Abstract
Objective
To assess the feasibility, efficacy, and safety of targeted embolization for cavernous sinus dural arteriovenous fistulas (CSDAVF).
Materials and Methods
This retrospective study investigated patients with CSDAVF who underwent endovascular treatment at a tertiary hospital between October 1991 and March 2023. Treatment strategies were determined based on clinical symptoms and shunt characteristics. Targeted or non-targeted curative embolization was performed to achieve complete shunt occlusion. Initially, targeted embolization, selective occlusion of the shunted pouch while preserving the normal cavernous sinus lumen, was conducted, should that fail, non-targeted embolization was performed. In contrast, palliative embolization solely reduced shunt flow. Clinical signs, imaging characteristics, and outcomes were evaluated according to the agreed treatment strategy.
Results
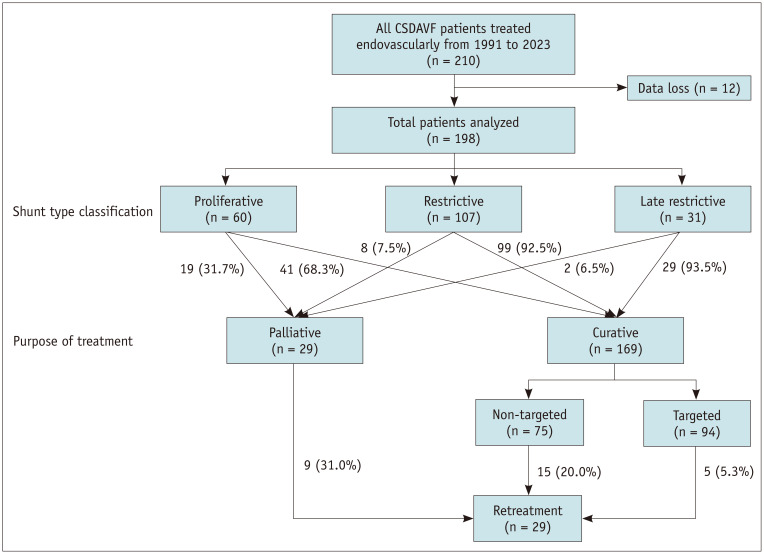
In total, 198 patients with CSDAVF (mean age 59.0 ± 12.1 years, 23.2% male) participated in this study. Of which, 94 patients (47.5%) were treated with targeted embolization, 75 (37.9%) with non-targeted embolization, and 29 (14.6%) with palliative treatment. For patients undergoing curative embolization, 55.7% (94/169) successfully achieved targeted embolization; this procedure was usually used to treat focal fistulas (restrictive or late-restrictive types), whereas diffuse fistulas (proliferative type) often underwent non-targeted or palliative embolization. For patients that underwent targeted embolization, the rate of complete or near-complete occlusion on immediate post-treatment digital subtraction angiography was 93.6% (88/94), with a complication rate of 2.1% (2/94), symptom improvement rate of 96.8% (91/94), and retreatment rate of 5.3% (5/94). No serious complications were reported during follow-up.
Conclusion
When successful, targeted embolization of CSDAVF causes low rates of cranial nerve palsy, retreatment, and good clinical outcomes.
Keywords: Cavernous sinus, Dural arteriovenous fistula, Endovascular treatment, Targeted embolization
INTRODUCTION
Cerebrovascular disorders represent a complex spectrum of pathological conditions, including cavernous sinus dural arteriovenous fistulas (CSDAVF), which present various symptoms, treatment options, and technical approaches [1,2]. Most cases of CSDAVF involve abnormal connections between dural arteries and the cavernous sinus (CS), leading to complications such as eye redness, proptosis, chemosis, bruit, retro-orbital pain, unilateral headache, diplopia, dilated conjunctival veins, transient sixth nerve palsy, elevated intraocular pressure, and/or diminished visual acuity [3,4]. As our understanding of CSDAVF deepens, a multidisciplinary approach becomes instrumental in the development of effective treatment strategies [5].
Traditionally, endovascular treatment (EVT) has been the primary therapeutic approach for CSDAVF [6]. Specifically, optimal treatment involves transvenous embolization, ideally focusing exclusively on the fistula as the preferred target [7,8,9]. However, transvenous embolization of the entire affected sinus is usually performed as a CSDAVF treatment, which may result in disturbance of normal venous drainage or permanent cranial nerve palsy [10]. In this regard, targeted embolization, involving the deliberate occlusion of specific vessels contributing to the fistula, has garnered attention for its precision and efficacy [10,11,12,13]. However, a comprehensive exploration of the outcomes associated with this treatment remains a critical area of research [10].
This observational study aimed to assess the feasibility, efficacy, and safety of targeted embolization for the treatment of CSDAVF.
MATERIALS AND METHODS
The Local Ethics Committee approved this study (IRB No. S2024-0517-0001), and the requirement for informed consent was waived because of the retrospective nature of this study.
Patient Selection
We retrospectively reviewed the data of patients with CSDAVF who underwent EVT and were admitted to a tertiary hospital between October 1991 and March 2023. Patients were included in this study if 1) they were ≥18 years, 2) diagnosis was made on transarterial six-vessel angiography, including the bilateral internal carotid, external carotid, and vertebral arteries, and 3) they underwent transarterial or transvenous EVT for curative or palliative therapy. An experienced neuro-interventionist made the final decisions. We excluded patients with no related data, including imaging or clinical outcomes, and Gamma Knife treatment was excluded from the treatment modalities assessed in our study. During the study, 210 patients underwent initial EVT; however, 12 patients were excluded because of their lack of adequate medical documentation.
Definitions
Targeted embolization refers to procedures in which an embolic agent is specifically directed toward the intended target area based on predefined imaging and anatomical criteria. This was intended to preserve the anatomical and functional integrity of the CS, selectively occluding the pathological arteriovenous connections within the CS.
In cases in which curative embolization was performed, we initially attempted targeted embolization. However, if access to the fistula site failed, if the shunt remained after successfully occluding the target area, or if there was a risk of shunt redirection due to incomplete occlusion, we proceeded with non-targeted embolization. This approach occludes the fistula and normal CS, often resulting in the occlusion of the entire ipsilateral CS.
Success rate was defined as the percentage of procedures in which the intended treatment outcome (complete or near-complete occlusion) was achieved immediately after digital subtraction angiography (DSA).
Treatment Procedure
Treatment is recommended for patients with CSDAVF who show retrograde venous drainage into the cortical and/or deep vein systems along with those experiencing severe symptoms, such as intolerable diplopia, severe headaches, neurological deficits, progressive visual loss, or increased intraocular pressure. In contrast, conservative management is recommended for patients with incidentally discovered CSDAVF or those with mild symptoms [14].
Curative treatment aims to completely occlude the shunt, which is typically chosen when the target is discrete and can be feasibly occluded without completely occluding the CS. By contrast, palliative treatment aims to reduce shunt flow, thereby decreasing venous pressure, improving symptoms, and reducing the risk of intracranial hemorrhage or edema. This approach is usually adopted when the shunt is too diffused for targeted embolization [15].
Embolization procedures for treating CSDAVF includes both transarterial and transvenous approaches. The transarterial route, primarily using polyvinyl alcohol (PVA) particles, is preferred for palliative embolization and targeted external carotid artery feeders. The transvenous method, chosen for curative embolization, is performed using the ipsilateral inferior petrosal sinus (IPS), irrespective of its occlusion status [16]. In case of failure, alternatives such as contralateral IPS, superior petrosal sinus, superior ophthalmic vein, or direct puncture were considered [5,17]. The main objective upon reaching the fistula was performing targeted embolization to occlude the shunted pouch using coils while sparing the CS lumen (Fig. 1) [9,10]. If this strategy did not adequately control the shunt, additional coils were placed in the CS to block venous reflux and occlude diffuse fistular points, thus adjusting treatment strategy to non-targeted embolization.
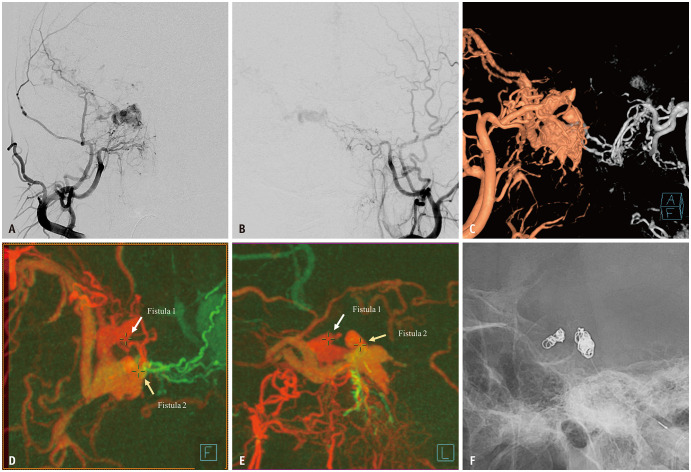
Fig. 1. A patient in their 60s underwent targeted embolization of cavernous sinus dural arteriovenous fistula after presenting with chemosis and diplopia. A, B: Bilateral external carotid arteriographies revealing an arteriovenous shunt with cortical reflux in the right cavernous sinus. C-E: Volume-rendered (C) and source fusion (D, E) images from three-dimensional rotational angiographies identifying two distinct fistulas (arrows) located anteromedially and posteromedially within the cavernous sinus. F: Transvenous coil embolization was selectively performed on each shunted pouch, achieving complete shunt occlusion (not shown).
We implemented several strategies to avoid potential complications, such as unintentional compartmental occlusion and shunt flow redirection. To mitigate these risks, we carefully reviewed CS anatomy preoperatively using both two-dimensional (2D) and three-D rotational angiography (3D-RA). This detailed imaging facilitated the development of our treatment strategy. During embolization, we were particularly careful to avoid occluding the sole access route to the fistula, ensuring that any remaining parts remained accessible. If there is a significant risk of redirecting shunt flow to sensitive areas, such as the cortical or ophthalmic veins, we may preemptively place coils at these veins orifices before occluding the main fistula. Should complications arise despite these precautions, shunt flow should be reduced using transarterial particles or other embolic materials, minimizing the risks associated with redirected shunt flow.
Clinical Variables
Demographic information and clinical symptoms were retrieved from medical records. To evaluate neurological procedural complications (new or worsened cranial nerve palsy, stroke/new neurological deficit, intracranial hemorrhage, and mortality), patient medical records at admission and 3-months post-EVT were assessed. The final clinical status was defined as resolution, partial improvement of symptoms related to the lesion, persistence of symptoms, or worsening of preexisting symptoms detected during follow-up [18]. Moreover, the main symptoms were classified into four patterns [19]. The orbital pattern induced by retrograde venous flow and/or pressure from the CS, including chemosis, exophthalmos, periorbital pain, and eyelid swelling. The cavernous pattern caused by cranial nerve deficits related to bulging or elevated pressure at the CS and/or to the steal phenomenon of the blood included ptosis, diplopia, anisocoria, and ophthalmoplegia [20]. The ocular pattern was related to increased venous pressure due to impaired drainage, causing decreased vision (either as a patient-reported symptom or interocular difference of >0.2, that is, a two-line difference in the Snellen visual acuity chart or its equivalent), increased intraocular pressure (>20 mmHg or an interocular difference of >5 mmHg), severe ocular pain, glaucoma, and retinal hemorrhage. The cerebral pattern is related to arteriovenous shunt reflux in the superficial middle cerebral or petrosal veins into the perimesencephalic and cerebellar veins, thus leading to infarction or venous congestion in basal ganglia, brainstem, or cerebellum, further leading to seizures or hemorrhage [19].
Imaging Variables
DSA performed pre-EVT was used for angioarchitecture analysis. The CSDAVFs were classified into the following three types according to Suh’s classification, depending on the type of arterial feeder and venous drainage: proliferative type, if having numerous arterial feeders and being drained by multiple venous outlets; restrictive type, if having multiple arterial feeders, lower in number than that of the proliferative type, converging to the wall of the dilated CS; and late-restrictive type, if having few arterial feeders with sluggish retrograde venous flow (Supplementary Fig. 1) [19]. Additionally, all identified lesions were classified as either focal or diffuse based on the extent of their involvement, which was determined by whether the fistulous sites (mural channels) were confined to a specific area or diffusely spanned the CS [21]. To analyze the detailed fistula configuration, we used 3D-RA and fusion of multiple 3D-RA series with different color codes (Fig. 1). This facilitated an understanding of fistular characteristics, including accurate fistula location, number, feeders, and draining routes. Multimodal MRI (Philips Healthcare, Eindhoven, Netherlands), including fluid-attenuated inversion recovery and time-of-flight magnetic resonance angiography (intracranial), was performed within 3 months of the procedure [22]. When magnetic resonance imaging was unfeasible due to implantable cardiac defibrillator or head and neck metal implants, and so on computed tomography angiography was performed.
Outcome Variables
Angiographically, complete occlusion was defined as total occlusion of the arteriovenous fistula; near-complete occlusion as a small residual stagnant shunt that was likely thrombosed; and partial occlusion as the presence of a residual fistula [23]. Both complete and near-complete occlusions were considered successful [5].
Interventional neuroradiologists and neurologists primarily performed clinical follow-up. In case of any delay in patient recovery or clinical suspicion of recurrent disease, imaging was repeated. Recurrence was defined as the reappearance of the CSDAVF after successful treatment, and retreatment was determined based on clinical symptoms and imaging results [24]. The primary endpoints were successful procedures, complications, and retreatment after initial CSDAVF treatment.
Statistical Analyses
For continuous variables, results were presented as mean ± standard deviation or median (interquartile range), whereas for categorical variables, results were expressed as the number of participants (%). The clinical characteristics of patients who underwent targeted and non-targeted embolization in the curative treatment group were compared. Additionally, the characteristics of patients in the curative and palliative treatment groups were compared. The chi-square test (Fisher’s exact test) was used for categorical variables, and the Student’s t-test was used for continuous variables. We used IBM SPSS Statistics software, version 21.0 (IBM Corp., Armonk, NY, USA). P-value <0.05 was considered statistically significant.
RESULTS
This study included 198 patients with CSDAVF. The mean age was 59.0 ± 12.1 years, and 46 patients (23.2% [46/198]) were male. The orbital pattern (74.2% [147/198]) was the most prevalent, followed by cavernous (56.6% [112/198]), ocular (15.2% [30/198]), and cerebral (1.5% [3/198]) patterns (Table 1). Regarding angiographic shunt findings, the restrictive type (54.0% [107/198]) was the most prevalent, followed by the proliferative (30.3% [60/198]) and late-restrictive types (15.7% [31/198]). The focal fistula type (59.1% [117/198]) occurred more frequently than the diffuse type (40.9% [81/198]).
Table 1. Characteristic of patients and cavernous sinus dural arteriovenous fistulas.
| Curative (n = 169) | P * | Palliative (n = 29) | P † | |||
|---|---|---|---|---|---|---|
| Targeted (n = 94) | Non-targeted (n = 75) | |||||
| Age, yr | 60.0 ± 11.5 | 56.9 ± 12.5 | 0.124 | 61.9 ± 12.3 | 0.159 | |
| Sex, male | 20 (21.3) | 22 (29.3) | 0.229 | 4 (13.8) | 0.193 | |
| Main symptom pattern | ||||||
| Orbital pattern | 77 (81.9) | 62 (82.7) | 0.899 | 8 (27.6) | <0.001 | |
| Cavernous pattern | 56 (59.6) | 50 (66.7) | 0.343 | 6 (20.7) | <0.001 | |
| Ocular pattern | 16 (17.0) | 13 (17.3) | 0.957 | 1 (3.4) | 0.057 | |
| Cerebral pattern | 1 (1.1) | 2 (2.7) | 0.433 | 0 (0.0) | 0.470 | |
| Fistula type | <0.001 | 0.001 | ||||
| Diffuse | 9 (9.6) | 52 (69.3) | 20 (69.0) | |||
| Focal | 85 (90.4) | 23 (30.7) | 9 (31.0) | |||
| Fistula location | 0.088 | 0.246 | ||||
| Right | 47 (50.0) | 37 (49.3) | 10 (34.5) | |||
| Left | 43 (45.7) | 28 (37.3) | 17 (58.6) | |||
| Both | 4 (4.3) | 10 (13.3) | 2 (6.9) | |||
| Suh’s classification | <0.001 | <0.001 | ||||
| Proliferative | 6 (6.4) | 35 (46.7) | 19 (65.5) | |||
| Restrictive | 66 (70.2) | 33 (44.0) | 8 (27.6) | |||
| Late restrictive | 22 (23.4) | 7 (9.3) | 2 (6.9) | |||
Data are presented as number (%) or mean ± standard deviation.
*Targeted vs. non-targeted groups, †Curative vs. palliative groups
Of 198 patients, 169 (85.4%) received curative treatment (94 targeted and 75 non-targeted), and 29 (14.6%) underwent palliative treatment (Fig. 2). Patients with symptoms were more likely to receive curative than palliative treatment (orbital symptoms, 82.2% (139/169) vs. 27.6% (8/29), P < 0.001; cavernous symptoms, 62.7% (106/169) vs. 20.7% (6/29), P < 0.001). Curative embolization prevailed in cases of the late-restrictive (93.5% [29/31]) and restrictive types (92.5% [99/107]) but was less prevalent in the proliferative type (31.7% [19/60]). In the curative embolization group, coil embolization was predominantly performed using transvenous embolization (84.6% [143/169]), whereas in the palliative group, transarterial PVA particle embolization prevailed (62.1% [18/29]) (Table 2).
Fig. 2. Classification and treatment diagram for patients with CSDAVF. CSDAVF = cavernous sinus dural arteriovenous fistula.
Table 2. Treatment characteristics and outcomes.
| Curative (n = 169) | P * | Palliative (n = 29) | P † | |||
|---|---|---|---|---|---|---|
| Targeted (n = 94) | Non-targeted (n = 75) | |||||
| Treatment approach | 0.515 | <0.001 | ||||
| Arterial | 5 (5.3) | 2 (2.7) | 22 (75.9) | |||
| Venous | 77 (81.9) | 66 (88.0) | 0 (0.0) | |||
| Combined | 12 (12.8) | 7 (9.3) | 7 (24.1) | |||
| Treatment material | 0.082 | <0.001 | ||||
| Coil | 77 (81.9) | 61 (81.3) | 1 (3.4) | |||
| PVA particle | 0 (0.0) | 0 (0.0) | 18 (62.1) | |||
| Coil + glue | 3 (3.2) | 1 (1.3) | 1 (3.4) | |||
| Coil + PVA particle | 7 (7.4) | 7 (9.3) | 5 (17.2) | |||
| Glue | 4 (4.3) | 0 (0.0) | 1 (3.4) | |||
| Onyx | 1 (1.1) | 0 (0.0) | 0 (0.0) | |||
| Coil + onyx | 1 (1.1) | 0 (0.0) | 0 (0.0) | |||
| Coil + glue + PVA particle | 1 (1.1) | 0 (0.0) | 0 (0.0) | |||
| Aborted | 0 (0.0) | 6 (8.0) | 0 (0.0) | |||
| Gelfoam | 0 (0.0) | 0 (0.0) | 2 (6.9) | |||
| Glue + PVA particle | 0 (0.0) | 0 (0.0) | 1 (3.4) | |||
| Onset to treatment time, mos | 5.2 ± 6.6 | 3.9 ± 4.7 | 0.172 | 4.0 ± 8.8 | 0.613 | |
| Treatment count | 1.1 ± 0.3 | 1.2 ± 0.6 | 0.215 | 1.4 ± 0.6 | 0.021 | |
| Immediate DSA result | 0.002 | <0.001 | ||||
| Complete occlusion | 53 (56.4) | 30 (40.0) | 1 (3.4) | |||
| Near complete occlusion | 35 (37.2) | 26 (34.7) | 9 (31.0) | |||
| Partial occlusion | 6 (6.4) | 13 (17.3) | 19 (65.5) | |||
| Persistent | 0 (0.0) | 6 (8.0) | 0 (0.0) | |||
| Periprocedural complications | 2 (2.1) | 7 (9.3) | 0.038 | 0 (0.0) | 0.203 | |
| Clinical outcome | 0.014 | 0.176 | ||||
| Resolution | 57 (60.6) | 31 (41.3) | 11 (37.9) | |||
| Partial improvement | 34 (36.2) | 33 (44.0) | 15 (51.7) | |||
| Persistent | 1 (1.1) | 6 (8.0) | 3 (10.3) | |||
| Worsened | 2 (2.1) | 5 (6.7) | 0 (0.0) | |||
| Recurrence | 8 (8.5) | 7 (10.1) | 0.852 | 6 (21.4) | 0.056 | |
| Retreatment | 5 (5.3) | 15 (20.0) | 0.003 | 9 (31.0) | 0.007 | |
| Mean clinical follow-up, mos | 27.6 ± 31.6 | 43.1 ± 59.2 | 0.043 | 42.2 ± 63.6 | 0.438 | |
Data are presented as number (%) or mean ± standard deviation.
*Targeted vs. non-targeted groups, †Curative vs. palliative groups.
PVA = polyvinyl alcohol, DSA = digital subtraction angiography
After initial EVT, the success rate of immediate DSA was 93.6% (88/94) in the targeted embolization group, 74.6% (56/75) in the non-targeted group, and 34.4% (10/29) in the palliative group. Regarding periprocedural complications, the non-targeted group had a complication rate of 9.3% (7/75), including new or worsened cranial nerve palsy in six patients (8.0% [6/75]), one with a permanent deficit and five with transient deficits, as well as a new neurologic deficit due to venous infarction in one patient (1.3% [1/75]). In contrast, the targeted embolization group exhibited a lower complication rate of 2.1% (2/94), primarily due to new or exacerbated cranial nerve palsy, all of which were transient deficits (2.1% [2/94]).
The rate of symptom improvement, including post-procedural resolution and partial improvement, was 91.4% (181/198), and was significantly higher in the targeted embolization group (96.8% [91/94]) than in the non-targeted group (Table 2). No serious complications involving intracranial hemorrhage or mortality were observed post-treatment. Retreatment was performed in 29 (14.6%) patients, with the proportion of patients undergoing retreatment being lower in the targeted embolization group (5.3% [5/94]) than in the non-targeted (20.0% [15/75]) and palliative (31.0% [9/29]) groups (Table 2).
DISCUSSION
This retrospective study analyzed 198 patients with CSDAVF based on the following treatment strategies: targeted (47.5%), non-targeted (37.9%), and curative or palliative embolization (14.6%). Targeted embolization was performed predominantly in cases of focal fistulas, whereas diffuse fistulas underwent non-targeted or palliative embolization. Post-procedural complications occurred in 2.1% and 9.3% of targeted and non-targeted embolization groups, respectively. In the targeted embolization group, clinical symptoms improved in 93.6% (88/94) of patients with no serious post-treatment complications, even though 5.3% (5/94) of patients required retreatment.
Transvenous targeted embolization has become the standard treatment for dural arteriovenous fistulas characterized by a distinct fistula opening [9,10,25]. This concept is straightforward and effective when the fistula site is clearly depicted through imaging and access to fistula is achievable. However, targeted embolization intended for all cases selected for curative treatment in our study was only successfully achieved in 55.6% (94/169) of cases. The presence of diffuse shunts increases the difficulty of performing targeted embolization as they obscure the precise location of the fistula, a difficulty compounded by the frequent occurrence of multiple fistulas [26]. Our results further support this finding, among those in the curative groups, targeted embolization was only achievable in 14.6% (6/41) of patients with the proliferative type, 66.7% (66/99) with the restrictive type, and 75.9% (22/29) with the late-restrictive type. Additionally, the occlusion outcomes for curative embolization in diffuse type CSDAVF were suboptimal, with total occlusion at 34.4% (21/61), near occlusion at 45.9% (28/61), and partial occlusion at 19.7% (12/61). Therefore, palliative embolization, with or without staged curative embolization, may be a feasible option for diffuse-type CSDAVF.
Excessive transvenous coil packing of the CS may lead to cranial nerve III, IV, and V palsies, which occurs approximately in 11%–15% of cases treated by packing the entire sinus, with some instances resulting in permanent damage [27,28]. While the exact mechanisms underlying these complications remains unclear, it is hypothesized that the compression of adjacent cranial nerves by packed coils and associated thrombosis may be the cause. Therefore, minimizing the use of coils to occlude the shunt could potentially reduce the risk of such complications. In our study, 8% of patients who underwent non-targeted embolization experienced cranial nerve palsy, which was substantially higher than that in the targeted embolization group (2.1%). A report on targeted embolization of CSDAVFs showed that transient abducens nerve palsy occurred in 25% of patients undergoing targeted embolization, whereas permanent cranial nerve palsy was observed in 25% of patients undergoing entire sinus packing [10].
Although targeted embolization shows promise, inherent risks are associated with the procedure, particularly owing to the complex anatomy of the CS. To mitigate these risks, we have incorporated several preventive and rescue strategies. Detailed preprocedural planning and controlled embolization techniques are critical to avoid unintentional compartmental occlusion and redirection of shunt flow into the cortical veins. Continuous monitoring of shunt flow during the procedure ensures prompt detection and management of complications. In cases of residual symptomatic shunt flow or complications, reaccesses and additional embolizations were performed.
Historically, embolization with PVA particles has not been regarded as a curative approach, because recanalization is often reported post-treatment [29,30]. Herein, PVA particles was used as a palliative treatment, initially performed in patients presenting with indistinct and diffuse fistulas. The rationale behind this strategy is that complete shunt occlusion often necessitates complete CS occlusion in diffuse CSDAVFs. Furthermore, an isolated reduction in shunt flow often significantly improves symptoms, allowing targeted embolization at subsequent stages. Thus, our study indicates that this method induced complete shunt occlusion in 69% of cases, possibly facilitating shunt maturation and subsequent spontaneous closure. Palliative embolization also effectively relieved symptoms in 89.7% of patients (26/29), providing adequate time for subsequent targeted embolization.
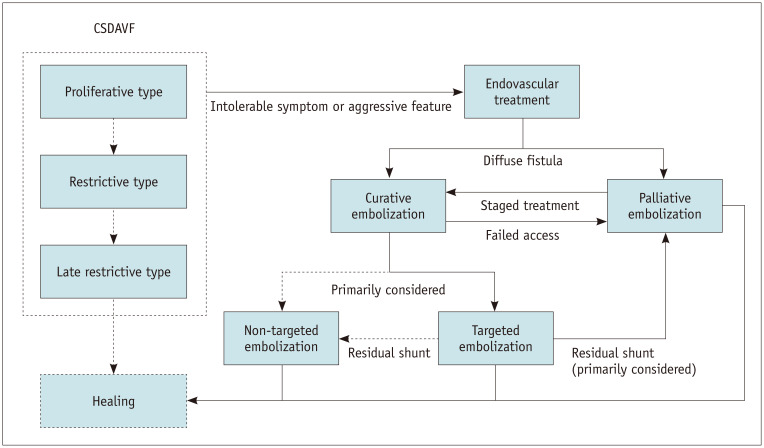
Based on our treatment outcomes and experience, we proposed a strategy for managing CSDAVF (Fig. 3). Regular monitoring is recommended in patients with mild symptoms. However, patients with intolerable symptoms or angiographic evidence of aggressive features such as cortical reflux may require treatment. For diffuse shunt types, palliative embolization is recommended because curative embolization may require the occlusion of all CS (non-targeted embolization). Targeted embolization should be the primary consideration. If access to the fistula is unfeasible, transarterial palliative embolization can reduce shunt flow and induce subsequent spontaneous occlusion. If the shunt persists, repeated palliative, curative, or Gamma Knife therapy may be an option. If targeted embolization fails to occlude the shunt, palliative embolization may be preferable to non-targeted embolization unless significant cortical or ophthalmic reflux persists after palliative treatment.
Fig. 3. Flowchart of the proposed treatment strategy for patients with CSDAVF. CSDAVF = cavernous sinus dural arteriovenous fistula.
This study had several limitations. First, its retrospective design may have led to a selection bias. A comparison of outcomes across treatment groups may be inappropriate because of differing characteristics, such as a greater proportion of focal and discrete fistulas in the targeted embolization group. This warrants cautious interpretation of the results. Intergroup comparisons may include various confounding factors that may affect their robustness. Second, the single-center design and limited number of patients and operators may restrict the generalizability of the results, despite the inclusion of a relatively large sample size compared to prior research. Third, Gamma Knife radiosurgery provides a noninvasive treatment option by delivering focused radiation to the target area, potentially reducing the risk of complications associated with invasive procedures. It is particularly beneficial for patients who are unsuitable for embolization because of anatomical constraints or comorbidities. However, Gamma Knife treatment was not included in the treatment modalities assessed in our study. Finally, fistula-type assessment is subjective and lacks standardized criteria, and dependence on 2D imaging before 2012 may further compromise the accurate evaluation of fistulas. Despite these limitations, our findings provide valuable insights into the potential of targeted embolization as a treatment option for CSDAVF.
In conclusion, our study showed that targeted embolization of CSDAVF is achievable in approximately two-thirds of cases of focal fistulas, leading to a high rate of complete or near-complete occlusion and lower rates of post-treatment cranial nerve palsy and retreatment. In addition, most patients who underwent targeted embolization experienced symptom improvement.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sangil Park, Yunsun Song.
- Data curation: Sangil Park.
- Formal analysis: Sangil Park.
- Investigation: all authors.
- Methodology: Sangil Park, Deok Hee Lee, Dae Chul Suh, Yunsun Song.
- Supervision: Yunsun Song.
- Visualization: Sangil Park, Kyubong Lee.
- Writing—original draft: Sangil Park, Yunsun Song.
- Writing—review & editing: Sangil Park, Boseong Kwon, Deok Hee Lee, Dae Chul Suh, Yunsun Song.
Funding Statement: None
Availability of Data and Material
Data are not publicly available because of ethical reasons. Further inquiries can be directed to the corresponding author.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2024.0351.
References
- 1.Andjelkovic AV, Stamatovic SM, Phillips CM, Martinez-Revollar G, Keep RF. Modeling blood-brain barrier pathology in cerebrovascular disease in vitro: current and future paradigms. Fluids Barriers CNS. 2020;17:44. doi: 10.1186/s12987-020-00202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JM, Park ES, Kwon SC. Endovascular management of cavernous sinus dural arteriovenous fistulas: overall review and considerations. J Cerebrovasc Endovasc Neurosurg. 2021;23:293–303. doi: 10.7461/jcen.2021.E2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iampreechakul P, Wangtanaphat K, Lertbutsayanukul P, Wattanasen Y, Siriwimonmas S. Spontaneous closure of a cavernous sinus dural arteriovenous fistula with spinal perimedullary drainage (Cognard V) during attempted transvenous embolization. Asian J Neurosurg. 2019;14:1268–1274. doi: 10.4103/ajns.AJNS_277_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyers PM, Halbach VV, Dowd CF, Lempert TE, Malek AM, Phatouros CC, et al. Dural carotid cavernous fistula: definitive endovascular management and long-term follow-up. Am J Ophthalmol. 2002;134:85–92. doi: 10.1016/s0002-9394(02)01515-5. [DOI] [PubMed] [Google Scholar]
- 5.Choi BS, Park JW, Kim JL, Kim SY, Park YS, Kwon HJ, et al. Treatment strategy based on multimodal management outcome of cavernous sinus dural arteriovenous fistula (CSDAVF) Neurointervention. 2011;6:6–12. doi: 10.5469/neuroint.2011.6.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimuta Y, Awa R, Sugata S, Nagayama T, Makiuchi T, Tomosugi T, et al. Long-term outcome after endovascular treatment of cavernous sinus dural arteriovenous fistula and a literature review. Acta Neurochir (Wien) 2017;159:2113–2122. doi: 10.1007/s00701-017-3336-4. [DOI] [PubMed] [Google Scholar]
- 7.Puri AS, Kühn AL, Hou SY, Wakhloo AK. Use of intermediate guide catheters as an adjunct in extracranial embolization to avoid onyx reflux into the anastomotic vasculature. A technical note. Interv Neuroradiol. 2014;20:424–427. doi: 10.15274/INR-2014-10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao Y, Niu Y, Zhu G, Chen Z. Endovascular treatment of a traumatic dural arteriovenous fistula of the superior sagittal sinus using dual lumen balloon microcatheter. Neurosciences (Riyadh) 2016;21:158–160. doi: 10.17712/nsj.2016.2.20150331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiyosue H, Tanoue S, Hori Y, Hongo N, Mori H. Shunted pouches of cavernous sinus dural AVFs: evaluation by 3D rotational angiography. Neuroradiology. 2015;57:283–290. doi: 10.1007/s00234-014-1474-4. [DOI] [PubMed] [Google Scholar]
- 10.Satow T, Murao K, Matsushige T, Fukuda K, Miyamoto S, Iihara K. Superselective shunt occlusion for the treatment of cavernous sinus dural arteriovenous fistulae. Neurosurgery. 2013;73(1 Suppl):ons100–ons105. doi: 10.1227/NEU.0b013e31828ba578. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M, Tamaki N, Kawaguchi T, Fujita S. Selective transvenous embolization of dural carotid-cavernous sinus fistulas with preservation of sylvian venous outflow. Report of three cases. J Neurosurg. 1998;89:825–829. doi: 10.3171/jns.1998.89.5.0825. [DOI] [PubMed] [Google Scholar]
- 12.Piske RL, Campos CM, Chaves JB, Abicalaf R, Dabus G, Batista LL, et al. Dural sinus compartment in dural arteriovenous shunts: a new angioarchitectural feature allowing superselective transvenous dural sinus occlusion treatment. AJNR Am J Neuroradiol. 2005;26:1715–1722. [PMC free article] [PubMed] [Google Scholar]
- 13.Agid R, Willinsky RA, Haw C, Souza MP, Vanek IJ, terBrugge KG. Targeted compartmental embolization of cavernous sinus dural arteriovenous fistulae using transfemoral medial and lateral facial vein approaches. Neuroradiology. 2004;46:156–160. doi: 10.1007/s00234-003-1131-9. [DOI] [PubMed] [Google Scholar]
- 14.Rhim JK, Cho YD, Park JJ, Jeon JP, Kang HS, Kim JE, et al. Endovascular treatment of cavernous sinus dural arteriovenous fistula with ipsilateral inferior petrosal sinus occlusion: a single-center experience. Neurosurgery. 2015;77:192–199. doi: 10.1227/NEU.0000000000000751. discussion 199. [DOI] [PubMed] [Google Scholar]
- 15.Tran TQ, Nguyen AM, Trinh TM, Huynh NT, Nguyen HV. Endovascular management of cavernous sinus dural arteriovenous fistula: a single-center experience. Interdiscip Neurosurg. 2024;36:101884 [Google Scholar]
- 16.Jia ZY, Song YS, Sheen JJ, Kim JG, Lee DH, Suh DC. Cannulation of occluded inferior petrosal sinuses for the transvenous embolization of cavernous sinus dural arteriovenous fistulas: usefulness of a frontier-wire probing technique. AJNR Am J Neuroradiol. 2018;39:2301–2306. doi: 10.3174/ajnr.A5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AlAli M, Kwon B, Song Y, Lee DH. Facilitated retrograde access via the facial vein for transvenous embolization of the cavernous sinus dural arteriovenous fistula with isolated ophthalmic venous drainage. Neurointervention. 2024;19:39–44. doi: 10.5469/neuroint.2023.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexandre AM, Sturiale CL, Bartolo A, Romi A, Scerrati A, Flacco ME, et al. Endovascular treatment of cavernous sinus dural arteriovenous fistulas. Institutional series, systematic review and meta-analysis. Clin Neuroradiol. 2022;32:761–771. doi: 10.1007/s00062-021-01107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh DC, Lee JH, Kim SJ, Chung SJ, Choi CG, Kim HJ, et al. New concept in cavernous sinus dural arteriovenous fistula: correlation with presenting symptom and venous drainage patterns. Stroke. 2005;36:1134–1139. doi: 10.1161/01.STR.0000166194.82027.63. [DOI] [PubMed] [Google Scholar]
- 20.Lasjaunias P, Chiu M, ter Brugge K, Tolia A, Hurth M, Bernstein M. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg. 1986;64:724–730. doi: 10.3171/jns.1986.64.5.0724. [DOI] [PubMed] [Google Scholar]
- 21.Rhim JK, Cho YD, Yoo DH, Kang HS, Cho WS, Kim JE, et al. Endovascular treatment of bilateral cavernous sinus dural arteriovenous fistula: therapeutic strategy and follow-up outcomes. Korean J Radiol. 2018;19:334–341. doi: 10.3348/kjr.2018.19.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eaton-Rosen Z, Melbourne A, Orasanu E, Cardoso MJ, Modat M, Bainbridge A, et al. Longitudinal measurement of the developing grey matter in preterm subjects using multi-modal MRI. Neuroimage. 2015;111:580–589. doi: 10.1016/j.neuroimage.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Deniwar MA, Kwon B, Song Y, Park JC, Lee DH. Use of a rigid-tipped microguidewire for the endovascular treatment of cavernous sinus dural arteriovenous fistulas with an occluded inferior petrosal sinus. J Korean Neurosurg Soc. 2022;65:688–696. doi: 10.3340/jkns.2021.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang HC, Lin CJ, Luo CB, Lee CC, Wu HM, Guo WY, et al. Treatment outcomes of cavernous sinus dural arteriovenous fistulas: comparison of radiosurgery and endovascular embolisation. Clin Neuroradiol. 2020;30:321–330. doi: 10.1007/s00062-019-00787-z. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro M, Raz E, Litao M, Becske T, Riina H, Nelson PK. Toward a better understanding of dural arteriovenous fistula angioarchitecture: superselective transvenous embolization of a sigmoid common arterial collector. AJNR Am J Neuroradiol. 2018;39:1682–1688. doi: 10.3174/ajnr.A5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawamura Y, Takigawa T, Hyodo A, Suzuki K. Targeted transvenous embolization of cavernous sinus dural arteriovenous fistula with liquid materials using a dual-lumen balloon microcatheter. Cureus. 2021;13:e13821. doi: 10.7759/cureus.13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DJ, Kim DI, Suh SH, Kim J, Lee SK, Kim EY, et al. Results of transvenous embolization of cavernous dural arteriovenous fistula: a single-center experience with emphasis on complications and management. AJNR Am J Neuroradiol. 2006;27:2078–2082. [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida K, Melake M, Oishi H, Yamamoto M, Arai H. Transvenous embolization of dural carotid cavernous fistulas: a series of 44 consecutive patients. AJNR Am J Neuroradiol. 2010;31:651–655. doi: 10.3174/ajnr.A1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fournier D, Terbrugge K, Rodesch G, Lasjaunias P. Revascularization of brain arteriovenous malformations after embolization with bucrylate. Neuroradiology. 1990;32:497–501. doi: 10.1007/BF02426463. [DOI] [PubMed] [Google Scholar]
- 30.Gruber A, Mazal PR, Bavinzski G, Killer M, Budka H, Richling B. Repermeation of partially embolized cerebral arteriovenous malformations: a clinical, radiologic, and histologic study. AJNR Am J Neuroradiol. 1996;17:1323–1331. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not publicly available because of ethical reasons. Further inquiries can be directed to the corresponding author.