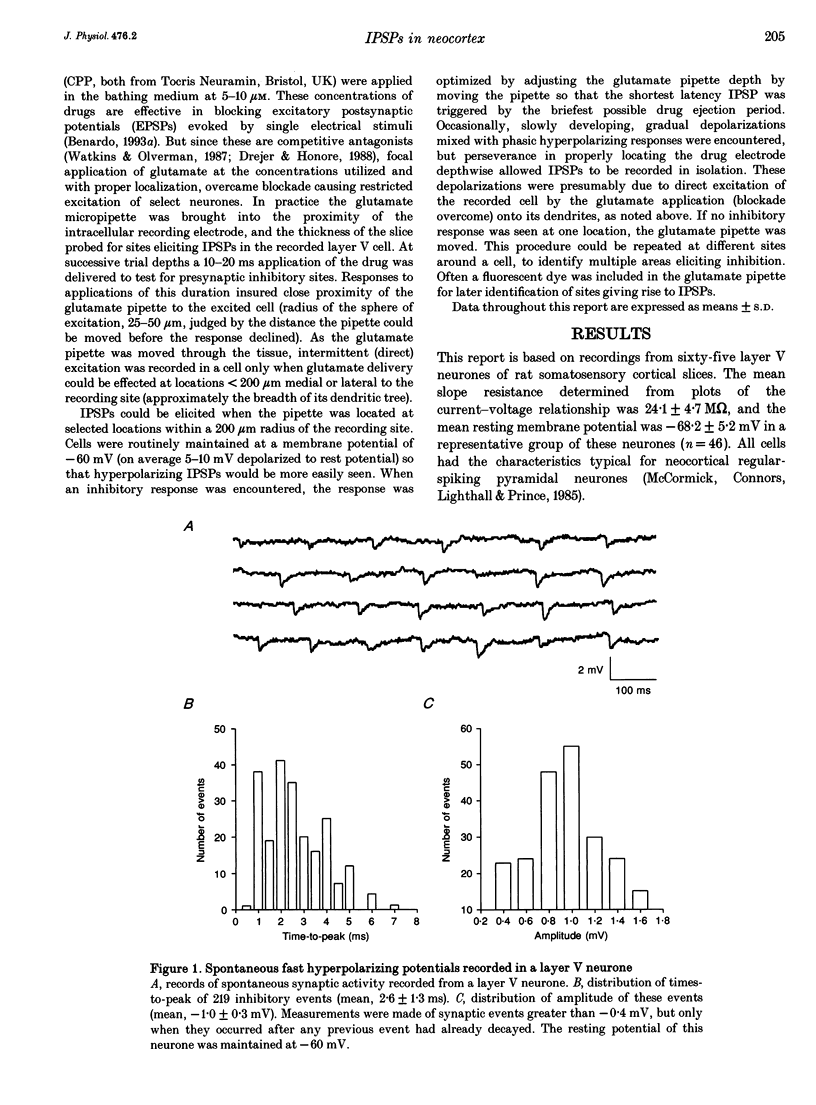
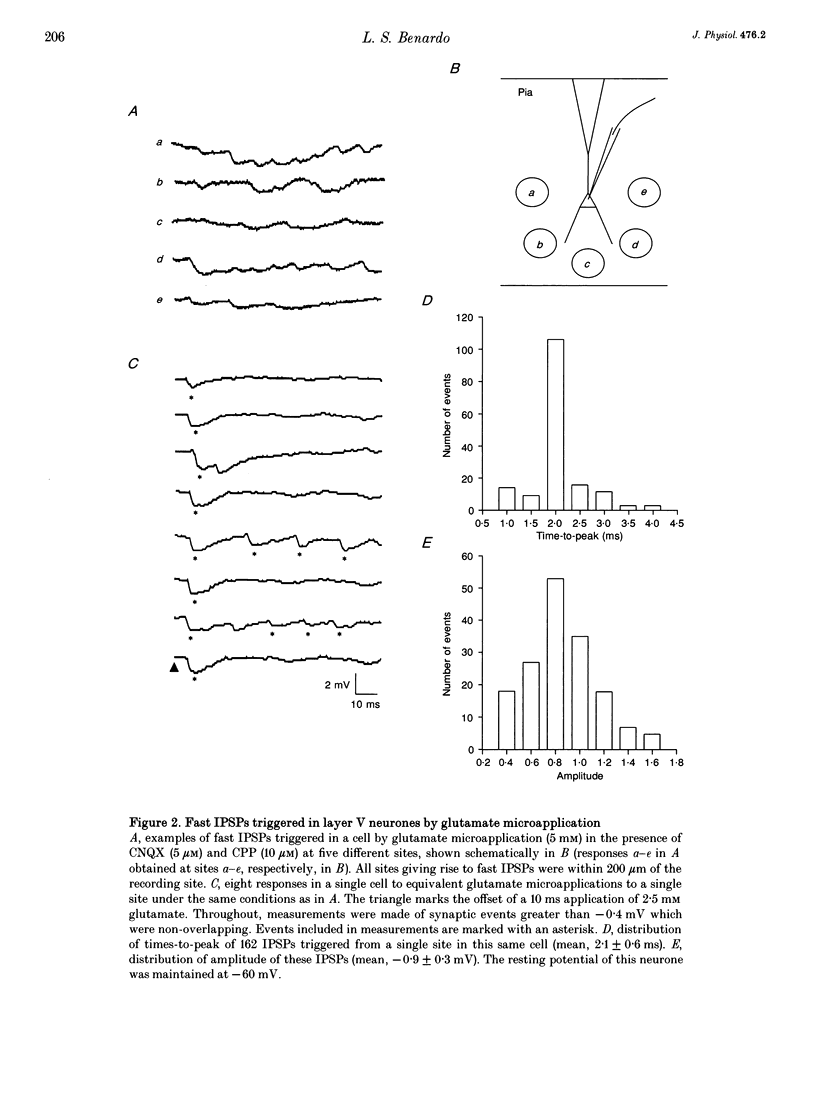
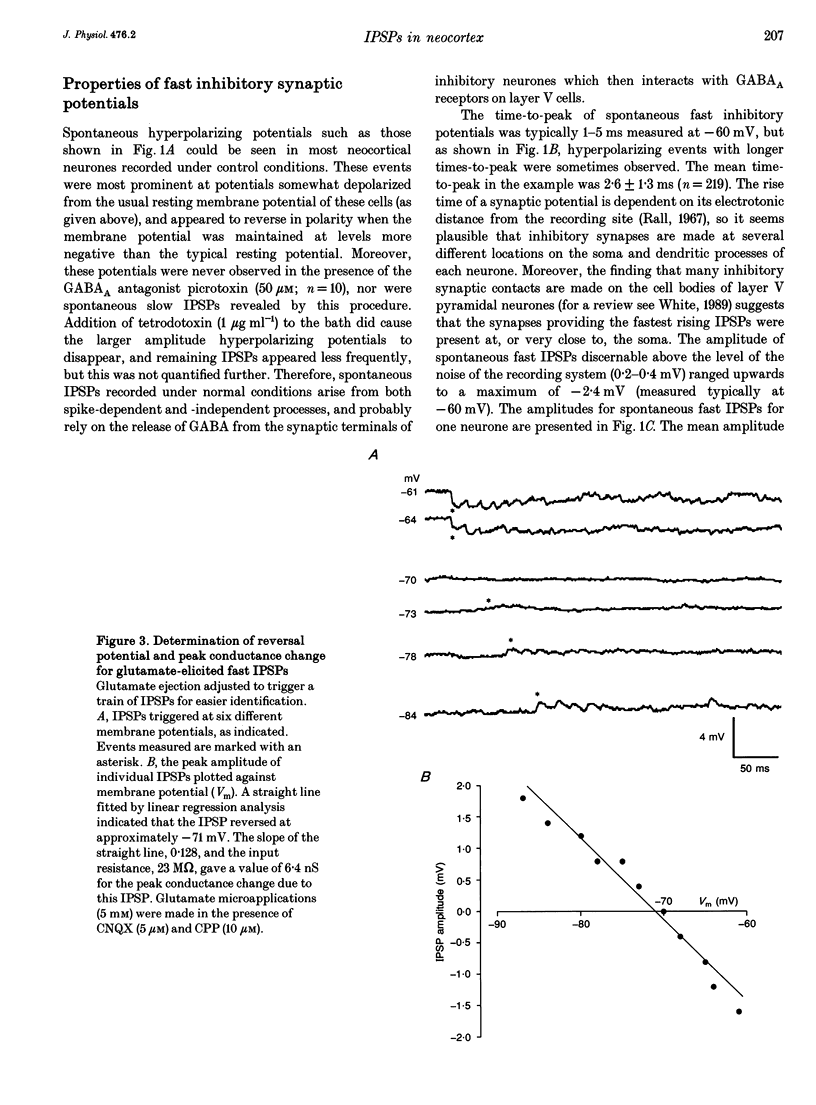
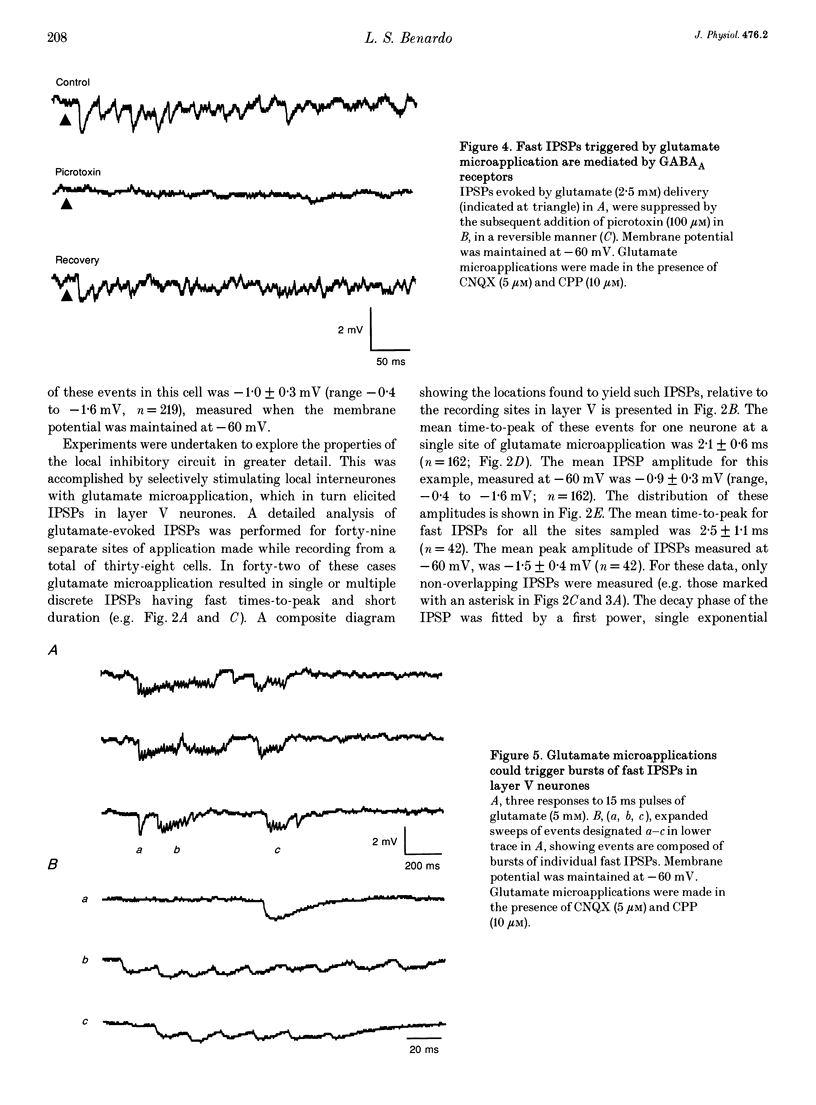
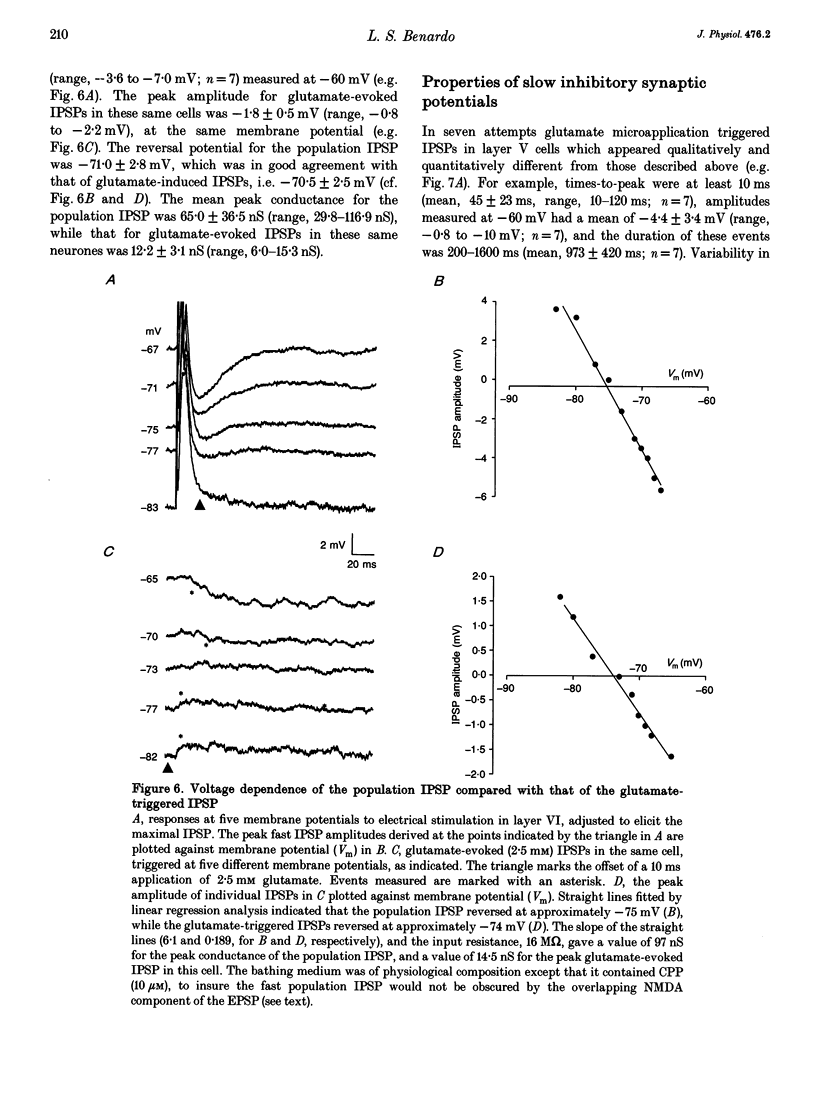
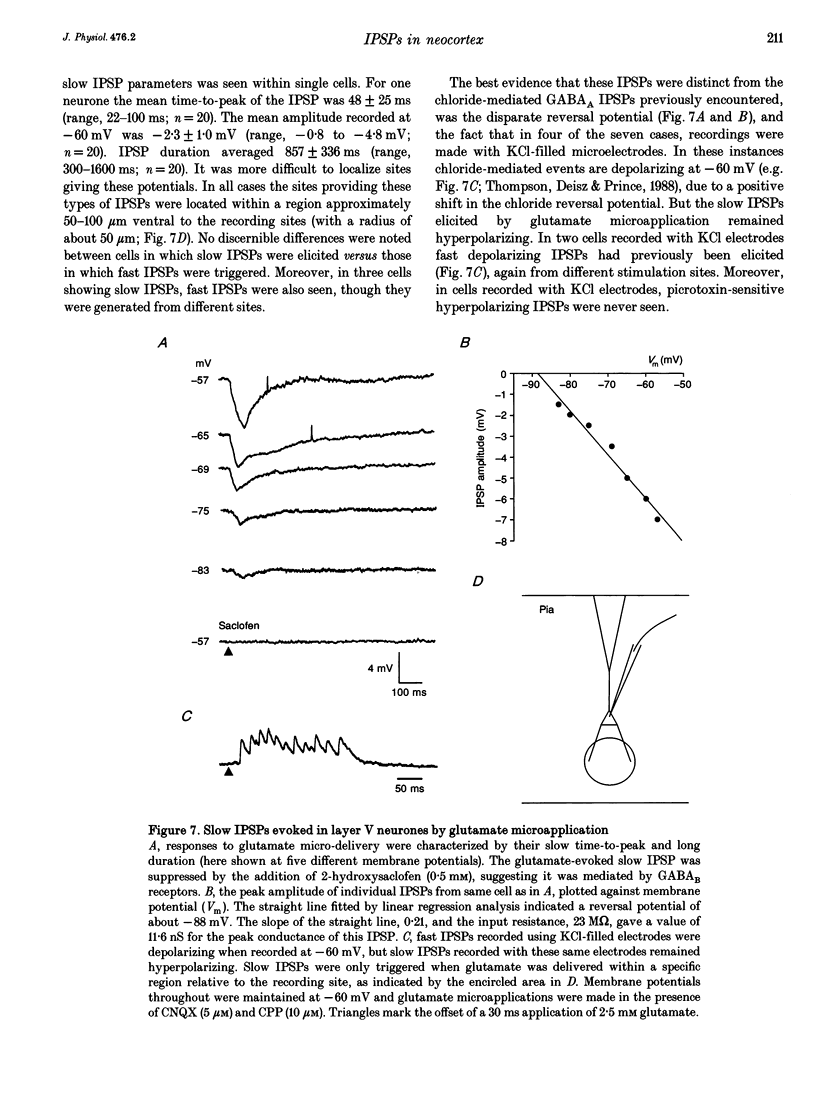
Abstract
Synaptic inhibition was investigated by stimulating inhibitory neurones with focal microapplications of glutamate, while recording from layer V pyramidal neurones of rat somatosensory cortical slices. One class of inhibitory postsynaptic potentials (IPSPs) thus elicited was characterized as a fast, chloride-mediated, GABAA IPSP in part by its fast time-to-peak (mean 2.5 ms) and brief duration, but primarily on the basis of its reversal potential at -68 mV, and its blockade by picrotoxin. The average peak amplitude for these fast IPSPs was -1.5 mV, measured at -60 mV. The peak conductance calculated for these events was about 10 nS. The conductance change associated with the maximal fast inhibitory postsynaptic potential resulting from electrical stimulation of afferent pathways ranged up to 116 nS. A second class of IPSP was encountered much less frequently. These glutamate-triggered events were characterized as slow, potassium-mediated GABAB IPSPs partly because of their longer times-to-peak (mean, 45 ms) and duration, but especially because of their extrapolated equilibrium potential at about -89 mV and blockade by 2-hydroxysaclofen. The average peak amplitude for these slow IPSPs was -2.3 mV, measured at -60 mV. The peak conductance for these events was about 8 nS. IPSPs resulting from the excitation of individual inhibitory interneurones were elicited by glutamate microapplication at particular locations relative to recording sites. Both fast and slow IPSPs were generated, but these occurred as separate events, and mixed responses were never seen. Thus, the two mechanistically distinct types of IPSPs which result from GABA interaction at GABAA and GABAB receptors on neocortical neurones may be mediated by separate classes of inhibitory neurones.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E. Gating of GABAergic inhibition in hippocampal pyramidal cells. Ann N Y Acad Sci. 1991;627:249–263. doi: 10.1111/j.1749-6632.1991.tb25929.x. [DOI] [PubMed] [Google Scholar]
- Avoli M. Inhibitory potentials in neurons of the deep layers of the in vitro neocortical slice. Brain Res. 1986 Apr 2;370(1):165–170. doi: 10.1016/0006-8993(86)91118-2. [DOI] [PubMed] [Google Scholar]
- Benardo L. S. Acetylcholine and norepinephrine mediate slow synaptic potentials in normal and epileptic neocortex. Neurosci Lett. 1991 May 27;126(2):137–140. doi: 10.1016/0304-3940(91)90538-5. [DOI] [PubMed] [Google Scholar]
- Benardo L. S. Characterization of cholinergic and noradrenergic slow excitatory postsynaptic potentials from rat cerebral cortical neurons. Neuroscience. 1993 Mar;53(1):11–22. doi: 10.1016/0306-4522(93)90280-s. [DOI] [PubMed] [Google Scholar]
- Benardo L. S. Recruitment of inhibition by enhanced activation of synaptic NMDA responses in the rat cerebral cortex. Brain Res. 1993 Nov 12;627(2):314–324. doi: 10.1016/0006-8993(93)90336-l. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Curtis D. R. Strychnine and cortical inhibition. Nature. 1967 May 27;214(5091):914–915. doi: 10.1038/214914a0. [DOI] [PubMed] [Google Scholar]
- Brink E., Harrison P. J., Jankowska E., McCrea D. A., Skoog B. Post-synaptic potentials in a population of motoneurones following activity of single interneurones in the cat. J Physiol. 1983 Oct;343:341–359. doi: 10.1113/jphysiol.1983.sp014896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y., Connors B. W. Horizontal spread of synchronized activity in neocortex and its control by GABA-mediated inhibition. J Neurophysiol. 1989 Apr;61(4):747–758. doi: 10.1152/jn.1989.61.4.747. [DOI] [PubMed] [Google Scholar]
- Christian E. P., Dudek F. E. Characteristics of local excitatory circuits studied with glutamate microapplication in the CA3 area of rat hippocampal slices. J Neurophysiol. 1988 Jan;59(1):90–109. doi: 10.1152/jn.1988.59.1.90. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Connors B. W. Initiation of synchronized neuronal bursting in neocortex. Nature. 1984 Aug 23;310(5979):685–687. doi: 10.1038/310685a0. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Malenka R. C., Silva L. R. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988 Dec;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. M., Curtis D. R., Voorhoeve P. E., Wilson V. J. Acetylcholine sensitivity of cerebellar neurones in the cat. J Physiol. 1966 Sep;186(1):139–165. doi: 10.1113/jphysiol.1966.sp008025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Leresche N. A role for GABAB receptors in excitation and inhibition of thalamocortical cells. Trends Neurosci. 1991 Jan;14(1):16–21. doi: 10.1016/0166-2236(91)90178-w. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Crawford J. M. Acetylcholine sensitivity of cerebellar neurones. Nature. 1965 May 1;206(983):516–517. doi: 10.1038/206516a0. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Felix D. The effect of bicuculline upon synaptic inhibition in the cerebral and cerebellar corticles of the cat. Brain Res. 1971 Nov;34(2):301–321. doi: 10.1016/0006-8993(71)90283-6. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Prince D. A. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989 May;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drejer J., Honoré T. New quinoxalinediones show potent antagonism of quisqualate responses in cultured mouse cortical neurons. Neurosci Lett. 1988 Apr 22;87(1-2):104–108. doi: 10.1016/0304-3940(88)90153-x. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Sutor B., Zieglgänsberger W. Baclofen reduces post-synaptic potentials of rat cortical neurones by an action other than its hyperpolarizing action. J Physiol. 1987 Mar;384:539–569. doi: 10.1113/jphysiol.1987.sp016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson J. S., Solís J. M., Nicoll R. A. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993 Feb;10(2):165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol. 1972 May;222(3):623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol. 1993 Feb;69(2):416–431. doi: 10.1152/jn.1993.69.2.416. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Hama K. Physiological heterogeneity of nonpyramidal cells in rat hippocampal CA1 region. Exp Brain Res. 1988;72(3):494–502. doi: 10.1007/BF00250594. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Hama K. Two subtypes of non-pyramidal cells in rat hippocampal formation identified by intracellular recording and HRP injection. Brain Res. 1987 May 12;411(1):190–195. doi: 10.1016/0006-8993(87)90700-1. [DOI] [PubMed] [Google Scholar]
- Kelly J. S., Krnjević K., Morris M. E., Yim G. K. Anionic permeability of cortical neurones. Exp Brain Res. 1969;7(1):11–31. doi: 10.1007/BF00236105. [DOI] [PubMed] [Google Scholar]
- Knowles W. D., Schwartzkroin P. A. Local circuit synaptic interactions in hippocampal brain slices. J Neurosci. 1981 Mar;1(3):318–322. doi: 10.1523/JNEUROSCI.01-03-00318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel D. D., Lacaille J. C., Schwartzkroin P. A. Ultrastructure of stratum lacunosum-moleculare interneurons of hippocampal CA1 region. Synapse. 1988;2(4):382–394. doi: 10.1002/syn.890020405. [DOI] [PubMed] [Google Scholar]
- Kuno M., Weakly J. N. Quantal components of the inhibitory synaptic potential in spinal mononeurones of the cat. J Physiol. 1972 Jul;224(2):287–303. doi: 10.1113/jphysiol.1972.sp009895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille J. C., Mueller A. L., Kunkel D. D., Schwartzkroin P. A. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987 Jul;7(7):1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille J. C., Mueller A. L., Kunkel D. D., Schwartzkroin P. A. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987 Jul;7(7):1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille J. C., Schwartzkroin P. A. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. I. Intracellular response characteristics, synaptic responses, and morphology. J Neurosci. 1988 Apr;8(4):1400–1410. doi: 10.1523/JNEUROSCI.08-04-01400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Connors B. W., Lighthall J. W., Prince D. A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985 Oct;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol. 1989 Nov;62(5):1018–1027. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- Michelson H. B., Wong R. K. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991 Sep 20;253(5026):1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- Miles R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J Physiol. 1990 Sep;428:61–77. doi: 10.1113/jphysiol.1990.sp018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol. 1986 Apr;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Unitary inhibitory synaptic potentials in the guinea-pig hippocampus in vitro. J Physiol. 1984 Nov;356:97–113. doi: 10.1113/jphysiol.1984.sp015455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Renaud L. P., Kelly J. S. Identification of possible inhibitory neurons in the pericruciate cortex of the cat. Brain Res. 1974 Oct 11;79(1):9–28. doi: 10.1016/0006-8993(74)90563-0. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Mathers L. H. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978 Nov 17;157(1):1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- Segal M. A subset of local interneurons generate slow inhibitory postsynaptic potentials in hippocampal neurons. Brain Res. 1990 Mar 12;511(1):163–164. doi: 10.1016/0006-8993(90)90236-5. [DOI] [PubMed] [Google Scholar]
- Segal M. Repetitive inhibitory postsynaptic potentials evoked by 4-aminopyridine in hippocampal neurons in vitro. Brain Res. 1987 Jun 30;414(2):285–293. doi: 10.1016/0006-8993(87)90008-4. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschênes M., Domich L., Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985 Dec;54(6):1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Sugita S., Johnson S. W., North R. A. Synaptic inputs to GABAA and GABAB receptors originate from discrete afferent neurons. Neurosci Lett. 1992 Jan 6;134(2):207–211. doi: 10.1016/0304-3940(92)90518-c. [DOI] [PubMed] [Google Scholar]
- Takahashi T. The minimal inhibitory synaptic currents evoked in neonatal rat motoneurones. J Physiol. 1992 May;450:593–611. doi: 10.1113/jphysiol.1992.sp019145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker J. G., Peacock W. J., Dudek F. E. Local synaptic circuits and epileptiform activity in slices of neocortex from children with intractable epilepsy. J Neurophysiol. 1992 Mar;67(3):496–507. doi: 10.1152/jn.1992.67.3.496. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Deisz R. A., Prince D. A. Relative contributions of passive equilibrium and active transport to the distribution of chloride in mammalian cortical neurons. J Neurophysiol. 1988 Jul;60(1):105–124. doi: 10.1152/jn.1988.60.1.105. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Deuchars J., West D. C. Single axon excitatory postsynaptic potentials in neocortical interneurons exhibit pronounced paired pulse facilitation. Neuroscience. 1993 May;54(2):347–360. doi: 10.1016/0306-4522(93)90257-g. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Wong R. K. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982 May 14;216(4547):745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Williams S., Lacaille J. C. GABAB receptor-mediated inhibitory postsynaptic potentials evoked by electrical stimulation and by glutamate stimulation of interneurons in stratum lacunosum-moleculare in hippocampal CA1 pyramidal cells in vitro. Synapse. 1992 Jul;11(3):249–258. doi: 10.1002/syn.890110309. [DOI] [PubMed] [Google Scholar]
- Yamamoto C. Quantal analysis of excitatory postsynaptic potentials induced in hippocampal neurons by activation of granule cells. Exp Brain Res. 1982;46(2):170–176. doi: 10.1007/BF00237173. [DOI] [PubMed] [Google Scholar]


