Abstract
Background and study aims Although small hypervascular tumors are suspected to be pancreatic neuroendocrine tumors (p-NENs), their diagnosis and treatment are challenging. This study evaluated the usefulness of endoscopic ultrasound-guided tissue acquisition (EUS-TA) for diagnosis of small p-NENs.
Methods All p-NEN lesions that underwent EUS-TA at our hospital between April 2018 and December 2023 were retrospectively analyzed. The diagnostic sensitivity of EUS-TA and the concordance rate of grading with EUS-TA and surgical specimens were examined. The lesions were grouped by size.
Results The diagnostic sensitivity of EUS-TA was analyzed for 82 lesions, of which 44 were compared with postoperative specimens for grading. The definitive diagnosis was neuroendocrine tumor (NET) in 75 lesions, neuroendocrine carcinoma in five lesions, and mixed neuroendocrine non-neuroendocrine neoplasm in two lesions. Thirty tumors were ≤10 mm, 30 were 10 to 20 mm, and 22 were >20 mm, and the diagnostic sensitivities were 96.7%, 96.7%, and 90.9%, respectively. Concordance rates for grading were 94.4%, 82.4%, and 77.8% for tumors ≤10 mm, 10 to 20 mm, and ≥20 mm, respectively, with Cohen’s kappa coefficients of 0.64, 0.48, and 0.40, respectively.
Conclusions EUS-TA showed adequate diagnostic sensitivity and grading agreement for p-NENs of all sizes, allowing for determination of appropriate treatment.
Keywords: Endoscopic ultrasonography, Pancreas, Tissue diagnosis, Fine-needle aspiration/biopsy
Introduction
Pancreatic neuroendocrine neoplasm (p-NEN) is a rare pancreatic tumor that accounts for 2% to 3% of all pancreatic neoplasms 1 . According to the 2019 World Health Organization (WHO) classification system, neuroendocrine neoplasms (NENs) are classified as neuroendocrine tumors (NETs), neuroendocrine carcinomas (NECs), and mixed neuroendocrine non-neuroendocrine neoplasms (MiNENs). NETs are further graded based on the Ki-67 index (G1: Ki-67 index <3%; G2: Ki-67 index 3–20%; G3: Ki-67 index >20%) 2 . Pancreatic NECs and MiNENs are associated with extremely poor prognoses and are typically treated with chemotherapy 3 4 . In contrast, surgical resection is generally performed for p-NET due to its malignant potential. However, the European Neuroendocrine Tumor Society (ENETS) suggests that this observation may also be considered for p-NETs that are <20 mm, nonfunctional, classified as G1 or low G2, asymptomatic, and predominantly in the head of the pancreas, show no radiologic malignant findings, and have no other patient-related factors 5 .
Recent advances in imaging diagnostic equipment have led to an increase in incidentally-diagnosed p-NET 6 7 , resulting in a parallel rise in detection of small p-NETs 8 . p-NETs ≤10 mm demonstrate malignancy and are graded from G1 to G3 9 . p-NET grading has been reported as an independent risk factor for metastasis 10 . Of all tumors, including those <5 mm, 33% exhibit regional lymph node metastasis and 11% exhibit distant metastasis 11 . Therefore, to establish a treatment strategy for p-NENs ≤10 mm in diameter, precise grading based on the Ki-67 index is necessary. However, no previous studies have assessed the diagnostic accuracy of endoscopic ultrasound-guided tissue acquisition (EUS-TA) specifically for p-NETs ≤10 mm. Therefore, this study analyzed the diagnostic utility of EUS-TA for p-NENs ≤10 mm and assessed the concordance rate for grading these p-NETs by comparing the EUS-TA results with the histological results.
Patients and methods
Patient selection
Data from patients referred to Tokyo Medical and Dental University Hospital between April 2018 and December 2023 based on a clinical suspicion of p-NEN for whom EUS-TA was performed were retrospectively analyzed. When surgery was conducted, definitive diagnosis was based on the surgical specimens. When surgery was not performed, definitive diagnosis was determined using biochemical, radiological, and somatostatin receptor imaging assessments conducted during a 6-month follow-up period. In cases with multiple lesions, all lesions for which EUS-TA was performed were included in the analysis.
Patient age, sex, endocrinological symptoms, cystic components, and surgical history and the primary tumor location, puncture site, needle gauge, needle type, number of punctures, EUS-TA diagnosis, final diagnosis, complication, and multiple endocrine neoplasia type 1 (MEN-1) data were extracted from patient charts.
EUS-TA procedure
EUS-TA was conducted using a GF-UCT260 ultrasound gastrovideoscope (Olympus, Tokyo, Japan) and an EU-ME2 PREMIER PLUS (Olympus, Tokyo, Japan) ultrasound processor was used. Acquire (Boston Scientific, Marlborough, Massachusetts, United States) 22- or 25-G needles, SharkCore (Medtronic, Dublin, Ireland) 22- or 25-G needles, and Trident (Century Medical, Tokyo, Japan) 22-G needles were used during fine-needle biopsy (FNB). EZ shot 3 plus(Olympus, Tokyo, Japan) 25-G needles were used for fine-needle aspiration. The puncture needle was selected at surgeon discretion. Basically, when performing EUS-TA, continuous suction was applied using a 20-mL syringe and the lesion was punctured with 20 rapid suction strokes. Rapid onsite specimen evaluation is not performed at our hospital; therefore, EUS-TA was considered complete when it was judged that an adequate amount of white tissue had been acquired from the tumor.
Histopathology and Ki-67 assessment
Specimens acquired using EUS-TA were rinsed with physiological saline solution. The solid part of the specimen, including white tissue, was fixed in 10% neutral buffered formalin and underwent histological analysis. After 24 hours of formalin fixation, histological samples were embedded in paraffin and treated as normal tissue blocks. Thin, 3-μm sections were sliced from the paraffin-embedded cell blocks and stained with hematoxylin and eosin.
Specimens collected using EUS-TA were evaluated by two or more experienced pathologists to determine the definitive diagnosis. p-NEN was diagnosed when chromogranin A (CGA), synaptophysin (SYN), and/or neural cell adhesion molecule (CD56) results were positive. The Ki-67 index was assessed using at least 500 cells in areas with the highest density of Ki-67+ cells (hotspots), as recommended by the WHO 12 . If 500 cells could not be evaluated, the tumor was not graded.
Surgical specimens were fixed with 10% neutral buffered formalin for 48 hours and sliced into sections <5 mm thick for lesion identification. After paraffin embedding, the specimens were processed in the same manner as EUS-TA specimens.
Immunostaining of the largest segment of the lesion was performed for diagnosis. Ki-67 hotspots were identified within the specimens and were evaluated using the same criteria as the EUS-TA specimens.
Analytic endpoint
EUS-TA sensitivity for diagnosis of p-NEN pathologically diagnosed using EUS-TA or surgery was determined. Then, the concordance rate of grading among cases with both EUS-TA and surgical specimen grades was investigated. These endpoints were compared among groups based on tumor diameter: ≤10 mm, 10 to 20 mm, and >20 mm.
Statistical analysis
Continuous variables are presented as median and interquartile range (IQR), and categorical variables are presented as frequency and percentage. Distributions of continuous and categorical variables among the three groups of lesion sizes were compared using the Wilcoxon rank-sum and chi-squared tests, respectively. Sensitivity and 95% confidence intervals (CI) for the diagnosis of p-NEN using EUS-TA were calculated. The concordance rate (95% CI) of p-NET grading between EUS-TA and surgical specimens was evaluated, as was the kappa coefficient (κ) to quantify consistency. For each categorical variable, we calculated odds ratios and 95% confidence intervals (95% CIs) for correct diagnosis of p-NEN using EUS-TA and the concordance rate of p-NET grading between EUS-TA and surgical specimens using univariable and multivariable logistic regression analyses. Statistical significance was set at P <0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, United States).
Results
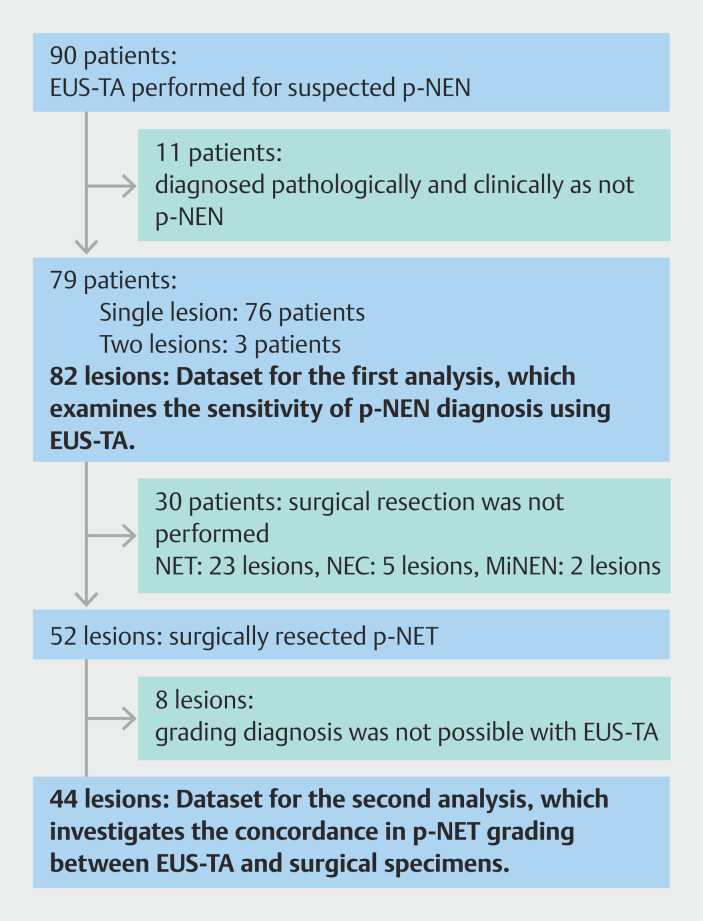
EUS-TA was performed in 90 patients with clinically suspected p-NEN. Eleven patients were excluded from this study because p-NEN was pathologically and clinically ruled out in these patients. Among the remaining 79 patients, three underwent EUS-TA for two lesions. Therefore, 82 lesions were included in this study ( Fig. 1 ), including 39 in male patients and 43 in female patients ( Table 1 ). Median patient age was 64 years (IQR: 54.3−73 years) and median lesion size was 12 mm (IQR: 9–22 mm). Fifty-two lesions (63.4%) were surgically resected. Among the remaining 30 lesions, two were MiNEN, five were NEC, and 23 were NET. The NET lesions were not resected due to evidence of metastasis (n=6), small size (n=10), MEN-1 (n=2), and placement on the surgical waitlist (n=5) ( Fig. 1 ). The final pathological diagnosis was NET G1 in 58 lesions, NET G2 in 15 lesions, NET G3 in one lesion, and NET of undiagnosed grade in one lesion.
Fig. 1.
Study flow chart. p-NEN, pancreatic neuroendocrine neoplasm; EUS-TA, endoscopic ultrasound-guided tissue acquisition; NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma; MiNEN, mixed neuroendocrine non-neuroendocrine neoplasm; p-NET, pancreatic neuroendocrine tumor.
Table 1 Characteristics of p-NEN lesions that underwent EUS-TA.
| Total | ≤10 mm | 10–20 mm | >20 mm | P value | ||
|
Statistically significant
P
values are indicated in bold.
EUS-TA, endoscopic ultrasound-guided tissue acquisition; FNA, fine-needle aspiration; FNB, fine-needle biopsy; IQR, interquartile range; MEN 1, multiple endocrine neoplasia type 1; MiNEN, mixed neuroendocrine non-neuroendocrine neoplasm; NEC, neuroendocrine carcinoma;NET, neuroendocrine tumor; p-NEN, pancreatic neuroendocrine tumor. | ||||||
| Number of lesions | 82 | 30 | 30 | 22 | ||
| Age (years) | Median (IQR) | 64 (54.3–73) | 64 (54.8–72.3) | 65 (56–74.8) | 61.5 (52–70.5) | 0.65 |
| Sex | Male/female | 39/43 | 14/16 | 15/15 | 10/12 | 0.94 |
| Size (mm) | Median (IQR) | 12 (9–22) | 8.4 (7.8–9.8) | 12.4 (12–15) | 35.5 (26.3–50) | |
| Symptoms | N (%) | 7 (8.5) | 2 (6.7) | 3 (10.0) | 2 (9.1) | 0.89 |
| Cystic component | N (%) | 4 (4.9) | 0 (0.0) | 1 (3.3) | 3 (13.6) | 0.07 |
| Metastasis | N (%) | 15 (18.3) | 0 (0.0) | 4 (13.3) | 11 (50.0) | <0.01 |
| Multiple lesion | N (%) | 14 (17.1) | 7 (23.3) | 2 (6.7) | 5 (22.7) | 0.16 |
| MEN1 | N (%) | 5 (6.1) | 3 (10.0) | 0 (0.0) | 2 (9.1) | 0.23 |
| Tumor location | 0.31 | |||||
|
N (%) | 26 (31.7) | 6 (20.0) | 12 (40.0) | 8 (36.4) | |
|
N (%) | 29 (35.4) | 12 (40.0) | 9 (30.0) | 8 (36.4) | |
|
N (%) | 27 (32.9) | 12 (40.0) | 9 (30.0) | 6 (27.3) | |
| Surgery | N (%) | 52 (63.4) | 22 (73.3) | 18 (60.0) | 12 (54.5) | 0.03 |
| Final diagnosis | 0.02 | |||||
|
N (%) | 58 (70.7) | 28 (93.3) | 20 (66.7) | 10 (45.5) | |
|
N (%) | 15 (18.3) | 2 (6.7) | 7 (23.3) | 6 (27.3) | |
|
N (%) | 1 (1.2) | 0 (0.0) | 1 (3.3) | 0 (0.0) | |
|
N (%) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 1 (4.5) | |
|
N (%) | 5 (6.1) | 0 (0.0) | 2 (6.7) | 3 (13.6) | |
|
N (%) | 2 (2.4) | 0 (0.0) | 0 (0.0) | 2 (9.1) | |
Thirty lesions were ≤10 mm, 30 were 10 to 20 mm, and 22 were >20 mm. Larger lesions were more likely to be associated with metastasis, leading to a significant decrease in the number of surgical interventions. Similarly, larger lesions were significantly associated with higher malignancy rates ( Table 1 ).
Needle type and needle gauge, number of punctures, puncture site, and complications were not significantly different between the lesion size groups ( Table 2 ). Representative images of diagnosis of p-NENs <10 mm are shown in Fig. 2 .
Table 2 Characteristics of EUS-TA procedures.
| Total | ≤10 mm | 10–20 mm | >20 mm | P value | ||
| EUS-TA, endoscopic ultrasound-guided tissue acquisition; FNA, fine-needle aspiration; IQR, interquartile range; MEN 1, multiple endocrine neoplasia type 1; MiNEN, mixed neuroendocrine/non-neuroendocrine neoplasm; NEC, neuroendocrine carcinoma; NET, neuroendocrine tumor. | ||||||
| Number of Cases | 82 | 30 | 30 | 22 | ||
| Needle type | 0.38 | |||||
|
n (%) | 3 (3.7) | 2 (6.7) | 0 (0.0) | 1 (4.5) | |
|
n (%) | 79 (96.3) | 28 (93.3) | 30 (100) | 21 (95.5) | |
| Needle gauge | 0.48 | |||||
|
n (%) | 75 (91.5) | 28 (93.3) | 26 (86.7) | 21 (95.5) | |
|
n (%) | 7 (8.5) | 2 (6.7) | 4 (13.3) | 1 (4.5) | |
| Number of punctures | 0.07 | |||||
|
n (%) | 14 (17.1) | 8 (26.7) | 1 (3.3) | 5 (22.7) | |
|
n (%) | 55 (67.1) | 20 (66.7) | 23 (76.7) | 12 (54.6) | |
|
n (%) | 13 (15.9) | 2 (6.7) | 6 (20.0) | 5 (22.7) | |
| Puncture site | 0.13 | |||||
|
n (%) | 62 (75.6) | 27 (90.0) | 21 (70.0) | 14 (63.6) | |
|
n (%) | 1 (1.2) | 0 (0.0) | 1 (3.3) | 0 (0.0) | |
|
n (%) | 19 (23.2) | 3 (10.0) | 8 (26.7) | 8 (36.4) | |
| Complications of EUS-TA | 0.45 | |||||
|
n (%) | 1 (1.2) | 0 (0.0) | 1 (3.3) | 0 (0.0) | |
|
n (%) | 2 (2.4) | 1 (3.3) | 1 (3.3) | 0 (0.0) | |
| EUS-TA diagnosis | 0.16 | |||||
|
n (%) | 57 (69.5) | 23 (76.7) | 23 (76.7) | 11 (50.0) | |
|
n (%) | 8 (9.8) | 3 (10.0) | 3 (10.0) | 2 (9.1) | |
|
n (%) | 1 (1.2) | 0 (0.0) | 1 (3.3) | 0 (0.0) | |
|
n (%) | 5 (6.1) | 3 (10.0) | 0 (0.0) | 2 (9.1) | |
|
n (%) | 5 (6.1) | 0 (0.0) | 2 (6.7) | 3 (13.6) | |
|
n (%) | 2 (2.4) | 0 (0.0) | 0 (0.0) | 2 (9.1) | |
|
n (%) | 4 (4.9) | 1 (3.3) | 1 (3.3) | 2 (9.1) | |
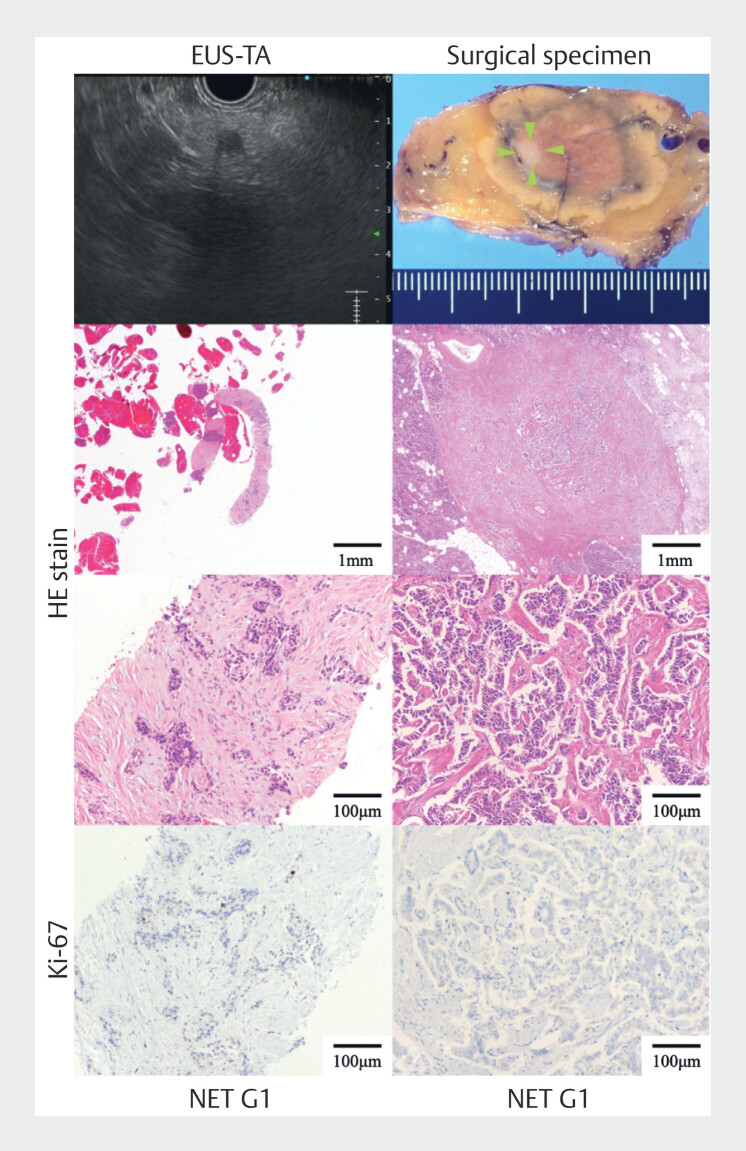
Fig. 2.
Representative images of diagnosis of small pancreatic neuroendocrine neoplasm using endoscopic ultrasound-guided tissue acquisition (EUS-TA). The smallest tumor diameter for which grading in this study could be assessed was 5 mm. The left row shows the EUS-TA specimen and the right row shows the surgical specimens (green arrowheads indicate the tumor site). The uppermost left row shows the EUS image and the uppermost right row shows the surgical specimen. The middle two rows are hematoxylin and eosin-stained sections and the bottom row is a section stained for Ki-67.
Sensitivity of EUS-TA
Overall sensitivity of EUS-TA for diagnosis of p-NEN was 95.1% (95% CI: 88.0–98.7). Among lesions ≤10 mm, 10–20 mm, and ≥20 mm, diagnostic sensitivity of EUS-TA was 96.7% (95% CI: 82.8–99.9), 96.7% (95% CI: 82.8–99.9), and 90.9% (95% CI: 70.8–98.9), respectively ( Table 3 ). Due to the small number of cases associated with each factor, both univariate and multivariate analyses lacked sufficient statistical power, making it impossible to conduct analyses of factors associated with the correct diagnosis of p-NEN using EUS-TA (Supplementary Table 1).
Table 3 Sensitivity of EUS-TA for p-NEN.
| Tumor size | Total (n) | True positive (n) | False negative (n) | Sensitivity (%) (95% CI) |
| EUS-TA, endoscopic ultrasound-guided tissue acquisition; p-NEN, pancreatic neuroendocrine neoplasm; 95% CI: 95% confidence interval. | ||||
| Overall | 82 | 78 | 4 | 95.1 (88.0–98.7) |
| ≤10 mm | 30 | 29 | 1 | 96.7 (82.8–99.9) |
| 10–20 mm | 30 | 29 | 1 | 96.7 (82.8–99.9) |
| >20 mm | 22 | 20 | 2 | 90.9 (70.8–98.9) |
Concordance of p-NET grading between EUS-TA and surgical specimens
Fifty-two patients with p-NET lesions underwent surgery. However, four of these lesions could not be diagnosed as p-NET using EUS-TA and four could not be graded using EUS-TA. The remaining 44 lesions were included in the second analysis in this study ( Fig. 1 ). Preoperatively, 39 lesions were classified as G1 and five lesions were classified as G2 ( Table 4 ). Postoperatively, 35 lesions were classified as G1 and nine were classified as G2. Undergrading was observed in lesions >10 mm and overgrading was observed in lesions ≤10 mm.
Table 4 Concordance rate for grading between EUS-FNA and surgical specimens.
| EUS-TA diagnosis | Total (n) | Surgical diagnosis (n) | Concordance rate | Kappa coefficient | |||
| G1 | G2 | % (95% CI) | κ | P | |||
|
Statistically significant
P
values are indicated in bold.
Gray backgrounds indicate lesions where grading did not match. 95% CI, 95% confidence interval; κ, Cohen's kappa coefficient; P, P value for kappa coefficient. | |||||||
| Overall | 44 | G1 | 34 | 5 | 86.4 (72.6–94.8) | 0.50 | <0.01 |
| G2 | 1 | 4 | |||||
| ≤10 mm | 18 | G1 | 16 | 0 | 94.4 (72.7–99.9) | 0.64 | <0.01 |
| G2 | 1 | 1 | |||||
| 10–20 mm | 17 | G1 | 12 | 3 | 82.4 (56.6–96.2) | 0.48 | 0.02 |
| G2 | 0 | 2 | |||||
| >20 mm | 9 | G1 | 6 | 2 | 77.8 (40.0–97.2) | 0.40 | 0.13 |
| G2 | 0 | 1 | |||||
The overall concordance rate for grading between EUS-TA and surgical specimens was 86.4% (95% CI: 72.6–94.8). For lesions ≤10 mm, 10–20 mm, and >20 mm, concordance rates were 94.4% (95% CI: 72.7–99.9), 82.4% (95% CI: 56.6–96.2), and 77.8 % (95% CI: 40.0–97.2), respectively. Substantial agreement was observed in the ≤10 mm group (κ=0.64; P <0.01), moderate agreement in all lesions (κ=0.50; P <0.01) and the 10–20 mm group (κ=0.48; P =0.02), and fair agreement in the >20 mm group (κ=0.40; P =0.13) ( Table 4 ). Due to the small number of cases associated with each factor, both univariate and multivariate analyses lacked sufficient statistical power, making it impossible to conduct analyses of factors associated with the concordance rate of p-NET grading between EUS-TA and surgical specimens (Supplementary Table 2).
Discussion
In this study, diagnostic sensitivity of EUS-TA for p-NEN was analyzed based on tumor size. The concordance rate of grading between EUS-TA and surgical specimens was also determined. Larger lesions were associated with higher malignancy and incidence of metastasis, resulting in a greater number of cases in which surgery was not conducted. Lesions >10 mm included those with metastasis. Among the lesions ≤10 mm, two were G2. G2 is a risk factor for metastasis even in small p-NENs 13 , and conservative management was not recommended for these lesions according to ENETS guidelines. Therefore, lesions of all sizes must be diagnosed and malignancy must be assessed via EUS-TA. Among patients with lesions ≤10 mm who underwent EUS-TA, pancreatitis was diagnosed in one patient and managed conservatively. Complication rates, including the hemorrhage rate, were not significantly different among lesion size groups, highlighting the safety of EUS-TA ( Table 2 ).
The overall diagnostic sensitivity of EUS-TA for p-NEN was high ( Table 3 ). Similar results were also obtained when limited to surgically resected p-NENs (Supplementary Table 3). FNB needles have been reported to have improved diagnostic performance 14 . Diagnostic sensitivity of EUS-TA using an FNB needle for p-NEN ranges from 90.3% to 100.0% 15 16 17 18 , which is similar to sensitivity determined in the current study. Sensitivity was high for each lesion size group in the current study. FNB needles were typically used for EUS-TA in this study, which may account for the high sensitivity in lesions ≤10 mm. In addition, p-NENs are easily visualized using EUS and demonstrate high cellularity, which may facilitate tissue acquisition, even from small lesions 19 . Slightly decreased sensitivity for lesions >20 mm is thought to be due to inclusion of cases with low cellularity due to tumor necrosis as the lesions increase in size.
Ki-67 grading was feasible using samples obtained via EUS-TA in most lesions in this study (66/71; 93.0%), including 93.1% of lesions ≤10 mm, 100% of lesions 10 to 20 mm, and 86.7% of lesions >20 mm. Grading of p-NET using an FNB needle has been reported as feasible in 84.7% to 90.3% of lesions in previous studies 19 20 , which is consistent with the current results. The concordance rate of p-NET grading between EUS-TA and surgical specimens in the ≤10 mm group indicated substantial agreement ( Table 4 ). In a previous systematic review, the concordance rate of the Ki-67 index for p-NET was 77.5% and the κ was 0.65. Among lesions ≤20 mm, the concordance rate was 84.5% and the κ was 0.59 20 . In the current study, the κ was highest among lesions ≤10 mm. EUS-TA achieved the wrong grade in six lesions in this study: one lesion in the ≤10-mm group was overgraded and five lesions in the >10 mm group were undergraded ( Table 4 ). In the overgraded case, it was found that the EUS-TA specimen had a high concentration of blood cell components, which may have contributed to overestimation of the Ki-67 index.
Undergrading is more problematic than overgrading because it can lead to misjudgment about cases that should be treated aggressively. As tumor size increases, intratumoral heterogeneity increases 21 22 , which may contribute to discrepancies in grading. Tissue must be collected from a wide area in large tumors by changing the puncture line or utilizing the fanning method as much as possible to collect tissue from tumor hotspots. In contrast, if the tumor is small, heterogeneity is less likely to occur. Therefore, undergrading is less likely to occur in tumors ≤10 mm when an adequate amount of tissue is collected, allowing for determination of the appropriate treatment strategy.
This study is not without limitations. First, this was a retrospective study and the sample size was limited. There were a few G2 and G3 lesions. Furthermore, the >20-mm group was smaller than the other groups. These factors may affect the concordance rates. Second, because p-NEN were not definitively diagnosed via surgery unless clinically suspected, specificity and accuracy could not be calculated in this study. However, this limitation has also been observed in previous studies.
Conclusions
In conclusion, with advances in diagnostic imaging, there is a growing demand for diagnosis of small lesions with a clinical suspicion of p-NEN. Some studies have reported aggression in some p-NENs <10 mm and have raised concerns about relying on surveillance alone 23 24 . EUS-TA demonstrated high diagnostic sensitivity and concordance rates for grading p-NENs <10 mm. Therefore, EUS-TA is useful for appropriate diagnosis of patients with tumors ≥G2 at risk of metastasis to determine an appropriate treatment strategy, even for small p-NENs. On the other hand, there is still no consensus regarding EUS-TA for small NETs. Therefore, it is necessary to make an informed decision on its indication by thoroughly explaining risks and benefits to the patient.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Supplementary Material
References
- 1.Fesinmeyer MD, Austin MA, Li CI et al. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1766–1773. doi: 10.1158/1055-9965.EPI-05-0120. [DOI] [PubMed] [Google Scholar]
- 2.Gill AJ, Klimstra DS, Lam AK WHO Classification of Tumours Editorial Board . Lyon: IARC Press; 2019. Neuroendocrine neoplasms; pp. 343–370. [Google Scholar]
- 3.Garcia-Carbonero R, Sorbye H, Baudin E et al. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology. 2016;103:186–194. doi: 10.1159/000443172. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Lou X, Qin Y et al. Mixed neuroendocrine nonneuroendocrine neoplasms of the pancreas: a case report and literature review of pancreatic mixed neuroendocrine nonneuroendocrine neoplasm. Gland Surg. 2021;10:3443–3452. doi: 10.21037/gs-21-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falconi M, Eriksson B, Kaltsas G et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103:153–171. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao JC, Hassan M, Phan A et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 7.Halfdanarson TR, Rubin J, Farnell MB et al. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008;15:409–427. doi: 10.1677/ERC-07-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallet J, Law CHL, Cukier M et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589–597. doi: 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- 9.Jutric Z, Grendar J, Hoen HM et al. Regional metastatic behavior of nonfunctional pancreatic neuroendocrine tumors: impact of lymph node positivity on survival. Pancreas. 2017;46:898–903. doi: 10.1097/MPA.0000000000000861. [DOI] [PubMed] [Google Scholar]
- 10.Pan M, Yang Y, Teng T et al. Development and validation of a simple-to-use nomogram to predict liver metastasis in patients with pancreatic neuroendocrine neoplasms: a large cohort study. BMC Gastroenterol. 2021;21:101. doi: 10.1186/s12876-021-01685-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gratian L, Pura J, Dinan M et al. Impact of extent of surgery on survival in patients with small non-functional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol. 2014;21:3515–3521. doi: 10.1245/s10434-014-3769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd RV, Osamura RY, Klöppel GN . Lyon, France: WHO; 2017. WHO Classification of Tumours of Endocrine Organs. [Google Scholar]
- 13.Javed AA, Pulvirenti A, Zheng J et al. A novel tool to predict nodal metastasis in small pancreatic neuroendocrine tumors: A multicenter study. Surgery. 2022;172:1800–1806. doi: 10.1016/j.surg.2022.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Levine I, Trindade AJ. Endoscopic ultrasound fine needle aspiration vs fine needle biopsy for pancreatic masses, subepithelial lesions, and lymph nodes. World J Gastroenterol. 2021;27:4194–4207. doi: 10.3748/wjg.v27.i26.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eusebi LH, Thorburn D, Toumpanakis C et al. Endoscopic ultrasound-guided fine-needle aspiration vs fine-needle biopsy for the diagnosis of pancreatic neuroendocrine tumors. Endosc Int Open. 2019;7:E1393–E1399. doi: 10.1055/a-0967-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witt BL, Factor RE, Chadwick BE et al. Evaluation of the SharkCore needle for EUS-guided core biopsy of pancreatic neuroendocrine tumors. Endosc Ultrasound. 2018;7:323–328. doi: 10.4103/eus.eus_51_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Leo M, Poliani L, Rahal D et al. Pancreatic neuroendocrine tumours: the role of endoscopic ultrasound biopsy in diagnosis and grading based on the WHO 2017 classification. Dig Dis. 2019;37:325–333. doi: 10.1159/000499172. [DOI] [PubMed] [Google Scholar]
- 18.Kamata K, Ashida R, Yasukawa S et al. Histological diagnosis and grading of pancreatic neuroendocrine tumor by endoscopic ultrasound-guided fine needle biopsy using a 25-gauge needle with a core trap: A multicenter prospective trial. Pancreatology. 2020;20:1428–1433. doi: 10.1016/j.pan.2020.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Hijioka S, Hara K, Mizuno N et al. Diagnostic performance and factors influencing the accuracy of EUS-FNA of pancreatic neuroendocrine neoplasms. J Gastroenterol. 2016;51:923–930. doi: 10.1007/s00535-016-1164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishii T, Katanuma A, Toyonaga H et al. Role of endoscopic ultrasound in the diagnosis of pancreatic neuroendocrine neoplasms. Diagnostics. 2021;11:316. doi: 10.3390/diagnostics11020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unno J, Kanno A, Masamune A et al. The usefulness of endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of pancreatic neuroendocrine tumors based on the World Health Organization classification. Scand J Gastroenterol. 2014;49:1367–1374. doi: 10.3109/00365521.2014.934909. [DOI] [PubMed] [Google Scholar]
- 22.Fujimori N, Osoegawa T, Lee L et al. Efficacy of endoscopic ultrasonography and endoscopic ultrasonography-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors. Scand J Gastroenterol. 2016;51:245–252. doi: 10.3109/00365521.2015.1083050. [DOI] [PubMed] [Google Scholar]
- 23.Arra DASM, Ribeiro HSC, Henklain G et al. Surgery or active surveillance for pNETs < 2 cm: preliminary results from a single center Brazilian cohort. J Surg Oncol. 2022;126:168–174. doi: 10.1002/jso.26931. [DOI] [PubMed] [Google Scholar]
- 24.Perinel J, Nappo G, Zerbi A et al. Sporadic nonfunctional pancreatic neuroendocrine tumors: risk of lymph node metastases and aggressiveness according to tumor size: a multicenter international study. Surgery. 2022;172:975–981. doi: 10.1016/j.surg.2022.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.