Abstract
Background and study aims Long-standing ulcerative colitis (UC) is associated with an increased risk of developing colorectal neoplasia. Both dye-based chromoendoscopy (DCE) and virtual chromoendoscopy (VCE) increase detection of neoplastic lesions. In this prospective randomized controlled trial (RCT), we compared the neoplasia detection rate between DCE and i-scan VCE in patients with long-standing UC.
Patient and methods In four European hospitals, 131 patients with long-standing UC (disease duration > 8 years) were randomized to either DCE with methylene blue 0.1% (n = 66) or i-scan VCE (n = 65). All procedures were performed by trained endoscopists. Biopsies were taken from all visible lesions and the surrounding mucosa.
Results The mean number of neoplastic lesions detected per colonoscopy was not significantly different between DCE (0.27) and i-scan VCE (0.37) ( P = 0.41). Similarly, there was no significant difference in neoplasia detection rate between DCE (19.7%) and VCE (27.7%) (odds ratio0.64, 95% confidence interval 0.28–1.50, P = 0.31). However, the per lesion neoplasia detection rate was significantly higher with i-scan VCE compared to DCE (27.6% vs 15.3%, P = 0.036). Both withdrawal and total procedure time were on average 10.0 and 9.9 minutes shorter using i-scan VCE (both P < 0.001).
Conclusions This multicenter, prospective RCT showed no significant difference in neoplasia detection between DCE and i-scan VCE in long-standing UC. However, use of i-scan VCE was associated with a lower false-positive rate and a significantly shorter procedure time compared with DCE. I-scan VCE, therefore, could be a valid replacement for DCE in UC surveillance colonoscopies.
Keywords: Endoscopy Lower GI Tract; Inflammatory bowel disease; CRC screening; Diagnosis and imaging (inc chromoendoscopy, NBI, iSCAN, FICE, CLE...)
Introduction
Patients with long-standing ulcerative colitis (UC) are at increased risk for development of colonic neoplastic lesions 1 . A recent systematic review estimated that the overall risk of UC-associated colorectal cancer (CRC) is 1.4% (95% confidence interval [CI] 1.2%-1.6%) in this patient population, increasing with disease duration and extent 1 . Therefore, periodic surveillance for detection of neoplasia is strongly recommended by gastroenterology societies, such as the European Crohn’s and Colitis Organization (ECCO) and the European Society of Gastrointestinal Endoscopy (ESGE) 2 3 . Surveillance should either consist of a pan-colonic dye-based chromoendoscopy (DCE) (e.g. 0.1% methylene blue or 0.1% to 0.5% indigo carmine) with targeted biopsies of any visible lesion and two biopsies every 10 cm to assess disease activity and extent, or alternatively white light endoscopy with random four-quadrant biopsies every 10 cm, as well as targeted biopsies of all visible lesions 2 3 4 . Multiple studies have shown that DCE has a superior neoplasia detection rate compared with standard definition white light endoscopy (SDWLE) with random biopsies and, therefore, is considered the current gold standard 5 6 . However, notwithstanding its superior neoplasia detection rate, adoption of DCE in UC surveillance has been slow. This is mainly because DCE, even though it is less laborious than random four-quadrant biopsies, remains very time-consuming and is unpractical in patients with suboptimal bowel preparation, because no additional rinsing of the colonic mucosa can be performed while staining.
In recent years, dye-less or virtual chromoendoscopy (VCE) has gained interest as a potential alternative technique for detection of neoplasia. Examples of these novel technologies include i-scan (Pentax, Tokyo, Japan), Blue Light Imaging (BLI – Fujifilm, Tokyo, Japan), and Narrow Band Imaging (NBI – Olympus, Tokyo, Japan). Of these, i-scan virtual chromoendoscopy allows the endoscopist to switch between three different modes/algorithms, namely Surface Enhancement (SE), Tone Enhancement (TE), and Contrast Enhancement (CE), at the flick of a switch. The SE mode facilitates the visualization of the edges of lesions. This helps to better delineate mucosal structure and tissue folds, making structures appear elevated and blood vessels more accentuated. The TE mode, on the other hand, changes colorization of the individual pixels and accentuates mucosal patterns and vascular structures, enabling better lesion characterization. Lastly, CE mode is useful for detection of depressed areas by colored representation of these low-density areas. This is mainly used to accentuate surface vessels and the surface texture of the mucosa 7 .
In the latest guidelines from ESGE, use of VCE, in addition to conventional DCE, was supported in UC 8 . Unfortunately only a few studies have compared the efficacy of i-scan versus DCE for detection of neoplasia in long-standing UC 9 10 11 . Therefore, we performed this multicenter, prospective, randomized controlled trial (RCT) to compare i-scan to conventional DCE in patients with long-standing ulcerative colitis (UC) undergoing routine surveillance colonoscopy for detection of neoplasia.
Patients and methods
Study design and patients
This study was designed as an international, multicenter, prospective, non-blinded RCT (ClinicalTrials.gov ID: NCT01882205). Participants were recruited in four European centers (Copenhagen University Hospital Herlev, Copenhagen, Denmark; Amphia Hospital, Breda, the Netherlands; Maastricht University Hospital, Maastricht, the Netherlands and University Hospitals Leuven, Leuven, Belgium) from September 2008 until April 2018. The study protocol was reviewed and approved by all local ethics committees and the ethics committee of UZ Leuven (ML4291); this study was performed in accordance with the principles of the Helsinki Declaration.
Because dysplasia and colorectal cancer rarely occurs within the first 8 years after disease onset, the consensus among guidelines is that surveillance colonoscopies should start after approximately 8 years of disease duration 8 12 13 14 15 . Therefore, all adult patients (aged ≥ 18 years) with long-standing UC (> 8 years for extensive colitis or > 10 years for left-sided colitis) who had not had a surveillance colonoscopy during the past year were eligible for inclusion. Exclusion criteria were active UC (defined as Mayo score > 1) extending above 20 cm from the anal margin 16 , inadequate bowel preparation (defined as stool remnants that could not be rinsed off, corresponding with Boston Bowel Preparation Score 17 (BBPS) ≤ 2 in at least one segment), personal history of colorectal cancer, known allergy, or intolerance to methylene blue. Patients refusing or incapable of giving written informed consent and pregnant or nursing women were also excluded.
The primary objective was to compare the total number of neoplastic lesions detected using DCE (methylene blue 0.1%) versus i-scan VCE in patients with long-standing UC.
Secondary objectives were to compare: the neoplasia detection rate (the number of patients with at least one neoplasia detected) between both groups; the per lesion neoplasia detection rate (ratio of number of neoplastic lesions/total number of lesions) between both groups; the total number of non-neoplastic lesions detected between both groups; the total number of biopsies taken per colonoscopy between both groups; and the total duration of the endoscopic procedure and the withdrawal time between both groups.
In addition, demographic and clinical data were collected from each participant through interview by a study investigator and review of digital patient medical records (when available). Variables, which were prospectively collected, consisted of patient age, sex, date of diagnosis, duration of disease in years, active medication at the time of endoscopy, as well as those previously prescribed, number of flares in the past 2 years, BPPS, withdrawal time, and total endoscopic procedure time.
Randomization
Patients were randomly assigned to the DCE or i-scan group in a 1:1 ratio. An independent researcher prepared sealed envelopes with the endoscopic surveillance method to be performed. These envelopes were grouped in sets of 20 (block randomization) and sent to the various participating centers. An independent research assistant randomly selected one envelope and opened it immediately before the colonoscopy.
Endoscopic procedure
Prior to endoscopy, patients were given a standard bowel preparation, using a split-dose polyethylene glycol-based solution, either at home or in the endoscopy department. All colonoscopies were performed using the commercially available EC3890Fi colonoscope from Pentax, Japan, which was connected to an HD screen via an EPK-i7000 processor using the HD serial digital interface (SDI) signal. All endoscopies were performed by a selected team of endoscopists, who underwent standardized onsite theoretical training in DCE and i-scan by RB as well as a minimum of three supervised CE procedures by RB before the endoscopists could participate in the trial. As per the study protocol, any visible mucosal abnormalities (detected during DCE or i-scan) were either biopsied or resected, and two extra biopsies were taken from the surrounding mucosa using disposable biopsy forceps (Boston Scientific Radial Jaw 4 standard capacity forceps). Lesions were classified according to the Kudo pit pattern classification 18 . With the exception of typical pseudopolyps with Kudo pit pattern 1, all visible lesions were biopsied or resected.
Dye-based chromoendoscopy with 0.1% methylene blue
In the DCE group, the procedure involved a normal HD white-light endoscopy (HD-WLE) with water cleansing during progression of the scope. Once the cecum was reached and the colon was adequately cleansed, a 7F spray catheter was inserted through the biopsy channel of the endoscope to spray the mucosa with a 0.1% solution (diluted with saline) of methylene blue, whereafter the scope was slowly withdrawn. After 1 minute, excess methylene blue was removed and the scope was reinserted in the stained segment to inspect the mucosa for suspicious lesions. Targeted biopsies of all visible lesions and extra biopsies of the mucosa surrounding the lesion were acquired for histological evaluation. The entire colon was endoscopically assessed in concordance with this protocol. Besides dying the entire colonic mucosa, no other manipulations were needed for this technique. The endoscopes used in the DCE-group did not differ from the ones used in the other randomization arm.
Virtual chromoendoscopy with i-scan
In patients randomized to the i-scan group, the same commercially available colonoscope (Pentax EC3890Fi colonoscope) was used. The procedure involved a standard HD-WLE during progression of the scope. Once the cecum was reached, the scope was slowly retracted in the i-scan TE-modus with surface enhancement on medium (range low-medium-high). Similar to the DCE group, targeted biopsies of all visible lesions and additional biopsies of the mucosa surrounding the lesion were acquired and histologically analyzed.
Histological examination
Biopsies were placed into separate containers, in concordance with guidelines on use of CE-directed screening colonoscopy for UC 2 4 . Histological samples were examined by expert gastrointestinal pathologists in the different centers. In case of doubt about presence of dysplasia, a second pathologist reviewed the tissue samples and a consensus diagnosis was made. A lesion was considered neoplastic if it belonged to any of the following pathological types: adenocarcinoma, any grade of dysplasia, indefinite for dysplasia, sessile serrated lesion/polyp, tubular adenoma, or colitis-associated dysplasia.
Statistical analysis
Sample size calculation
Upon initiation of the study, no scientific data were available about the potential lesion detection rate with DCE or i-scan VCE for UC surveillance. To calculate the required sample size, we applied a power calculation similar to the one we used in previous studies of DCE in long-standing UC surveillance 5 . Assuming a neoplasia incidence of 10%, we calculated that 67 patients had to be enrolled per group (134 patients in total) to allow for a threefold superior neoplasia detection rate for either technique with a power of 80% (beta error 0.2; alpha error 0.05).
Rates of neoplastic lesions, detected with either endoscopic technique, were analyzed using a statistics software program (GraphPad Prism v9.5.1). Analyses were conducted on a “per patient” and “per lesion” basis. The “per patient” analysis was done by calculating the percentage of patients in whom true neoplastic lesions were detected from biopsies of endoscopically suspicious lesions. On the other hand, in “per lesion” analysis, the percentage of neoplastic lesions among all endoscopically suspicious lesions was calculated. Fisher’s exact test and χ 2 test, where deemed appropriate, were applied for dichotomous variables. Two-sided t -test (or Mann-Whitney U for data with a non-normal distribution) was used for continuous variables. P < 0.05 was considered significant.
Results
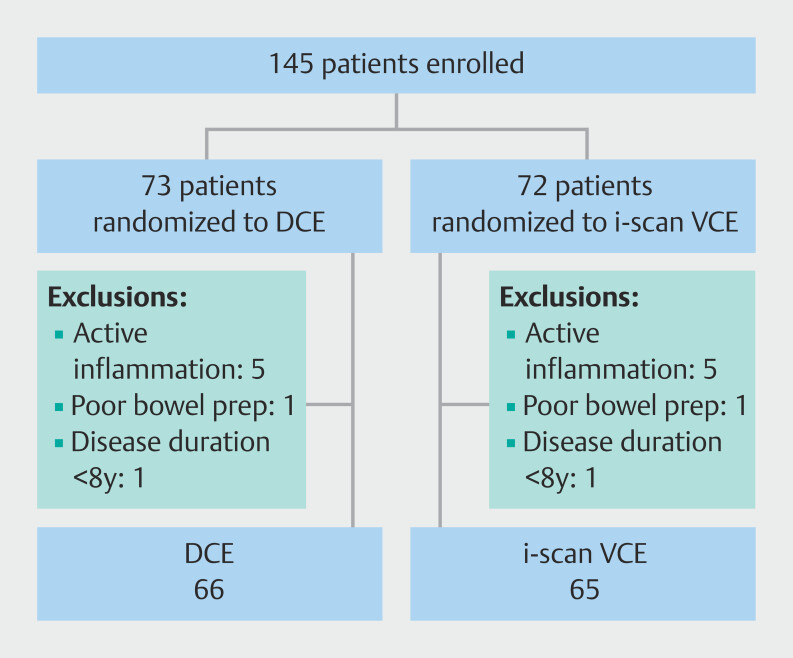
Between September 2008 and April 2018, 145 patients with long-standing UC were included into the study ( Fig. 1 ). Of these patients, 14 were excluded after randomization (7 in the DCE group and 7 in the i-scan VCE group): 10 patients had active inflammation at the time of endoscopic evaluation, two patients had a disease duration < 8 years, and two patients were excluded because of poor bowel preparation (BBPS ≤ 2 in at least one segment). For the final analysis, 131 patients were included (66 in the DCE group and 65 in the i-scan VCE group). Baseline characteristics did not significantly differ between both groups ( Table 1 ). Cecal intubation rate was 100%.
Fig. 1.
Study flowchart
Table 1 Baseline characteristics of DCE group vs i-scan VCE group.
| DCE (n = 66) | i-scan VCE (n = 65) | |
| ASA, aminosalicylic acid; DCE, dye-based chromoendoscopy; PSC, primary sclerosing cholangitis. | ||
| Age in years (median, IQR (P25-P75)) | 50.0 (39.8–63.0) | 49.0 (40.5–58.5) |
| Sex (M/F) | 21/42 | 24/37 |
| Disease duration in years (median, IQR (P25-P75)) | 17.2 (12.0–22.5) | 17.8 (12.8–25.5) |
| Age at onset in years (median, IQR (P25-P75)) | 31.2 (21.8 – 40.4) | 29.2 (22.7–37.5) |
| PSC | 4 | 5 |
| therapy | ||
|
34 (51.5%) | 41 (54.7%) |
|
18 (27.3%) | 10 (15.4%) |
|
11 (16.7%) | 10 (15.4%) |
| Quality of preparation | ||
|
43 (66.1%) | 50 (76.9%) |
|
22 (33.9%) | 15 (23.1%) |
Primary objective
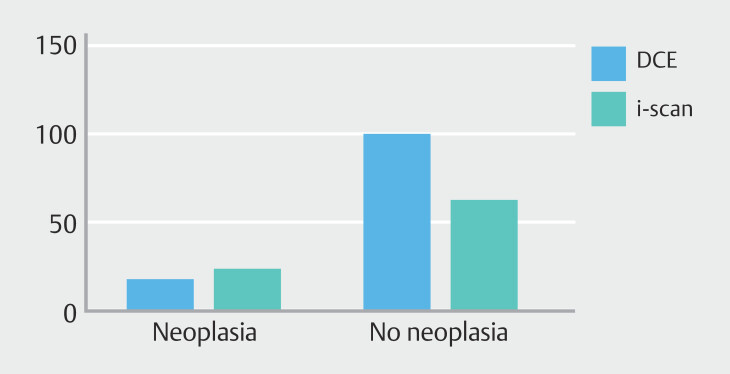
A total of 205 lesions were detected during 131 colonoscopies ( Fig. 2 ), corresponding to an average of 1.56 lesions detected per patient (/per colonoscopy). All detected lesions were resected or biopsied and histologically examined. Of the total 205 detected lesions, 42 lesions (20.5%) were neoplastic ( Table 2 ).
Fig. 2.
Number of detected lesions (neoplastic vs non neoplastic) in the DCE group and the i-scan VCE group.
Table 2 Overview of lesion type and number per group.
| DCE | i-scan | |
| DCE, dye-based chromoendoscopy; HGD, high-grade dysplasia; MGD, moderate-grade dysplasia; LGD, low-grade dysplasia. | ||
| Neoplastic lesion | 18 | 24 |
|
16 (88.8%) | 21 (87.5%) |
| HGD | 0 | 1 |
| MGD | 0 | 1 |
| LGD | 16 | 18 |
| Indefinite | 0 | 0 |
| Unknown | 0 | 1 |
|
1 (5.6%) | 2 (8.3%) |
|
0 | 1 (4.2%) |
|
1 (5.6%) | 0 |
| Non-neoplastic lesions | 100 | 63 |
|
44 (44.0%) | 26 (41.2%) |
|
4 (4.0%) | 5 (8.0%) |
|
52 (52.0%) | 32 (50,8%) |
| Total lesions | 118 | 87 |
In the DCE group (n = 66), 18 neoplastic lesions were detected in 13 patients. In the i-scan VCE group (n = 65) 24 neoplastic lesions were detected in 18 patients. The mean (SD) number of neoplastic lesions detected per colonoscopy was 0.27 (0.62) in the DCE group and 0.37 (0.72) in the i-scan VCE group ( P = 0.41).
Secondary objectives
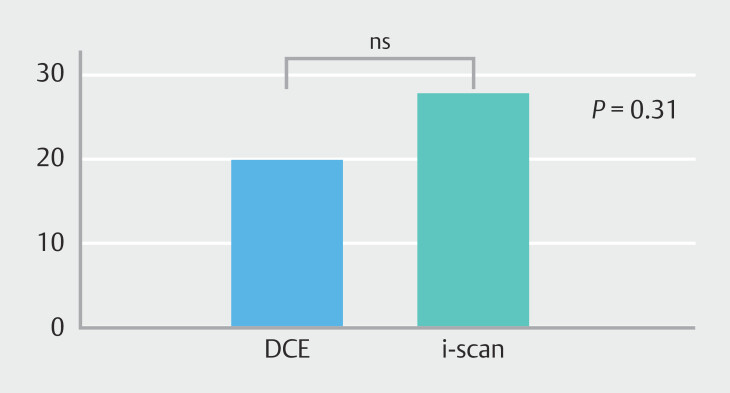
Based on the detected number of neoplastic lesions, the neoplasia detection rate (ratio of the number of colonoscopies with at least one neoplasia detected over the total number of colonoscopies) in the DCE group was 19.7% (13/66) versus 27.7% (18/65) in the i-scan group, which was not statistically significantly different ( P = 0.31) ( Fig. 3 ).
Fig. 3.
Neoplasia detection rate in the DCE group vs the i-scan VCE group.
However, when calculating the per lesion neoplasia detection rate, which is often used as an indirect parameter to estimate the false-positive rate of detections, a significant difference was found between the groups. In the DCE group 18 lesions were neoplastic from the 118 total biopsies. In the i-scan VCE group, 87 biopsies were performed, of which 21 were neoplastic. This resulted in a per lesion neoplasia detection rate of 0.15 (18/118) in the DCE group compared with 0.28 (24/87) in the i-scan VCE group ( P = 0.036).
Similarly, when comparing the number of non-neoplastic lesions per colonoscopy between the groups, a significant difference was found: In the DCE group, a mean number of 1.52 non-neoplastic lesions (95% CI 1.13 to 1.90) were biopsied per colonoscopy, compared with 0.97 non-neoplastic lesions (95% CI 0.66 to 1.28) per colonoscopy in the i-scan VCE group ( P = 0.024). However, there was no significant difference in the total number of lesions (neoplastic and non-neoplastic) when comparing DCE (n = 118) with i-scan VCE (n = 87) ( P = 0.11).
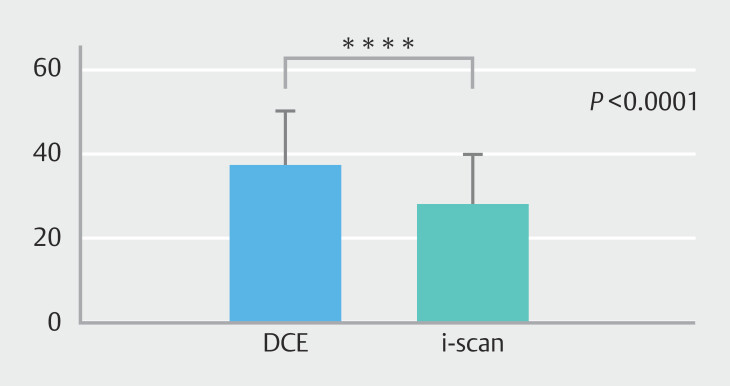
Lastly, both withdrawal and total procedure time were significantly different in the i-scan VCE group compared with the DCE group. On average, withdrawal time was 10.0 minutes shorter in the i-scan group ( P < 0.0001, 95% CI -13.43 to -6.47) and total procedure time, on average, was 9.9 minutes shorter in the i-scan group ( P < 0.0001, 95% CI -14.19 to -5.65) ( Fig. 4 ).
Fig. 4.
Total procedure time in the DCE group vs the i-scan VCE group.
Discussion
In this RCT, we did not detect any statistically significant difference between DCE and i-scan VCE for detection of neoplasia in patients with long-standing UC in clinical remission. This finding concurs with the available literature comparing DCE and i-scan VCE, showing a lack of difference in neoplasia detection rate between these two techniques 9 10 11 . In addition, other studies have compared alternative VCE technologies with similar results 19 20 21 . Even though our study was not designed for non-inferiority, the comparable rates of neoplasia detection in i-scan VCE compared with conventional DCE in a large sample size advocates for adoption of i-scan VCE as an alternative to traditional DCE in UC surveillance. At least, certainly numerically, there is no decreased detection with i-scan, which is different from previous publications 9 . However, our results show that i-scan VCE had a significantly better per lesion neoplasia detection rate compared with DCE (0.28 vs 0.15, P = 0.036) and a lower number of non-neoplastic lesions detected per colonoscopy (0.97 vs 1.52, P = 0.024). In concordance with one of our previous studies comparing DCE with NBI in long-standing UC surveillance 19 , DCE detected numerous hyperplastic lesions (37% of total resected lesions in DCE), suggesting that DCE highlights minimal changes in the colonic mucosa that have no prognostic significance, ultimately leading to lower accuracy and associated higher costs. Moreover, our study showed a significant reduction in average withdrawal time (-10.0 minutes, P < 0.001) and total procedure time (-9.9 minutes, P < 0.001) in favor of i-scan VCE. Other studies comparing DCE and VCE confirm that use of VCE is associated with a significant reduction in procedure time compared with DCE 10 11 19 20 21 .
One of the strengths of our study is that it was prospective, randomized, and multicenter. Moreover, colonoscopies were performed by expert endoscopists who had received prior standardized training in DCE and i-scan VCE, limiting endoscopist-related confounding factors such as individual endoscopist experience and learning curve. Our study did also have limitations. It was powered for superiority in neoplasia detection. Nevertheless, our group sizes fell short of the target group size set during power calculation. A type II error, therefore, cannot be excluded. Yet because our total sample size was > 130 patients, any possible missed differences in both groups would probably be clinically irrelevant, given the high number needed to treat. It can also not be fully ruled out that some lesions were missed with use of DCE or i-scan VCE because no random biopsies were taken. Still, this risk is considered negligible because the rate of neoplasia detection using random biopsies is very small and no additional neoplasia was detected in the lesion-surrounding biopsies [22 . ]. Finally, due to the inclusion criteria for our study, our data only apply to patients with quiescent UC and adequate bowel preparation.
Conclusions
In conclusion, this trial did not show any significant difference in neoplasia detection when using i-scan VCE compared with conventional DCE in detecting neoplasia in surveillance colonoscopy in long-standing UC. Moreover, use of i-scan is associated with significantly better per lesion neoplasia detection and is timesaving compared with conventional DCE. Hence, our results support use of i-scan VCE as an alternative to conventional DCE in patients undergoing surveillance colonoscopy for long-standing UC.
Footnotes
Conflict of Interest - Alexander Jans: No COI - Pieter Sinonquel: No COI - Tom Seerden: No COI - John Karstensen: JGK receives consultancy fees from Ambu, SNIPR BIOME and Boston Scientific. JGK receives speaker fees from Norgine. - Séverine Vermeire: SV has received grants from AbbVie, J&J, Pfizer, Takeda and Galapagos. SV has received consulting and/or speaking fees from AbbVie, Abivax, AbolerIS Pharma, AgomAb, Alimentiv, Arena Pharmaceuticals, AstraZeneca, Avaxia, BMS, Boehringer Ingelheim, Celgene, CVasThera, Cytoki Pharma, Dr Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead, GSK, Hospira, Imidomics, Janssen, J&J, Lilly, Materia Prima, MiroBio, Morphic, MrMHealth, Mundipharma, MSD, Pentax, Pfizer, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance, Tillots Pharma AG and Zealand Pharma. - Raf Bisschops: RB has received grants from Pentax, Fujifilm, Medtronic and Norgine. RB has received consultation fees from Pentax, Fujifilm, Medtronic, Norgine, CDx diagnostics, Ipsen and Ferring - Marieke Pierik: MP has received non-restricted research grants from Horizon 2020, ZONMW (Dutch national research fund), Maag Lever Darm stichting, Takeda, Johnson and Johnson, Abbvie and Galapagos, non-financial support from Immunodiagnostics, speaker’s fee from BMS, Janssen Cilag and Takeda all outside the submitted work. - Alexander De Bodelier: No COI - Gert De Hertogh: GDH receives consultancy fees from PENTAX Medical for support in pathology analysis. - Ingrid Demedts: No COI - Stine Sloth: No COI - Rogier De Ridder: No COI - Hilde Willekens: No COI
References
- 1.Zhou Q, Shen ZF, Wu BS et al. Risk of colorectal cancer in ulcerative colitis patients: a systematic review and meta-analysis. Gastroenterol Res Pract. 2019;5363261 doi: 10.1155/2019/5363261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magro F, Gionchetti P, Eliakim R et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649–670. doi: 10.1093/ecco-jcc/jjx008. [DOI] [PubMed] [Google Scholar]
- 3.Dekker E, Nass K, Lacucci M et al. Performance measures for colonoscopy in inflammatory bowel disease patients: European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2022;54:904–915. doi: 10.1055/a-1874-0946. [DOI] [PubMed] [Google Scholar]
- 4.Annese V, Daperno M, Rutter MD et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Kiesslich R, Fritsch J, Holtmann M et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880–888. doi: 10.1053/gast.2003.50146. [DOI] [PubMed] [Google Scholar]
- 6.Rutter MD, Saunders BP, Schofield G et al. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256–260. doi: 10.1136/gut.2003.016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kodashima S, Fujishiro M. Novel image-enhanced endoscopy with i-scan technology. World J Gastroenterol. 2010;16:1043–1049. doi: 10.3748/wjg.v16.i9.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisschops R, East JE, Hassan C et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy. 2019;51:1155–1179. doi: 10.1055/a-1031-7657. [DOI] [PubMed] [Google Scholar]
- 9.Iacucci M, Kaplan GG, Panaccione R et al. A randomized trial comparing high definition colonoscopy alone with high definition dye spraying and electronic virtual chromoendoscopy for detection of colonic neoplastic lesions during IBD surveillance colonoscopy. Am J Gastroenterol. 2018;113:225–234. doi: 10.1038/ajg.2017.417. [DOI] [PubMed] [Google Scholar]
- 10.López-Serrano A, Suárez MJ, Besó P et al. Virtual chromoendoscopy with iSCAN as an alternative method to dye-spray chromoendoscopy for dysplasia detection in long-standing colonic inflammatory bowel disease: a case–control study. Scand J Gastroenterol. 2021;56:820–828. doi: 10.1080/00365521.2021.1925339. [DOI] [PubMed] [Google Scholar]
- 11.González-Bernardo O, Riestra S, Vivas S et al. Chromoendoscopy with indigo carmine vs virtual chromoendoscopy (iSCAN 1) for neoplasia screening in patients with inflammatory bowel disease: A prospective randomized study. Inflamm Bowel Dis. 2021;27:1256–1262. doi: 10.1093/ibd/izaa291. [DOI] [PubMed] [Google Scholar]
- 12.Murthy SK, Feuerstein JD, Nguyen GC et al. AGA Clinical Practice Update on Endoscopic Surveillance and Management of Colorectal Dysplasia in Inflammatory Bowel Diseases: Expert Review. Gastroenterology. 2021;161:1043–1.051E7. doi: 10.1053/j.gastro.2021.05.063. [DOI] [PubMed] [Google Scholar]
- 13.Gordon H, Biancone L, Fiorino G et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J Crohns Colitis. 2023;17:827–854. doi: 10.1093/ecco-jcc/jjac187. [DOI] [PubMed] [Google Scholar]
- 14.Lamb CA, Kennedy NA, Raine T et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutter MD, Saunders BP, Wilkinson KH et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–1038. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 16.Langner C, Magro F, Driessen A et al. The histopathological approach to inflammatory bowel disease: A practice guide. Virchows Archiv. 2014;464:511–527. doi: 10.1007/s00428-014-1543-4. [DOI] [PubMed] [Google Scholar]
- 17.Lai EJ, Calderwood AH, Doros G et al. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620–625. doi: 10.1016/j.gie.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo S ei, Tamura S, Nakajima T et al. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8–14. doi: 10.1016/s0016-5107(96)70222-5. [DOI] [PubMed] [Google Scholar]
- 19.Bisschops R, Bessissow T, Joseph JA et al. Chromoendoscopy versus narrow band imaging in UC: A prospective randomised controlled trial. Gut. 2018;67:1087–1094. doi: 10.1136/gutjnl-2016-313213. [DOI] [PubMed] [Google Scholar]
- 20.Gulati S, Dubois P, Carter B et al. A randomized crossover trial of conventional vs virtual chromoendoscopy for colitis surveillance: Dysplasia detection, feasibility, and patient acceptability (CONVINCE) Inflamm Bowel Dis. 2019;25:1096–1106. doi: 10.1093/ibd/izy360. [DOI] [PubMed] [Google Scholar]
- 21.Pellisé M, López-Cerón M, Rodríguez De Miguel C et al. Narrow-band imaging as an alternative to chromoendoscopy for the detection of dysplasia in long-standing inflammatory bowel disease: A prospective, randomized, crossover study. Gastrointest Endosc. 2011;74:840–848. doi: 10.1016/j.gie.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Van Den Broek FJC, Stokkers PCF, Reitsma JB et al. Random biopsies taken during colonoscopic surveillance of patients with longstanding ulcerative colitis: Low yield and absence of clinical consequences. Am J Gastroenterol. 2014;109:715–722. doi: 10.1038/ajg.2011.93. [DOI] [PubMed] [Google Scholar]