Abstract
We report a case of an uncommonly aggressive presentation of the rare entity of synchronous papillary (PTC) and follicular thyroid carcinomas (FTC) in a 67-year-old female initially presenting with thyrotoxicosis from Graves’ disease. She was found to have two thyroid nodules with extensive intra-cardiac tumour thrombus, symptomatic left pelvis bony metastasis with pathological fracture, pulmonary metastases and mediastinal lymph node metastases. Further investigations suggested a diagnosis of synchronous papillary and metastatic follicular thyroid cancer. Treatment with radical surgery followed by adjuvant therapeutic radioiodine ablation was proposed, but the patient declined all forms of cancer-specific therapy and was elected solely for a palliative approach to treatment. We discuss the diagnostic considerations in arriving at the diagnosis of synchronous thyroid malignancy – in this case the clear features of PTC and the strong probability of FTC due to invasiveness and metastatic follicular lesions. This case underscores potential limitations of the ACR TI-RADS system, notably with certain ultrasonographic features suggesting malignancy that might not be adequately captured. Notably, the aggressive presentation of DTC in this case may be contributed by the concurrent presence of Graves’ Disease, suggesting heightened vigilance when assessing potential thyroid malignancies in such patients.
Keywords: papillary thyroid carcinoma, follicular thyroid carcinoma, tumour thrombus, Graves’ disease
INTRODUCTION
Thyroid cancer is the most common endocrine malignancy, with differentiated thyroid carcinoma (DTC), comprising papillary, follicular and Hurthle cell subtypes, accounting for 90% of cases.1 However, the simultaneous occurrence of papillary and follicular thyroid carcinomas is extremely rare. This case report presents an unusual and aggressive case of synchronous papillary and likely follicular thyroid carcinomas with Graves’ disease and discusses the diagnostic challenges, management decisions and clinical implications.
CASE
A 67-year-old female with no significant past medical history presented to the emergency department with symptoms of bilateral lower limb swelling for a week, unintentional weight loss of 6 kg, heat intolerance, palpitations, anxiety and intermittent mild facial swelling. She reported a secondary concern of left buttock pain radiating to the left thigh for 2 weeks, with no preceding trauma. She denied any smoking history or family history of thyroid disease or malignancy.
Upon examination, she was tachycardic and hypertensive but afebrile and not hypoxic. Bilateral pitting edema to the mid-shin was noted and she was mildly tremulous. However, she had no proximal weakness or thyroid eye disease. She had an asymmetrical goiter, larger on the right side, with a palpable, non-tender, firm nodule measuring approximately 2 x 2 cm. A markedly distended right external jugular vein was also noted. There was no cervical lymphadenopathy discernible clinically. Lastly, she did not have any pelvic tenderness nor pain on axial loading of her left hip and neurological examination of both lower limbs was unremarkable with a negative straight-leg raise test.
Diagnostic assessment
Initial investigations revealed primary hyperthyroidism with free thyroxine (FT4) level of 22.7 pmol/L [reference range 8.8-14.4 pmol/L] and completely suppressed thyroid stimulating hormone (TSH) levels of less than 0.010 mU/L [reference range 0.65-3.70 mU/L]. She had a positive TSH receptor antibody level of 4.57 IU/L [reference range <1.76 IU/L] and was therefore diagnosed with Graves’ disease. Liver and renal function and full blood count were normal.
N-terminal pro-brain natriuretic peptide was elevated, but troponin levels were not raised on serial measurement. Chest x-ray showed cardiomegaly but no pulmonary congestion or lung lesions. Lumbar spine, pelvis and left hip radiographs were unremarkable. An electrocardiogram showed sinus tachycardia with the “S1Q3T3” triad, but no right axis deviation.
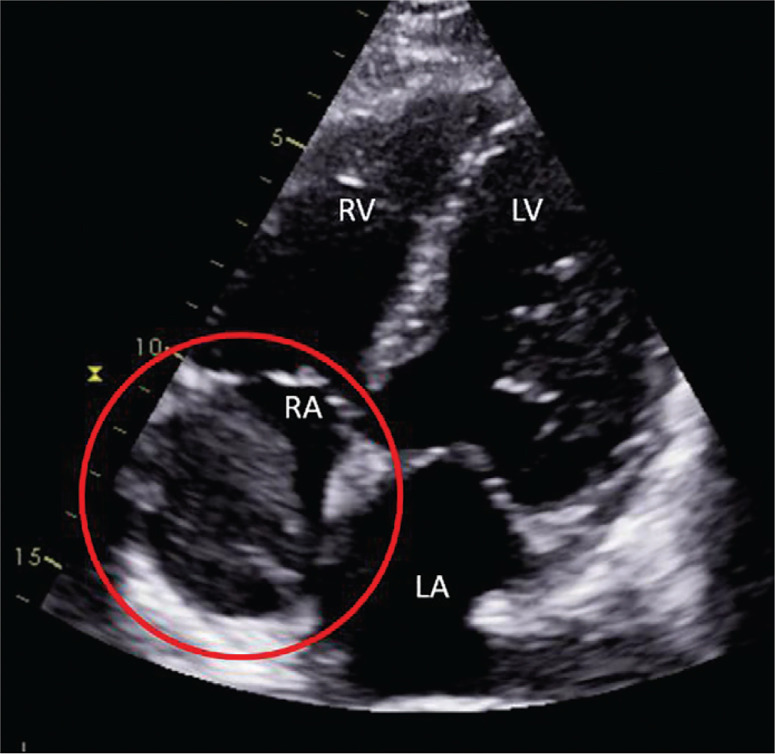
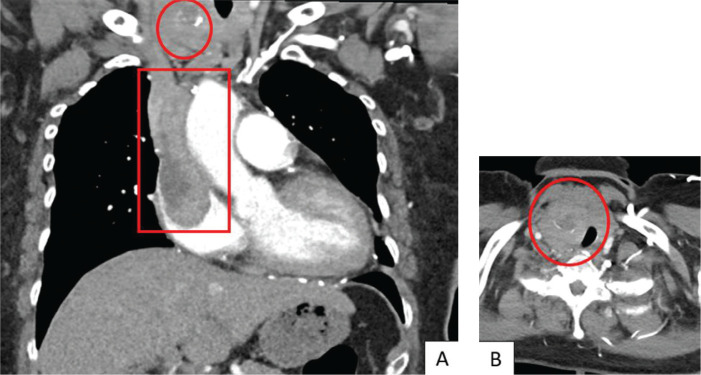
A transthoracic echocardiogram showed normal left ventricular structure and function without significant valvulopathy. However, an echodense mass measuring 4.4 x 2.4 cm was reported in the right atrium, with apparent extension into the inferior vena cava (Figure 1). Urgent computed tomography pulmonary angiography did not show any evidence of pulmonary embolism. The hypodense right atrial mass was further characterized (5.3 x 3.2 x 9.0 cm in dimension) and was demonstrated to extend up the superior vena cava (SVC) (Figure 2A). Asymmetric enlargement of the right thyroid lobe was observed, with associated nodularity and dystrophic calcification (Figure 2B). Lastly, multiple pulmonary nodules were noted in the left lower lobe (1.2 cm), left upper lobe (0.6 cm) and right lower lobe (0.4 cm).
Figure 1.
Apical 4-chamber view of transthoracic echocardiogram. The echodense mass is highlighted with the red circle.
RV = Right Ventricle, LV = Left Ventricle, RA = Right Atrium, LA = Left Atrium.
Figure 2.
(A) Coronal view showing extensive RA thrombus with SVC extension (red box) and nodular calcification in thyroid (red circle). (B) Axial view showing asymmetric thyroid enlargement with calcified right thyroid nodule (red circle).
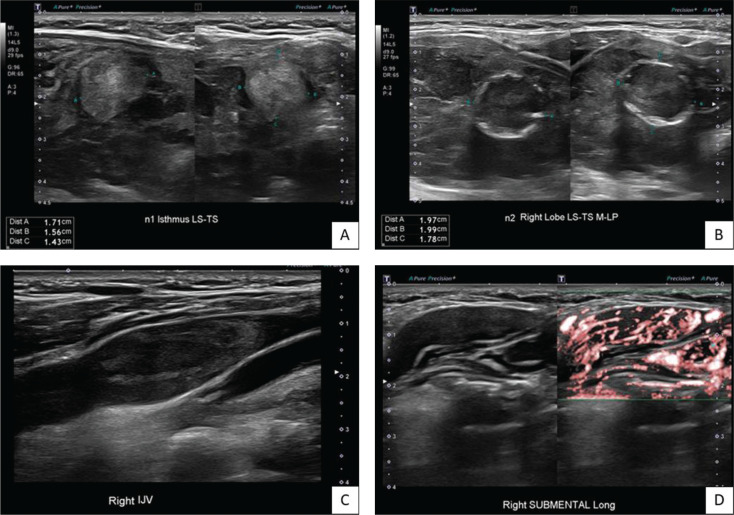
Ultrasonography of the thyroid gland (US Thyroid) showed 2 thyroid nodules. The first was a heterogenous hyperechoic solid isthmic nodule measuring 1.7 x 1.6 x 1.4 cm (wider than tall) with ill-defined margins, classified as TIRADS-3 (Figure 3A). The second was a solid iso-tohypoechoic solid right lower pole nodule measuring 2.0 x 2.0 x 1.8 cm (wider than tall) with a disrupted eggshell calcification pattern, classified as TIRADS-4 (Figure 3B). This corresponded to the nodule observed on CT. A right internal jugular vein (IJV) thrombus measuring 3.4cm with internal vascularity suggestive of tumour thrombus was reported (Figure 3C). Lastly, an abnormal lymph node was noted at the right submental/submandibular region (Level IA/B) measuring 3.5 x 1.1 x 1.7 cm and demonstrating internal vascularity (Figure 3D). Increased vascularity of the thyroid parenchyma was noted, in keeping with Graves’ disease.
Figure 3.
(A) Isthmic nodule (B) Right thyroid lower pole nodule (C) Right internal jugular vein thrombus (D) Right submental lesion with internal vascularity.
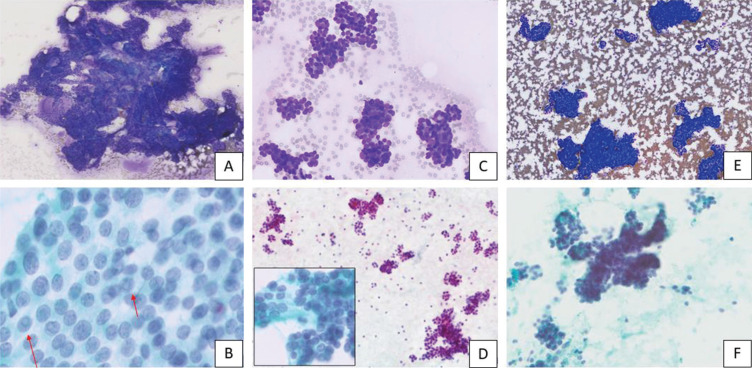
Fine needle aspiration of both thyroid nodules and the right submental lesion was performed. Cytology of the isthmic nodule showed features consistent with papillary thyroid carcinoma (PTC) (Bethesda VI) with classic microscopic features of pale nuclei with intranuclear pseudoinclusions and nuclear grooves (Figure 4A and 4B). Cytology of the right lower pole nodule showed follicular cells predominantly arranged in microfollicles and trabeculae (Figure 4C and 4D) characteristic of a follicular neoplasm (Bethesda IV). Cytology of the right submental lesion showed metastatic epithelial cells arranged in trabeculae and follicles, without classic nuclear features of PTC, in keeping with metastasis from the follicular neoplasm at the right lower pole (Figure 4E and 4F).
Figure 4.
(A) Diff Quik; 5x magnification – Papillary clusters of thyroid follicular cells. (B) Papanicolaou; 40x magnification – Follicular cells from papillary thyroid carcinoma showing pale ovoid nuclei with intranuclear pseudoinclusions (arrows) and nuclear grooves. (C) Diff Quik; 10x magnification – Thyroid follicular cells arranged in microfollicles and trabecular groups. (D) Papanicolaou; 5x magnification – Predominance of microfollicles. Follicular cells from “nodule 2” have round nuclei with evenly dispersed, granular chromatin (inset, 40x magnification). (E) Diff Quik; 5x magnification – Metastatic epithelial cells arranged in trabeculae and crowded clusters. (F) Papanicolaou; 10x magnification – Metastatic epithelial cells arranged in trabeculae and microfollicles.
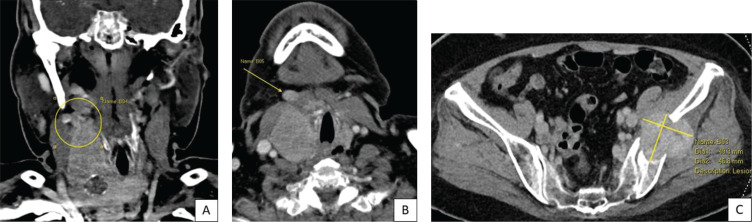
Staging was completed with computed tomography of the neck, thorax, abdomen and pelvis. The right IJV thrombus was further delineated to extend superiorly up to the right common facial, facial, anterior jugular and superior thyroid veins and inferiorly down to the right brachiocephalic vein and SVC. This thrombus was noted to be inseparable from the superior margin of the right thyroid gland (Figure 5A). The right submental lymph node previously reported on US Thyroid was suggested to instead represent the thrombosed right anterior jugular vein (Figure 5B). Mediastinal lymphadenopathy was also noted. A soft tissue mass measuring 4.9 x 4.7 cm centred on the left ilium with bony destruction and pathological fracture was seen (Figure 5C). This was the most likely cause of her reported left buttock pain. Lastly, a cardiac MRI was performed. The right atrial mass showed internal enhancement with gadolinium contrast, suggesting internal vascularity and hence tumour (as opposed to bland) thrombus.
Figure 5.
CT Neck (A) Coronal view showing extensive tumour thrombus inseparable from superior border of right thyroid lobe (yellow circle). (B) Axial view showing the abnormal right submental structure, either thombosed anterior jugular vein or submental lymph node. (C) Destructive bony metastasis in left ilium.
The final diagnosis for this patient was a synchronous papillary and likely follicular thyroid cancer (FTC), complicated by extensive tumour thrombus from the right common facial vein to the right atrium, a symptomatic left pelvic bony metastasis with pathological fracture and likely pulmonary, mediastinal and possibly right submental lymph node metastases. This was on a background of primary hyperthyroidism secondary to Graves’ disease.
Treatment and outcome
The patient was started on oral carbimazole 20 mg once daily at the time of admission. The beta-adrenergic blockade was withheld in view of the initial suspicion of acute decompensated heart failure. Her FT4 levels normalized by the time of her discharge from the hospital.
A multidisciplinary tumour board recommended total thyroidectomy with lateral neck dissection, followed by median sternotomy, cardiopulmonary bypass, venotomy and thrombectomy – an approach previously reported in a similar case.2 After consideration of the risks of surgery against the likelihood of a poor outcome without surgery and possible sudden cardiac death in the event of tumour embolization, the patient elected against proceeding with the recommended surgical resection and also declined further discussion of chemotherapy or molecularlytargeted therapy.
She remains on ongoing follow-up for the last 18 months since the time of her diagnosis. TRAb levels normalized rapidly and carbimazole was discontinued 8 months after her initial presentation due to persistent hypothyroidism despite rapid progressive reductions in carbimazole doses. A repeat US Thyroid was arranged 6 months after her diagnosis, which showed further tumour progression in the thyroid gland, possibly the cause of her progressive hypothyroidism. A repeat transthoracic echocardiogram also showed further progression of the right atrial mass with right ventricular inflow tract obstruction. Despite extensive counselling on the possibility of sudden cardiorespiratory collapse, she remained opposed to treatment. Overall management remains expectant, and she is mentally prepared for the likelihood of a poor eventual outcome.
DISCUSSION
Synchronous PTC and FTC are exceedingly rare, with fewer than 10 cases reported in the extant literature.3,4 Whilst also rare, synchronous papillary and medullary thyroid carcinomas (MTC) are better reported.5 This is thought to be due to the differences in the molecular pathogenesis of PTC and FTC4. PTC is more commonly associated with BRAF mutations (40-45%) and RET/PTC (10-20%) fusions and thus shares homologous mutations with MTC (germline RET/PTC mutations in patients with MEN2 and 40-45% of patients with sporadic MTC). FTC on the other hand is more associated with mutations of RAS (40-50%) and PAX8/PPARγ fusions (30-35%).6
The diagnosis of PTC in this case is clear, given the presence of distinctive nuclear features on cytology as described. However, the conclusive diagnosis of FTC (as opposed to follicular adenoma) requires the demonstration of invasiveness. Hence, surgical resection and subsequent microscopic examination are necessary to make the diagnosis. Despite the lack of conclusive histopathology, there are a few supporting features that make this a strong possibility. Firstly, the abnormal right thyroid lobe is inseparable from the tumour thrombus in the right IJV, suggesting invasiveness. Secondly, the right submental lesion demonstrates clear follicular cytopathology without any nuclear features of PTC. As this is a metastatic lesion, by logical extension, a metastatic follicular lesion would necessarily be a FTC. Lastly, the presence of bony metastasis at the time of diagnosis is a clinical feature more closely associated with FTC.7 The differential diagnosis of a follicular-variant PTC was considered less likely given the lack of nuclear features of PTC. There were also no features of poorly differentiated thyroid carcinoma.8
This case also highlights potential limitations of the American College of Radiology Thyroid Imaging Reporting and Data System (ACR TI-RADS).9 Using the ACR TI-RADS system, the isthmic nodule was classified as TIRADS-3 and the right thyroid nodule was classified as TIRADS-4, which would confer a cancer risk of <5% and 5-20% respectively9 in the absence of other suspicious ultrasonographic features. In particular, the appearance of the right thyroid nodule should be considered. Peripheral or “eggshell” calcifications have classically been considered a feature suggestive of benignity.10 However, more recent evidence suggests that in nodules without any other internal calcifications, disruption of the peripheral calcification and a peripheral halo are much more predictive of malignancy than other features captured by ACR TIRADS such as hypoechogenicity, lobulated margins or a taller-than-wide shape.11 Both these features were observed in this case. Additionally, the isthmic nodule would have been recommended for surveillance alone by size (<2.5 cm) and TIRADS-3 classification.
Secondly, this was an unusually aggressive presentation of DTC. It is rare to observe tumour thrombi at the time of DTC diagnosis, with a large observational series reporting a prevalence of less than 1%.12 TSH is a key stimulator of DTC growth, which explains the rationale for TSH suppression in patients at risk of recurrent disease after initial treatment. TSH receptor antibodies are homologous to TSH and can activate cellular TSH receptors in the same way. It has therefore been suggested that the presence of concomitant Graves’ disease might be associated with increased aggressiveness of thyroid cancer at the time of presentation, with higher rates of multifocality, local invasion and metastases.13,14 It may be prudent to be more cautious when evaluating and treating possible thyroid malignancies in patients with Graves’ disease.
CONCLUSION
This case presents an exceptionally aggressive manifestation of rare synchronous PTC and likely FTC in a patient with Graves’ disease, illustrating some diagnostic challenges and the potential influence of Graves’ disease on thyroid malignancy severity.
Funding Statement
Funding Source None.
Ethical Consideration
Patient consent was obtained before submission of the manuscript.
Statement of Authorship
All authors certified fulfillment of ICMJE authorship criteria.
CRediT Author Statement
GSGR: Conceptualization, Investigation, Writing – original draft preparation; STYT: Writing – review and editing, Supervision; ECJC: Resources, Writing – original draft preparation, Visualization; SM: Resources, Writing - review and editing; CCL: Writing – review and editing, Supervision
Author Disclosure
The authors declared no conflict of interest.
Data Availability Statement
No datasets were generated or analyzed for this study.
References
- 1.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. PMID: 26462967 PMCID: DOI: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamagami Y, Tori M, Sakaki M, Ohtake S, Nakahara M, Nakao K. Thyroid carcinoma with extensive tumor thrombus in the atrium. Gen Thorac Cardiovasc Surg. 2008;56(11):555–8. PMID: 19002756 DOI: 10.1007/s11748-008-0307-y [DOI] [PubMed] [Google Scholar]
- 3.Feng JW, Ye J, Hu J, Liu SY, Jiang Y, Hong LZ. Synchronous papillary thyroid carcinoma and follicular thyroid carcinoma: Case report and review of literature. Int J Clin Exp Pathol. 2020;13(11):2767–71. PMID: 33284887 PMCID: [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelaal A, El Ansari W, Abusabeib A, Farghaly H, Tabeb AAM. Simultaneous occurrence of follicular and papillary thyroid carcinomas in same thyroid lobe: A case series of six patients from Qatar. Int J Surg Case Rep. 2020;73:65–70. PMID: 32645594 PMCID: . DOI: 10.1016/j.ijscr.2020.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adnan Z, Arad E, Dana J, Shendler Y, Baron E. Simultaneous occurrence of medullary and papillary thyroid microcarcinomas: A case series and review of the literature. J Med Case Rep. 2013;7:26. PMID: 23336429 PMCID: DOI: 10.1186/1752-1947-7-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7(10):569–80. PMID: 21878896 DOI: 10.1038/nrendo.2011.142 [DOI] [PubMed] [Google Scholar]
- 7.Schlumberger MJ, Torlantano M. Papillary and follicular thyroid carcinoma. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(4):601–13. PMID: 11289737 DOI: 10.1053/beem.2000.0105 [DOI] [PubMed] [Google Scholar]
- 8.Christofer Juhlin C, Mete O, Baloch ZW. The 2022 WHO classification of thyroid tumors: Novel concepts in nomenclature and grading. Endocr Relat Cancer. 2023;30(2):e220293. PMID: 36445235 DOI: 10.1530/ERC-22-0293 [DOI] [PubMed] [Google Scholar]
- 9.Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14(5):587–95. PMID: 28372962 DOI: 10.1016/j.jacr.2017.01.046 [DOI] [PubMed] [Google Scholar]
- 10.Gooding GA. Ultrasonic appearance of a thyroid nodule invested in eggshell calcification. J Clin Ultrasound. 1978;6(1):41–3. PMID: 416047 DOI: 10.1002/jcu.1870060112 [DOI] [PubMed] [Google Scholar]
- 11.Kim BM, Kim MJ, Kim EK, et al. Sonographic differentiation of thyroid nodules with eggshell calcifications. J Ultrasound Med. 2008;27(10):1425–30. PMID: 18809952 DOI: 10.7863/jum.2008.27.10.1425 [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi K, Hirokawa M, Yabuta T, et al. Tumor thrombus of thyroid malignancies in veins: Importance of detection by ultrasonography. Thyroid. 2011;21(5):527–31. PMID: 21476893 DOI: 10.1089/thy.2010.0099 [DOI] [PubMed] [Google Scholar]
- 13.Belfiore A, Garofalo MR, Giuffrida D, et al. Increased aggressiveness of thyroid cancer in patients with Graves’ disease. J Clin Endocrinol Metab. 1990;70(4):830–5. PMID: 2180978 DOI: 10.1210/jcem-70-4-830 [DOI] [PubMed] [Google Scholar]
- 14.Staniforth JUL, Erdirimanne S, Eslick GD. Thyroid carcinoma in Graves’ disease: A meta-analysis. Int J Surg. 2016;27:118–25. PMID: 26626367 DOI: 10.1016/j.ijsu.2015.11.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed for this study.