods B. et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 (V617F) mice. Circ Res. 2018;123:e35–47. doi: 10.1161/CIRCRESAHA.118.313283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edelmann B, Gupta N, Schnoeder TM, Oelschlegel AM, Shahzad K, Goldschmidt J. et al. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. J Clin Invest. 2018;128:4359–71. doi: 10.1172/JCI90312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Jia J, Yu Z, Duanmu Z, He H, Chen S. et al. Inhibition of JAK2/STAT3/SOCS3 signaling attenuates atherosclerosis in rabbit. BMC Cardiovasc Disord. 2020;20:133. doi: 10.1186/s12872-020-01391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solli CN, Chamat-Hedemand S, Elming H, Ngo A, Kjaer L, Skov V. et al. High JAK2V617F variant allele frequency is associated with coronary artery but not aortic valve calcifications in patients with Philadelphia-negative myeloproliferative neoplasms. Eur J Haematol. 2023;111:400–6. doi: 10.1111/ejh.14019. [DOI] [PubMed] [Google Scholar]
- 19.Antonioli E, Guglielmelli P, Poli G, Bogani C, Pancrazzi A, Longo G. et al. Influence of JAK2V617F allele burden on phenotype in essential thrombocythemia. Haematologica. 2008;93:41–8. doi: 10.3324/haematol.11653. [DOI] [PubMed] [Google Scholar]
- 20.Kittur J, Knudson RA, Lasho TL, Finke CM, Gangat N, Wolanskyj AP. et al. Clinical correlates of JAK2V617F allele burden in essential thrombocythemia. Cancer. 2007;109:2279–84. doi: 10.1002/cncr.22663. [DOI] [PubMed] [Google Scholar]
- 21.Antonioli E, Guglielmelli P, Pancrazzi A, Bogani C, Verrucci M, Ponziani V. et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia. 2005;19:1847–9. doi: 10.1038/sj.leu.2403902. [DOI] [PubMed] [Google Scholar]
- 22.Barbui T, Carobbio A, Finazzi G, Vannucchi AM, Barosi G, Antonioli E. et al. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and pentraxin 3. Haematologica. 2011;96:315–8. doi: 10.3324/haematol.2010.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell PJ, Griesshammer M, Dohner K, Dohner H, Kusec R, Hasselbalch HC. et al. V617F mutation in JAK2 is associated with poorer survival in idiopathic myelofibrosis. Blood. 2006;107:2098–100. doi: 10.1182/blood-2005-08-3395. [DOI] [PubMed] [Google Scholar]
- 24.Vannucchi AM, Antonioli E, Guglielmelli P, Longo G, Pancrazzi A, Ponziani V. et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952–9. doi: 10.1038/sj.leu.2404854. [DOI] [PubMed] [Google Scholar]
- 25.Wolanskyj AP, Lasho TL, Schwager SM, McClure RF, Wadleigh M, Lee SJ. et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol. 2005;131:208–13. doi: 10.1111/j.1365-2141.2005.05764.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhao S, Zhang X, Xu Y, Feng Y, Sheng W, Cen J. et al. Impact of JAK2V617F mutation burden on disease phenotype in chinese patients with JAK2V617F-positive polycythemia vera (PV) and essential thrombocythemia (ET) Int J Med Sci. 2016;13:85–91. doi: 10.7150/ijms.10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee AJ, Kim SG, Nam JY, Yun J, Ryoo HM, Bae SH. Clinical features and outcomes of JAK2 V617F-positive polycythemia vera and essential thrombocythemia according to the JAK2 V617F allele burden. Blood Res. 2021;56:259–65. doi: 10.5045/br.2021.2021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yow KS, Liu X, Chai CN, Tung ML, Yan B, Christopher D. et al. Relationship of JAK2 (V617F) allelic burden with clinicohaematological manifestations of Philadelphia-negative myeloproliferative neoplasms. Asian Pac J Cancer Prev. 2020;21:2805–10. doi: 10.31557/APJCP.2020.21.9.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popova-Labachevska M, Panovska-Stavridis I, Eftimov A, Kapedanovska NA, Cevreska L, Ivanovski M. et al. Evaluation of the JAK2V617F mutational burden in patients with Philadelphia chromosome negative myeloproliferative neoplasms: a single-center experience. Balkan J Med Genet. 2019;22:31–6. doi: 10.2478/bjmg-2019-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ha JS, Kim YK, Jung SI, Jung HR, Chung IS. Correlations between Janus kinase 2 V617F allele burdens and clinicohematologic parameters in myeloproliferative neoplasms. Ann Lab Med. 2012;32:385–91. doi: 10.3343/alm.2012.32.6.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trifa AP, Bănescu C, Voina CM, Popa Ș, Török-Vistai T, Bojan AS. et al. Modest contribution of JAK2 V617F allele burden to the occurrence of major thrombosis in polycthemia vera and essential thrombocythemia. Blood Cells Mol Dis. 2018;73:45–6. doi: 10.1016/j.bcmd.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Latagliata R, Polverelli N, Tieghi A, Palumbo GA, Breccia M, Sabattini E. et al. Comparison of JAK2 V617 -positive essential thrombocythaemia and early primary myelofibrosis: the impact of mutation burden and histology. Hematol Oncol. 2018;36:269–75. doi: 10.1002/hon.2430. [DOI] [PubMed] [Google Scholar]
- 33.Tefferi A, Vannucchi AM, Barbui T. Essential thrombocythemia treatment algorithm 2018. Blood Cancer J. 2018;8:2. doi: 10.1038/s41408-017-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T. et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–96. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 35.Gisslinger H, Gotic M, Holowiecki J, Penka M, Thiele J, Kvasnicka HM. et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood. 2013;121:1720–8. doi: 10.1182/blood-2012-07-443770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birgegård G, Besses C, Griesshammer M, Gugliotta L, Harrison CN, Hamdani. et al. Treatment of essential thrombocythemia in Europe: a prospective long-term observational study of 3649 high-risk patients in the Evaluation of Anagrelide Efficacy and Long-term Safety study. Haematologica. 2018;103:51–60. doi: 10.3324/haematol.2017.174672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cascavilla N, De Stefano V, Pane F, Pancrazzi A, Iurlo A, Gobbi M. et al. Impact of JAK2(V617F) mutation status on treatment response to anagrelide in essential thrombocythemia: an observational, hypothesis-generating study. Drug Des Devel Ther. 2015;9:2687–94. doi: 10.2147/DDDT.S79576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antonioli E, Carobbio A, Pieri L, Pancrazzi A, Guglielmelli P, Delaini F. et al. Hydroxyurea does not appreciably reduce JAK2 V617F allele burden in patients with polycythemia vera or essential thrombocythemia. Haematologica. 2010;95:1435–8. doi: 10.3324/haematol.2009.021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zalcberg IR, Ayres-Silva J, de Azevedo AM, Solza C, Daumas A, Bonamino M. Hydroxyurea dose impacts hematologic parameters in polycythemia vera and essential thrombocythemia but does not appreciably affect JAK2-V617F allele burden. Haematologica. 2011;96:e18–20. doi: 10.3324/haematol.2010.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quintás-Cardama A, Kantarjian H, Manshouri T, Luthra R, Estrov Z, Pierce S. et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27:5418–24. doi: 10.1200/JCO.2009.23.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quintás-Cardama A, Abdel-Wahab O, Manshouri T, Kilpivaara O, Cortes J, Roupie AL. et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon α-2a. Blood. 2013;122:893–901. doi: 10.1182/blood-2012-07-442012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verger E, Cassinat B, Chauveau A, Dosquet C, Giraudier S, Schlageter MH. et al. Clinical and molecular response to interferon-α therapy in essential thrombocythemia patients with CALR mutations. Blood. 2015;126:2585–91. doi: 10.1182/blood-2015-07-659060. [DOI] [PubMed] [Google Scholar]
- 43.Deininger M, Radich J, Burn TC, Huber R, Paranagama D, Verstovsek S. The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood. 2015;126:1551–4. doi: 10.1182/blood-2015-03-635235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verstovsek S, Passamonti F, Rambaldi A, Barosi G, Rumi E, Gattoni E. et al. Ruxolitinib for essential thrombocythemia refractory to or intolerant of hydroxyurea: long-term phase 2 study results. Blood. 2017;130:1768–71. doi: 10.1182/blood-2017-02-765032. [DOI] [PMC free article] [PubMed] [Google Scholar]


