Abstract
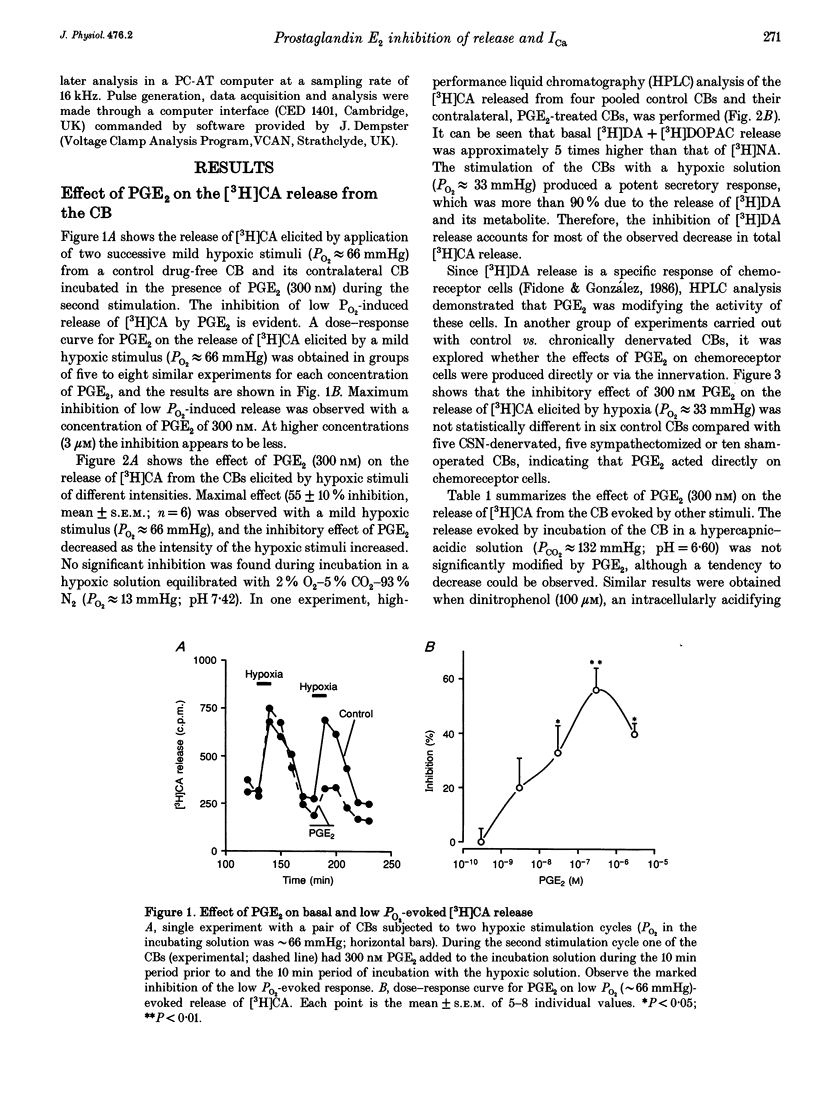
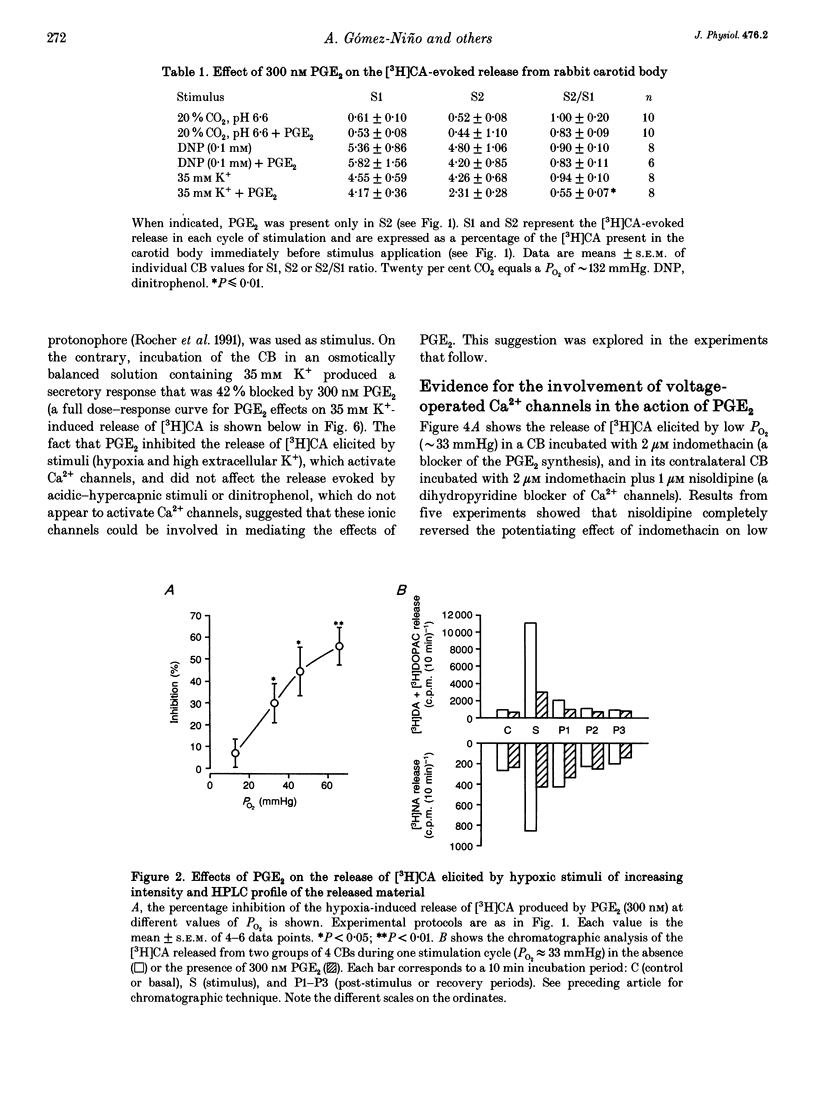
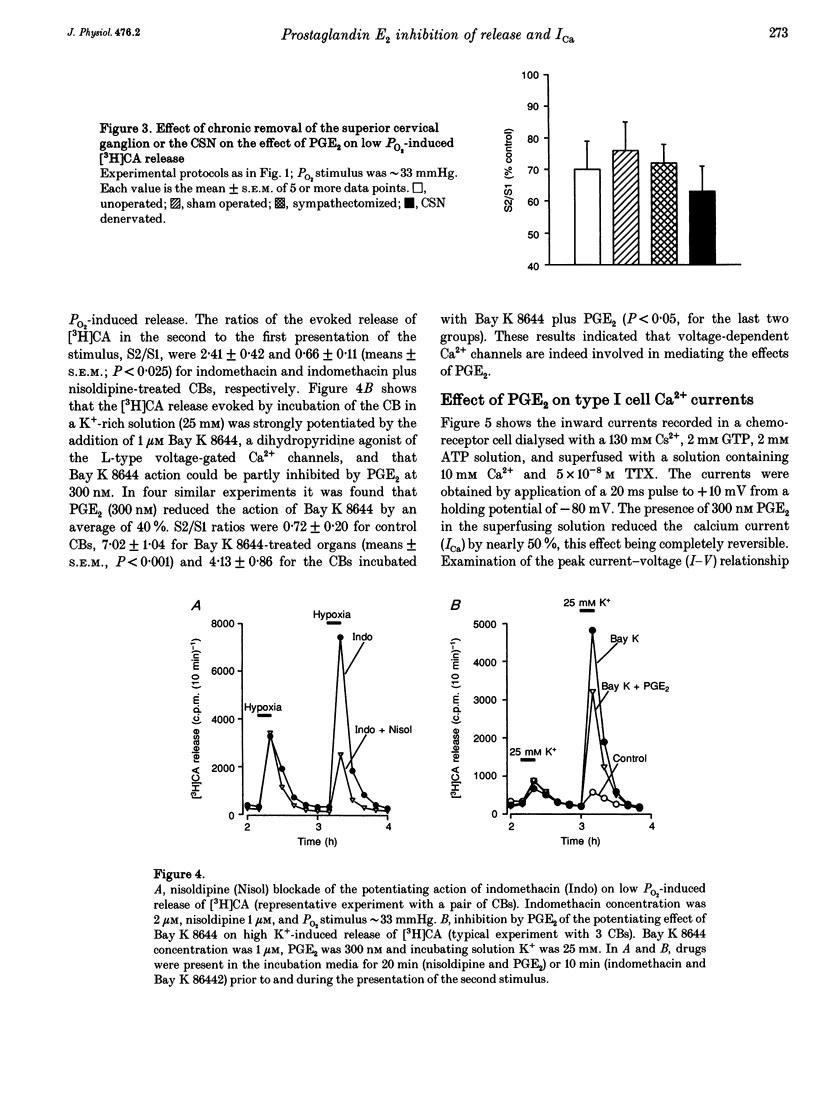
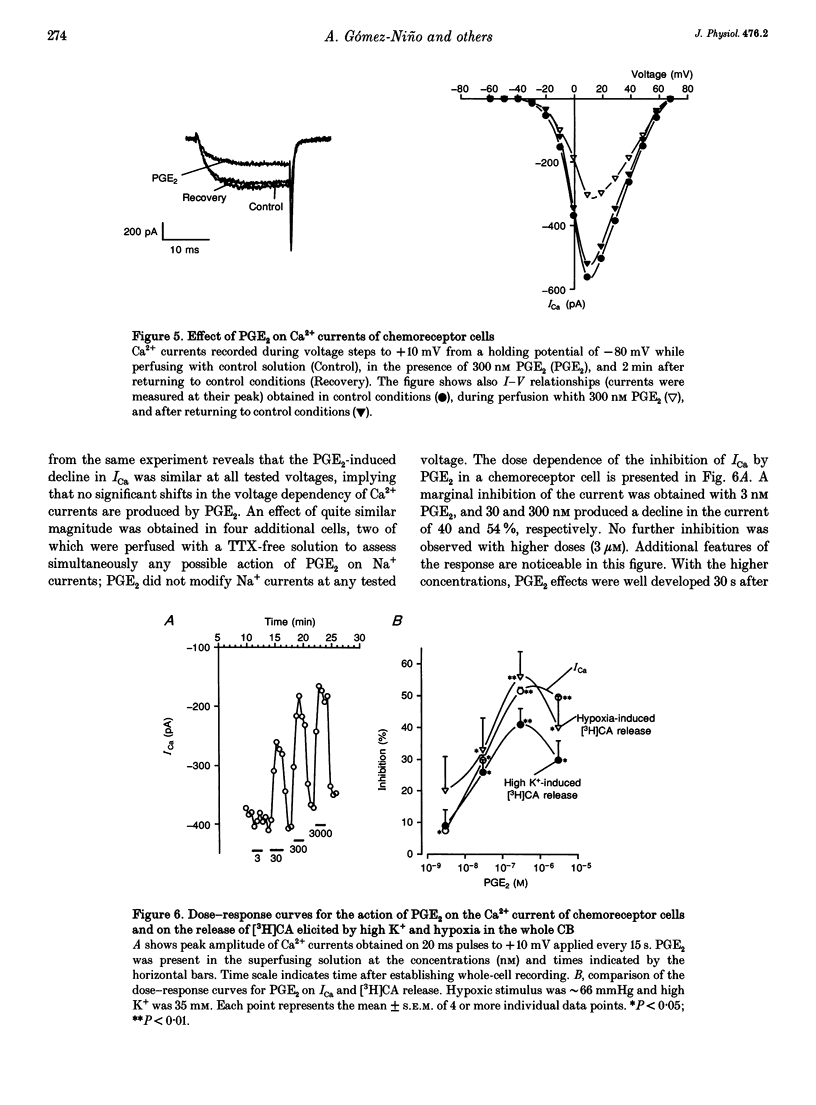
Basal release of [3H]catecholamine ([3H]CA) from rabbit carotid bodies (CBs), previously incubated in the presence of [3H]tyrosine, was not significantly modified by prostaglandin E2 (PGE2). On the contrary, PGE2 (3-300 nM) produced a dose-dependent inhibition of the low PO2-evoked release of [3H]CA. The inhibition was greatest (55%) at a low intensity of hypoxic stimulation (incubating solution PO2 approximately 66 mmHg) and decreased with increasing intensities of hypoxia. Chronic denervation of the CB did not modify the response to PGE2. The release of [3H]CA induced by incubating the CBs in a hypercapnic-acidic solution (PCO2 approximately 132 mmHg; pH = 6.60) and by dinitrophenol (100 microM) was not significantly modified by 300 nM PGE2. PGE2 (300 nM) inhibited the release of [3H]CA elicited by incubating the CBs in a high K+ (35 mM)-containing solution. The release response elicited by high K+ (25 mM) was strongly augmented by a dihydropyridine agonist of Ca2+ channels, Bay K 8644, at a concentration of 1 microM. The Bay K 8644 effect was partly inhibited by PGE2 (300 nM). Using whole-cell recordings in freshly dispersed or short-term cultured chemoreceptor cells from adult rabbits it was found that Ca2+ currents (ICa) were reversibly inhibited by bath application of PGE2. A good parallelism exits between the dose-response curves for PGE2 inhibition of ICa in isolated chemoreceptor cells and high extracellular [K+]- or hypoxia-evoked release of [3H]CA from the whole CB.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almaraz L., Gonzalez C., Obeso A. Effects of high potassium on the release of [3H]dopamine from the cat carotid body in vitro. J Physiol. 1986 Oct;379:293–307. doi: 10.1113/jphysiol.1986.sp016254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J., Burch R. M., Jelsema C. L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988 Mar;11(3):117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Delpiano M. A., Acker H. Extracellular pH changes in the superfused cat carotid body during hypoxia and hypercapnia. Brain Res. 1985 Sep 9;342(2):273–280. doi: 10.1016/0006-8993(85)91126-6. [DOI] [PubMed] [Google Scholar]
- Delpiano M. A., Acker H. Hypoxia increases the cyclic AMP content of the cat carotid body in vitro. J Neurochem. 1991 Jul;57(1):291–297. doi: 10.1111/j.1471-4159.1991.tb02127.x. [DOI] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982 Dec;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García A. G., Sala F., Reig J. A., Viniegra S., Frías J., Fontériz R., Gandía L. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature. 1984 May 3;309(5963):69–71. doi: 10.1038/309069a0. [DOI] [PubMed] [Google Scholar]
- González C., Almaraz L., Obeso A., Rigual R. Oxygen and acid chemoreception in the carotid body chemoreceptors. Trends Neurosci. 1992 Apr;15(4):146–153. doi: 10.1016/0166-2236(92)90357-e. [DOI] [PubMed] [Google Scholar]
- Gutman Y., Boonyaviroj P. Mechanism of PGE inhibition of catecholamine release from adrenal medulla. Eur J Pharmacol. 1979 Apr 15;55(2):129–136. doi: 10.1016/0014-2999(79)90384-4. [DOI] [PubMed] [Google Scholar]
- Gómez-Niño A., Almaraz L., González C. In vitro activation of cyclo-oxygenase in the rabbit carotid body: effect of its blockade on [3H]catecholamine release. J Physiol. 1994 Apr 15;476(2):257–267. doi: 10.1113/jphysiol.1994.sp020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R. Prostaglandin modulation of Ca2+ channels in rat sympathetic neurones is mediated by guanine nucleotide binding proteins. J Physiol. 1992 Dec;458:339–359. doi: 10.1113/jphysiol.1992.sp019421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Mochizuki-Oda N., Hori K., Ozaki K., Miyakawa A., Negishi M. Characterization of prostaglandin E2-induced Ca2+ mobilization in single bovine adrenal chromaffin cells by digital image microscopy. J Neurochem. 1991 Feb;56(2):531–540. doi: 10.1111/j.1471-4159.1991.tb08182.x. [DOI] [PubMed] [Google Scholar]
- Koyama Y., Kitayama S., Dohi T., Tsujimoto A. Evidence that prostaglandins activate calcium channels to enhance basal and stimulation-evoked catecholamine release from bovine adrenal chromaffin cells in culture. Biochem Pharmacol. 1988 May 1;37(9):1725–1730. doi: 10.1016/0006-2952(88)90435-2. [DOI] [PubMed] [Google Scholar]
- López-Barneo J., López-López J. R., Ureña J., González C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988 Jul 29;241(4865):580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- López-López J. R., De Luis D. A., Gonzalez C. Properties of a transient K+ current in chemoreceptor cells of rabbit carotid body. J Physiol. 1993 Jan;460:15–32. doi: 10.1113/jphysiol.1993.sp019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik K. U., Sehic E. Prostaglandins and the release of the adrenergic transmitter. Ann N Y Acad Sci. 1990;604:222–236. doi: 10.1111/j.1749-6632.1990.tb31996.x. [DOI] [PubMed] [Google Scholar]
- McQueen D. S., Belmonte C. The effects of prostaglandins E2, A2 and F2 alpha on carotid baroreceptors and chemoreceptors. Q J Exp Physiol Cogn Med Sci. 1974 Jan;59(1):63–71. doi: 10.1113/expphysiol.1974.sp002242. [DOI] [PubMed] [Google Scholar]
- Mochizuki-Oda N., Mori K., Negishi M., Ito S. Prostaglandin E2 activates Ca2+ channels in bovine adrenal chromaffin cells. J Neurochem. 1991 Feb;56(2):541–547. doi: 10.1111/j.1471-4159.1991.tb08183.x. [DOI] [PubMed] [Google Scholar]
- Obeso A., Almaraz L., Gonzalez C. Effects of 2-deoxy-D-glucose on in vitro cat carotid body. Brain Res. 1986 Apr 16;371(1):25–36. doi: 10.1016/0006-8993(86)90806-1. [DOI] [PubMed] [Google Scholar]
- Obeso A., Rocher A., Fidone S., Gonzalez C. The role of dihydropyridine-sensitive Ca2+ channels in stimulus-evoked catecholamine release from chemoreceptor cells of the carotid body. Neuroscience. 1992;47(2):463–472. doi: 10.1016/0306-4522(92)90260-9. [DOI] [PubMed] [Google Scholar]
- Peers C., Green F. K. Inhibition of Ca(2+)-activated K+ currents by intracellular acidosis in isolated type I cells of the neonatal rat carotid body. J Physiol. 1991 Jun;437:589–602. doi: 10.1113/jphysiol.1991.sp018613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong E., Vesin M.-F., Droz B. Prostaglandin E2 Receptors in the Chicken Spinal Cord: 1. Biochemical Characterization. Eur J Neurosci. 1990 Oct;2(11):897–903. doi: 10.1111/j.1460-9568.1990.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Pérez-García M. T., Almaraz L., González C. Cyclic AMP modulates differentially the release of dopamine induced by hypoxia and other stimuli and increases dopamine synthesis in the rabbit carotid body. J Neurochem. 1991 Dec;57(6):1992–2000. doi: 10.1111/j.1471-4159.1991.tb06414.x. [DOI] [PubMed] [Google Scholar]
- Pérez-García M. T., Almaraz L., González C. Effects of different types of stimulation on cyclic AMP content in the rabbit carotid body: functional significance. J Neurochem. 1990 Oct;55(4):1287–1293. doi: 10.1111/j.1471-4159.1990.tb03137.x. [DOI] [PubMed] [Google Scholar]
- Pérez-García M. T., Gómez-Niño A., Almaraz L., González C. Neurotransmitters and second messenger systems in the carotid body. Adv Exp Med Biol. 1993;337:279–287. doi: 10.1007/978-1-4615-2966-8_39. [DOI] [PubMed] [Google Scholar]
- Pérez-García M. T., Obeso A., López-López J. R., Herreros B., González C. Characterization of cultured chemoreceptor cells dissociated from adult rabbit carotid body. Am J Physiol. 1992 Dec;263(6 Pt 1):C1152–C1159. doi: 10.1152/ajpcell.1992.263.6.C1152. [DOI] [PubMed] [Google Scholar]
- Reimann W., Steinhauer H. B., Hedler L., Starke K., Hertting G. Effect of prostaglandins D2, E2 and F2alpha on catecholamine release from slices of rat and rabbit brain. Eur J Pharmacol. 1981 Feb 19;69(4):421–427. doi: 10.1016/0014-2999(81)90445-3. [DOI] [PubMed] [Google Scholar]
- Rigual R., López-López J. R., Gonzalez C. Release of dopamine and chemoreceptor discharge induced by low pH and high PCO2 stimulation of the cat carotid body. J Physiol. 1991 Feb;433:519–531. doi: 10.1113/jphysiol.1991.sp018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher A., Obeso A., Gonzalez C., Herreros B. Ionic mechanisms for the transduction of acidic stimuli in rabbit carotid body glomus cells. J Physiol. 1991 Feb;433:533–548. doi: 10.1113/jphysiol.1991.sp018442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K., Montague W., Pallot D. J. Biochemical studies on the release of catecholamines from the rat carotid body in vitro. Biochim Biophys Acta. 1989 Sep 4;1013(1):42–46. doi: 10.1016/0167-4889(89)90125-0. [DOI] [PubMed] [Google Scholar]
- Shirahata M., Fitzgerald R. S. Dependency of hypoxic chemotransduction in cat carotid body on voltage-gated calcium channels. J Appl Physiol (1985) 1991 Sep;71(3):1062–1069. doi: 10.1152/jappl.1991.71.3.1062. [DOI] [PubMed] [Google Scholar]
- Ureña J., López-López J., González C., López-Barneo J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. J., Cheng G. F., Yoshizaki K., Dinger B., Fidone S. The role of cyclic AMP in chemoreception in the rabbit carotid body. Brain Res. 1991 Feb 1;540(1-2):96–104. doi: 10.1016/0006-8993(91)90495-h. [DOI] [PubMed] [Google Scholar]


