Visual Abstract
Key Words: pericardial fluid, immune mediators, growth factors, coronary artery disease, fibrosis
Highlights
-
•
Human PF contains inflammatory mediators, including cytokines, chemokines, and growth factors.
-
•
Reflecting a dynamic space, disease states can influence the inflammatory mediator profile of human pericardial fluid.
-
•
Human PF can drive profibrotic processes in vitro via the TGF-β pathway.
Summary
Human pericardial fluid (PF) is a rich reservoir of biologically active markers. The acellular compartment of PF can drive cardiac fibroblast activity in vitro. This process is mediated through the transforming growth factor-β pathway. Of clinical importance, the PF of patients with coronary artery disease has an increased profibrotic capacity compared with the PF of patients without coronary artery disease.
Pericardial fluid (PF) is defined as the net ultrafiltrate of plasma from the epicardial capillary bed, with contributions from the parietal pericardial layer, myocardial interstitial fluid, and secretions of pericardial mesothelial cells.1 Owing to its anatomical proximity to the heart, PF provides a unique milieu that may reflect cardiac diseases, but also has a homeostatic effect on cardiac function. PF is a highly enriched reservoir of physiologically active substances. These bioactive molecules regulate cardiac function via intracrine actions and exhibit dysregulation in various disease states.2,3 The pericardium (PT), myocardial tissue, and epicardial and epiaortic adipose tissue can contribute to the contents of the PF.4 PF has a slow turnover rate,5 which can help to promote its potential role in containing diagnostic biomarkers for heart conditions and pericardial diseases and is an attractive target for future therapeutics and interventions. To date, most of the literature on PF has been generated by studies focused on benign or malignant pericardial effusions and various manifestations of pericarditis.6, 7, 8
As an evolving area in cardiovascular research, contents of the pericardial space have been assessed for their potential post-myocardial injury reparative capacity. A recent study by our group has found that Gata6+ pericardial cavity macrophages can relocate to the injured heart and prevent fibrosis after myocardial injury.9 Conversely, another study has used genetic lineage tracing, and found that the same population of macrophages have minimal reparative properties.10 Moreover, there is mounting evidence suggesting that the contents of the pericardial space after cardiac surgery can contribute to postoperative atrial fibrillation.11, 12, 13, 14, 15, 16 Our group has also shown that an acute ischemic event can alter the immune cell composition of human PF.17 Furthermore, our group and others have demonstrated that the pericardial space is replenished by inflammatory markers, such as immune cells, cytokines, chemokines, and growth factors, after cardiac surgery.18,19 We described how the postoperative pericardial inflammatory profile evolves 48 hours after surgery. We determined that the local postsurgical inflammatory response differs between conventional cardiac surgery and minimally invasive cardiac surgery.18 Notably, the inflammatory response in the pericardial space after cardiac surgery differs from postcardiopulmonary bypass systemic inflammation,20 and there is emerging evidence suggesting that biomarkers in the pericardial space after surgery may have usefulness in predicting length of hospital stay.21 Taken together, these findings further support the concept that the pericardial space is a dynamic niche that can evolve in response to injury and manipulation. However, the direct biological activity of the fluid on neighboring cells of the heart has not been pursued in detail.
In the present study, we focus on comprehensively characterizing the soluble mediators of the acellular component of native human PF. To do so, we collected PF from patients with coronary artery disease (CAD) who underwent coronary artery bypass grafting (CABG) and those undergoing valvular surgery. Given that this is a pilot study, done using clinical samples, our first objective was to determine the immune profile of acellular component of PF, or PF supernatant (PFsup). We analyzed PFsup for chemokines, cytokines, growth factors, matrix metalloproteases (MMPs), and tissue inhibitors of MMPs (TIMPs). To better understand these factors' source(s), we also analyzed cultured media from the PT, epiaortic adipose tissue, and myocardial tissue by using the right atrial appendage as a surrogate. Furthermore, using cardiac-derived fibroblasts, we found that PFsup can drive fibrotic activity in vitro. Importantly, we noted that the kinetics of PFsup-mediated effects differed in CABG patients compared with valve surgery patients. To elucidate the mechanism underlying our observations, we conducted bulk RNA-sequencing on cardiac fibroblasts exposed to PFsup, which implicated the transforming growth factor (TGF)-β pathway. Through validating bulk RNA-sequencing hits, we were able to show that bone morphogenic protein (BMP) may be involved in potentiating cardiac fibroblast activity in vitro.
Methods
Patient sample acquisition
Patients undergoing CABG, valvular, and concomitant CABG and valvular surgeries at the Foothills Medical Centre (Calgary, Canada) were enrolled in the study prospectively after providing written informed consent. The experiments were conducted under the approval of the Conjoint Health Research Ethics Board at the University of Calgary underlying the Declaration of Helsinki (Ethics ID: REB16-1906, approved 12 February 2021). The entire volume of native PF was obtained from all patients before the institution of cardiopulmonary bypass and the administration of heparin or steroids. An excised segment of the PT and right atrial appendage were also collected from patients. Inclusion criteria were age >18 years and patients undergoing surgery through a conventional full median sternotomy. We excluded patients who received insulin or immunosuppressive medications, patients with a history of inflammatory or rheumatic disease, patients who required dialysis, patients with active infective endocarditis, and those who underwent emergent surgery or redo surgery. Intraoperatively, the PT was incised at full length anteriorly and teed off with the creation of the pericardial cradle. In all patients, the native PF was removed after pericardiotomy. After collection, PF samples were transported on ice to the laboratory in a sodium heparin tube (Greiner Bio-One) and immediately processed. Processing entailed spinning the PF at 1,500 rounds per minute for 4 minutes at 3°C. The acellular component was retrieved and stored at −80°C for further analysis. A segment of PT was incised when creating the pericardial cradle, while right atrial appendage was obtained at the time of cannulating the right atrial appendage (prior to the institution of cardiopulmonary bypass). For analysis purposes, these tissues were normalized based on weight.
Cytokines, MMPs, TIMPs, and TGF-β
Concentrations of chemokines, cytokines, MMPs, TIMPs, and TGF-β in PFsup (of all 115 enrolled patients) and cultured media were measured with blinded multiplex analysis (EveTechnologies). Interleukin (IL)-1β, IL-1 receptor antagonist (IL-1Ra), IL-5, IL-6, IL-8, IL-10, IL-13, interferon-gamma (IFN)-γ, tumor necrosis factor alpha, and monocyte chemoattractant (MCP)-1 were included in our assays. Further, we probed for MMP-1, -2, -3, -7, -8, -9, -10, MP-12, and -13. We also assessed for TIMP-1, -2, and -4. Finally, we also probed for TGF-β1 and TGF-β2 concentrations. The MMPs and TIMPs analysis was carried out for 81 patients.
Messenger RNA isolation
Conditioned media from the rat tail collagen type I macrogel wells was aspirated and transferred into 1.5-mL Eppendorf tubes for later multiplex analysis. Gels were then transferred into 1.5-mL Eppendorf tubes using a 1-mL pipette. Gels were homogenized in RLT buffer before mRNA isolation using an RNeasy kit (Qiagen) per the manufacturer’s protocol. Collected mRNA was then stored in a −80°C freezer until later use.
Bulk-RNA sequencing
RNA samples were initially assessed for quality using the Agilent TapeStation 4200, with RNA Integrity Numbers ranging between 9.2 and 10.0. RNA quantification was assessed using a Promega QuantiFluor RNA plate assay, ranging from 15.3 to 71.0 ng/μL in 30 μL per sample. DNA carryover in a random subset of 5 samples was assessed relative to these RNA quantifications, using a high sensitivity Qubit double stranded DNA fluorescence assay (Promega), yielding carry-over estimates of 6.78% to 7.01%.
Bulk-RNA sequencing reads were pseudomapped to NCBI RefSeq human transcript models using Kallisto 0.42.4.22 Mapping counts were analyzed using Sleuth,23 with a regression model of the 3-level nominal media factor: control, PFsup, PFsup, and TGF-β1 receptor inhibitor (TGF-βR1i). Differentially expressed transcripts passed the aforementioned regression model with the Wald and likelihood ratio test P values of <0.05 after correction for multiple testing using the Benjamini-Hochberg post hoc test. Transcripts were annotated using Ingenuity Pathway Analysis (Qiagen). Pathways were considered significant with an enrichment P value of <0.01 after Benjamini-Hochberg correction, and up- and down-regulation was called when the absolute z-score was >2 (P < 0.05). The NCBI GEO accession number for the RNA sequencing is GSE262161.
Western Blotting
Human atrial fibroblasts cultured alone or treated with PFsup were washed with phosphate-buffered saline and incubated on ice with 100 μL of 1× RIPA buffer containing protease and phosphatase inhibitors to solubilize cells and isolate proteins. After scraping the cells from the culture plate with a cell scraper, the RIPA buffer was centrifuged for 1 min at 8,000×g to precipitate insoluble proteins. A 30-microliter aliquot was mixed with 10 μL of 4× Lamelli buffer and heated for 10 minutes at 95°C to denature the proteins. The resulting samples underwent SDS-PAGE gel electrophoresis (1.5 mm, 10% gel) to separate the proteins based on molecular weight. The subsequent proteins were transferred to nitrocellulose membrane for Western blotting of SMAD3 and GAPDH. The nitrocellulose membranes were blocked for a minimum of 2 hours using 5% skim milk powder in 1× TBS with 0.05% Tween 20. The primary antibodies used in this study were applied to the blots for 2 hours in the 5% skim milk, 1× TBS+Tween 20 solution. The antibodies used in this study were: rabbit anti-SMAD3 antibody (Cell; Cat. no. 9523) at 1:1,000 dilution and mouse anti-GAPDH (Sigma-Aldrich; Cat. no. 8795) at 1:2,000 dilution. Secondary antibodies used were goat anti-mouse horseradish peroxidase (Sigma-Aldrich; Cat. no. AP308P) and goat anti-rabbit horseradish peroxidase (Sigma-Aldrich; Cat. no. A6154) antibodies at 1:5,000 dilution. Stripping was performed using Restore PLUS Western Blot Stripping Buffer (Thermo Fisher Scientific). After probing each blot for SMAD3 expression, the same blot was stripped using Restore Plus Western blot stripping buffer (Thermo Fisher Scientific) according to the manufacturers protocol, and subsequently probed for GAPDH expression. The blots were visualized using a Supersignal West-Femto chemiluminescence kit (Thermo Fisher Scientific), and the resulting images were quantified using NIH-Image J software.
Human cardiac myofibroblast isolation
Human cardiac fibroblasts were isolated from right atrial appendage taken from consenting patients undergoing cardiac surgery at Foothills Medical Center as previously described.24,25 All experiments involving human tissue were approved by the Conjoint Health Research Ethics board at the University of Calgary and conformed to the Declaration of Helsinki. Informed consent was required. In brief, samples were minced into 0.5- to 1.0-mm fragments and suspended in Iscove’s IMDM (Lonza) supplemented with 10% fetal bovine serum (Gibco by Life Technologies) and 50,000 U penicillin-streptomycin (Life Technologies). Tissue suspensions were plated and cultured at 37°C in 5% CO2. Passages 1 through 4 were used for experiments. All cells were serum starved for 24 hours before experimental use.
3-dimensional collagen gel contraction model
The 3-dimensional collagen gel contraction model has been validated previously as a functional measure of myofibroblast activity, in which percent gel contraction positively correlates to myofibroblasts activity levels.26, 27, 28, 29 Briefly, a collagen gel solution was prepared by combining on ice: 10% 10× minimal essential medium (Gibco), rat tail type I collagen (BD Biosciences) to a final concentration of 1.8 mg/mL, 1 N NaOH to a pH of 7 to 8, and phosphate-buffered saline. Human cardiac myofibroblasts, serum starved for 24 h, were incorporated to achieve a concentration of 1.0 × 105 cells per well, and the solution was aliquoted into a 24-well plate (Falcon). The collagen gel was polymerized at 37°C for 1 hour. Once polymerized, a treatment solution of 500 μL of serum-free media alone (representing baseline myofibroblast activity) or in combination with recombinant human TGF-β1 (TGF-β; Gibco; 10 ng/mL; positive control known to increase myofibroblast activity) was added to each of the collagen gel-containing wells. Collagen gels were released from the 24-well plate after 24 hours and imaged an additional 24 hours after release. Images were analyzed using ImageJ (version 1.44g; NIH). Collagen remodeling was indicated by percent contraction, calculated according to the following: %Contraction = [(surface area of the well − surface area of the collagen gel)/surface area of the well] × 100.
Statistical analysis
Continuous data are shown in figures as box and whisker plots displaying the median, interquartile range, minimum and maximum or as mean ± SD. Patient characteristics are expressed as mean ± SD or count (percentage) where appropriate. The Student t test was performed to compare concentrations of mediators in PFsup. Statistical analysis was conducted using GraphPad Prism 9 Software. We considered P values of <0.05 as significant.
Results
Patient demographics
One hundred fifteen patients were enrolled in the study; 71 (61.7%) were male, and their mean age was 62.2 ± 14.5 years. Eighty-six had hypertension, 35 had noninsulin-dependent diabetes, and 84 had dyslipidemia. All patients underwent either CABG surgery, valvular surgery, or a combination of both. None were actively prescribed steroids or immunosuppressive medications or had contracted COVID-19 in the preceding 3 months. Baseline patient characteristics are summarized in Table 1.
Table 1.
Baseline Patient Demographics for the 115 Patients Who Had Multiplex Analysis Performed on Their PFsup
| Mean age (y) | 62.2 ± 14.5 |
| Sex | |
| Male | 71 |
| Female | 44 |
| Type of surgery | |
| Isolated CABG | 72 |
| Isolated valve surgery | 34 |
| CABG + valve surgery | 9 |
| Hypertension | 86 |
| Dyslipidemia | 84 |
| Diabetes | |
| Type I | 0 |
| Type II | 35 |
| Insulin dependent | 0 |
| Smoking | |
| Active | 27 |
| Remote | 37 |
| Atrial fibrillation | |
| Paroxysmal | 0 |
| Permanent | 10 |
| Severe LV dysfunction | 14 |
| Chronic kidney disease | |
| eGFR <45 mL/min | 15 |
| Dialysis dependent | 0 |
| Chronic obstructive lung disease | 11 |
| Peripheral arterial disease | 7 |
| Cerebrovascular disease | 10 |
| Rheumatoid disease | 0 |
| Autoimmune disease | 0 |
| Immunosuppressed | 0 |
| Active infective endocarditis | 0 |
| Preoperative medication use | |
| Steroids | 0 |
| Aspirin | 90 |
| NSAIDs | 0 |
| Anti-inflammatory | 0 |
| Antibiotics | 0 |
Values are mean ± SD or n.
CABG = coronary artery bypass grafting; eGFR = estimated glomerular filtration rate; LV = left ventricle; NSAID = nonsteroidal anti-inflammatory drug; PFsup = pericardial fluid supernatant.
The human PF contains a unique soluble mediator profile
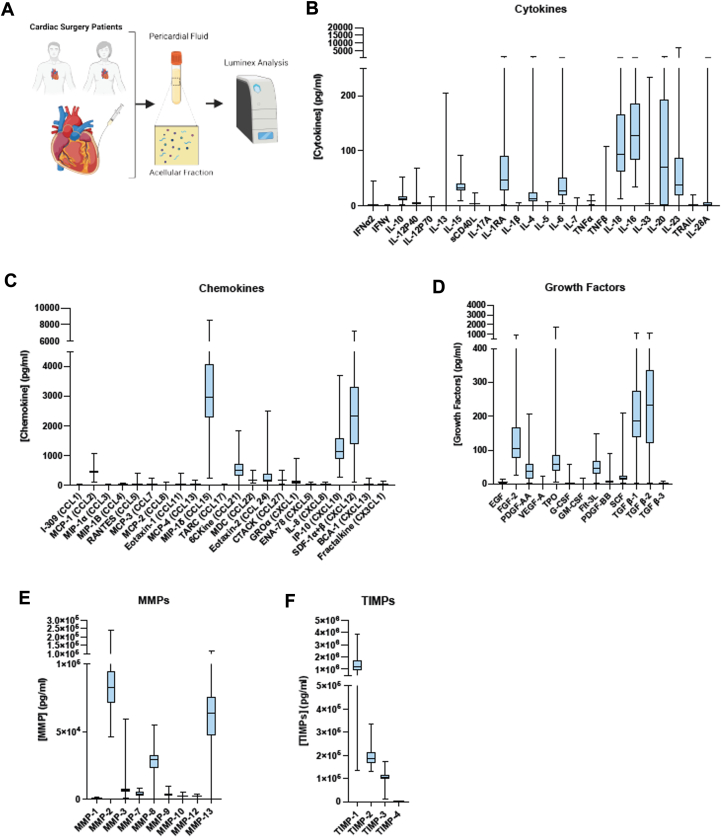
To comprehensively characterize the inflammatory profile of human PF, the acellular portion was probed for determining the concentration of various mediators (Figure 1A). Suggestive of a homeostatic environment, both anti-inflammatory and regulatory cytokines, such as IL-10 and IL-1RA, and proinflammatory cytokines, including IL-6, -18, and -23, were detected in PFsup (Figure 1B). Chemokines related to myeloid (eg, MCP-1, IP-10, and MIP-1δ) and lymphocytes (eg, IP-10, CCL21, and CXCL12) recruitment and retention were elevated in the PF, consistent with the presence of these cells within the environment (Figure 1C). These immune mediators were accompanied by numerous growth factors and extracellular matrix (ECM) remodeling regulators. Notably, the concentration of TGF-β1, TGF-β2, and fibroblast growth factor (FGF)-2 was higher in human PFsup (Figure 1D). MMP-2 and -13, 2 collagenases involved in the breakdown of ECM, had a higher pericardial concentration compared with other MMPs (Figure 1E). TIMPs can dampen MMP activity locally, so we measured the concentration of TIMPs in the same PFsup samples (Figure 1F). TIMP-1, which participates in wound healing, cell morphology and survival, angiogenesis, and inflammatory responses, was the most abundant form.
Figure 1.
Overall Study Design
(A) Schematic diagram showing the overall study design. (B to F) Cytokines, chemokines, growth factors, MMPs, and TIMPs identified to be present in the acellular compartment of patients undergoing cardiac surgery. For cytokines, chemokines, and growth factors, the data are derived from pericardial fluid supernatant (PFsup) collected from 115 undergoing coronary artery bypass grafting (CABG), valve surgery, and combined CABG and valve surgery. The dataset for MMPs and TIMPs was generated from analyzing the PFsup of 81 patients. Data are presented as box plots.
The soluble mediator profile of human PF is distinct from circulating markers in serum
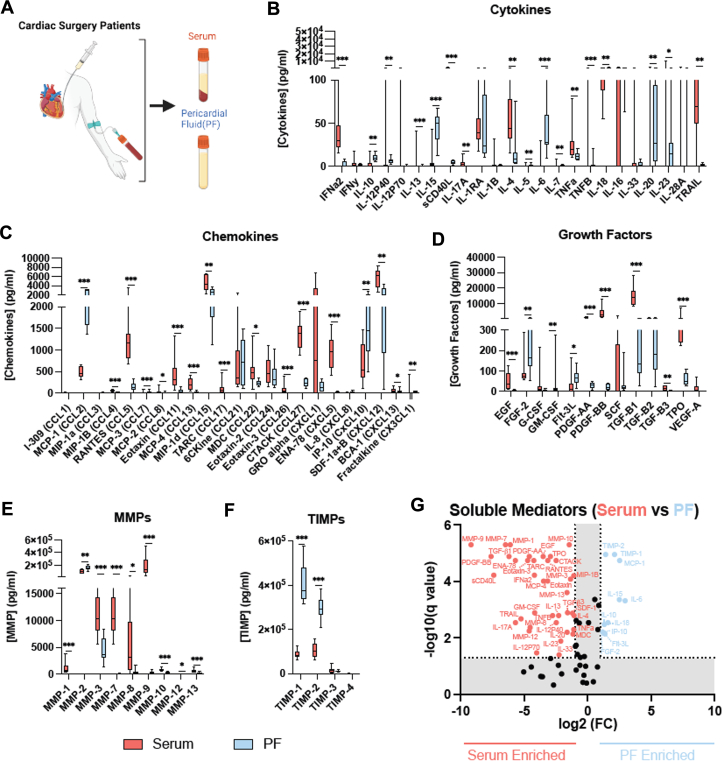
To compare between the mediator profile in PFsup and serum, in addition to collecting PF, blood was obtained from 10 patients enrolled in the study (Figure 2A). With respect to cytokines, significant differences were noted IFNα2, IL-6, IL-10, IL-15, IL-18, tumor necrosis factor alpha, soluble CD40L, and tumor necrosis factor-related apoptosis-inducing ligand (Figure 2B). Notably, the pericardial concentration of IL-6, -10, and -15 was markedly higher compared with their respective concentrations in serum. Differences were also observed for chemokines, where most of the mediators that were probed for had significantly different concentrations in the PF compared with serum (Figure 2C). Similarly, there were marked differences in the pericardial vs serum concentrations of growth factors (Figure 2D). Relevant to fibroblast activity and fibrosis, FGF-2 had was found at a higher concentration in the pericardial space, whereas TGF-β1 had a significantly higher concentration in serum. Among MMPs, MMP-2 was found to have the highest concentration in PFsup (Figure 2E), where its concentration was also significantly higher compared with serum levels of MMP-2. Important for ECM remodeling, compared with serum, TIMP-1 and -2 levels were markedly higher in the pericardial space (Figure 2F). As a snapshot, Figure 2G shows distinct patterns for soluble meditators enriched in serum vs those found at higher concentrations in PFsup. Taken together, these observations provide objective evidence supporting that the PF milieu is: 1) enriched with soluble mediators; and 2) its profile of these mediators is different when compared with the systemic circulation.
Figure 2.
Collection of PF and Blood Samples
(A) Schematic diagram depicting the collection of PF and blood samples from 10 patients undergoing cardiac surgery. (B to F) Comparison in concentrations of cytokines, chemokines, growth factors, MMPs, and TIMPs found in PF (blue) vs serum (red). (G) Soluble mediators enriched in serum (red) vs PF enriched (blue). Multiple Mann-Whitney U tests were performed with a false discovery rate by 2-stage step-up (Benjamini, Krieger, and Yekutieli). Data are presented as box plots. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001. Abbreviations as in Figure 1.
PT, pericardial cells, and atrial appendage can produce and secrete inflammatory markers into PFsup
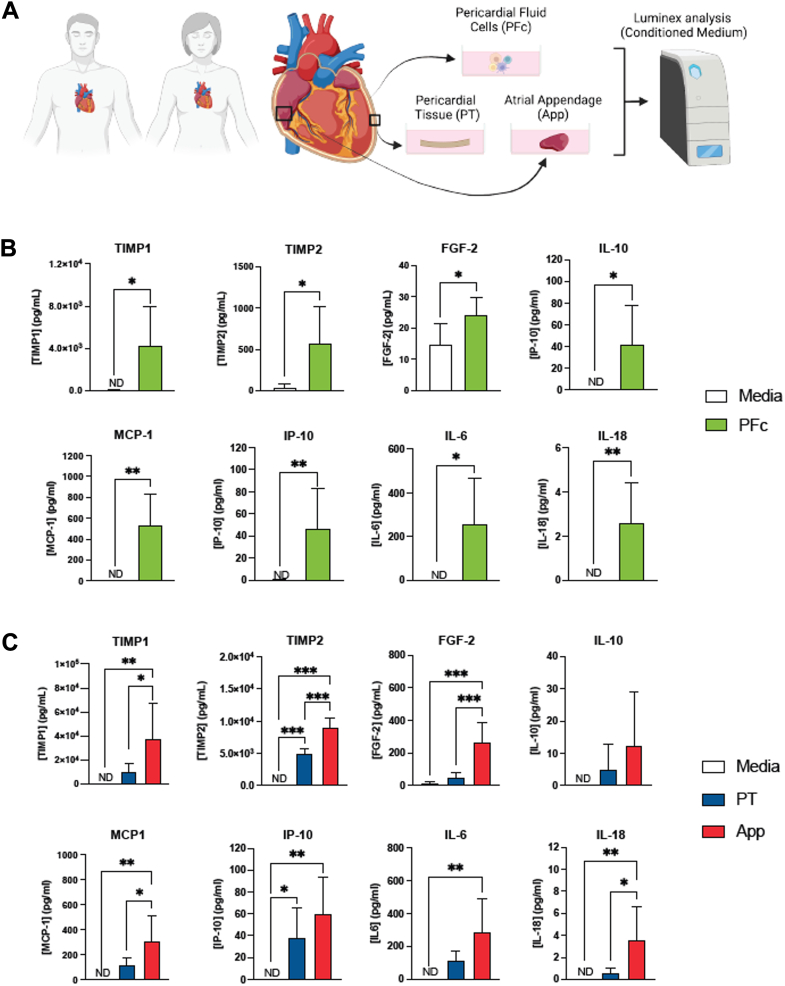
Given that PF demonstrates a unique mediator profile, the potential local sources of these molecules were explored. A segment of the PT, atrial appendage, and PF cells (PFc) were collected from 6 patients and cultured in media to measure mediator release at 24 hours (Figure 3A). Compared with control (culture media without any PFc), in the media containing PFc, the concentration of TIMP-1, TIMP-2, MCP-1, FGF-2, IP-10, IL-6, IL-10, and IL-18 was significantly higher (Figure 3B). For the same mediators, except for IL-10, there were significant differences when comparing between control vs atrial appendage (Figure 3C). PT conditioned media demonstrated higher levels of TIMP-2 and IP-10 compared with control. Comparing the conditioned media between tissue sources, atrial appendage tissue secreted more TIMP-1, TIMP-2, FGF-2, MCP-1, and IL-18 than PT.
Figure 3.
Collection and Culture of Different Pericardial Tissues
(A) Schematic showing the collection and culture of different pericardial tissue, including PF cells (PFcs), pericardium (PT), and right atrial appendage (APP). These tissue sources were collected from 6 patients. (B) PFcs were cultured in media and compared with media alone (controls). PFcs produced and secreted a significantly higher concentration of various mediators. The Student t test was done for statistical purposes and a P value of <0.05 was considered significant. (C) Segments of pericardium and right atrial appendage were also collected and cultured. Cultured media from each tissue source was analyzed using multiplex and probed for their concentration of soluble mediators. There were significant differences noted between the tissue sources and specific factors. Analysis was done after normalizing based on weight for each tissue source. One-way analysis of variance with Tukey’s post hoc test was done. Data are presented as mean ± SD. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
PFsup can drive human cardiac fibrotic activity in vitro via TGF-β
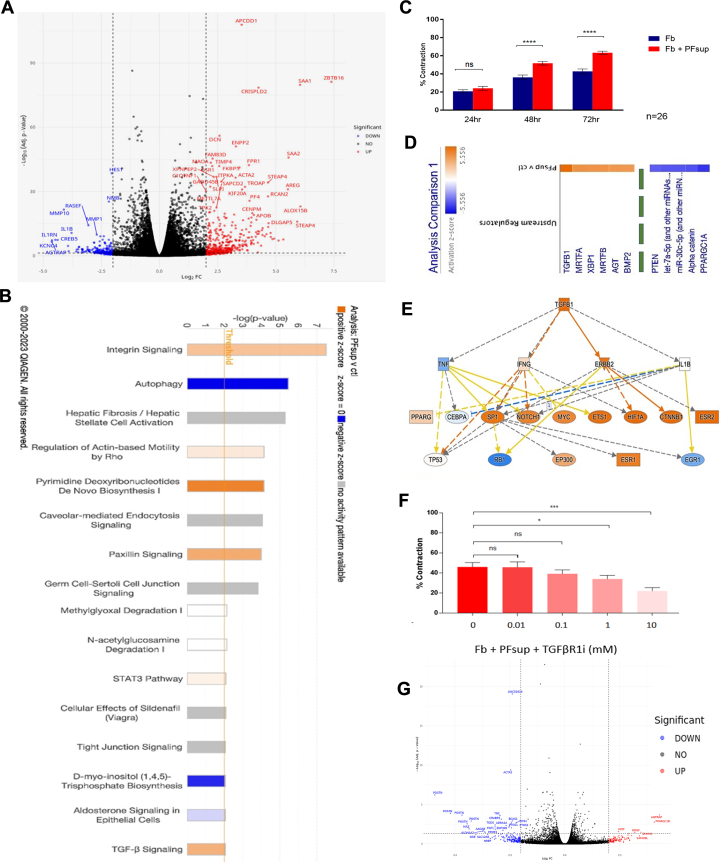
Many of the factors present in PF have links to tissue remodeling. Within the neighboring heart, cardiac fibroblasts play a central role in the regulation of the remodeling process. As such, the impact of the PF on the modulation of human cardiac fibroblast phenotype and activation was assessed following exposure of these cells to PFsup. Bulk RNA sequencing analysis of human cardiac fibroblasts revealed significant transcriptional changes between PFsup and control media exposure (Figure 4A). A total of 335 differentially expressed genes (DEGs) were identified; 265 were enriched in the PFsup condition and 70 genes were enriched in the control media setting (log 2-fold change, and adjusted P < 0.05). Ingenuity pathway analysis of these DEGs identified pathways linked to tissue remodeling and fibrotic in cardiac fibroblasts exposed to PFsup (Figure 4B). The pathways that were enriched with PFsup were related to cell-cell/cell-ECM junction and fibrotic . Control media were linked with enriched pathways related to autophagy and inositol triphosphate biosynthesis. To evaluate the PFsup mediated effect we then used a well-characterized three-dimensional collagen gel contraction assay that provides an assessment of these pathways with contractile force generation with functional fibroblast-collagen integration. These functions are good predictors of fibrotic activity in vivo. Human cardiac fibroblasts were seeded in a rat tail collagen l3D matrix and exposed to PFsup. At 48 and 72 hours, there was significant gel contraction (Figure 4C). Such an observation is indicative of increased fibroblast activity when these cells are exposed to PFsup in vitro.
Figure 4.
Bulk RNA-seq
(A) Bulk RNA sequencing (RNA-seq) was performed on human cardiac fibroblasts, identifying up-regulated and down-regulated genes. Analysis was done on 6 distinct human samples for right atrial appendage-derive cardiac fibroblasts and PFsup collected from 6 patients. (B) Canonical pathways were interrogated in silico, where transforming growth factor (TGF)-β was one of the hits. (C) Human cardiac fibroblasts were seeded in a rat tail collagen type I macrogel and treated with the acellular compartment of PF, also known as PFsup. Gel contraction was used as a marker of fibrotic activity in vitro. At 48 and 72 hours after treatment, there was a significant increase in fibrotic activity. Experiments were done on 26 distinct patient samples and analysis was done using the Student t test where a P value of <0.05 was taken to be significant. Data are presented as mean ± SD. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001. (D) Upstream genes were sorted by z-score and TGF-β1 was found to be highest in fibroblasts treated with PFsup compared with controls. The list is truncated (as evidenced by the green dashed line) to show the ligands with the highest and lowest z-score (top and bottom 5). The complete list of ligands is provided in Supplemental Figure 1. (E) Further in silico analysis of TGF-β1 revealed the well-established upstream regulators, such as IFN-γ. (F) Inhibition of TGF-β receptor 1 (TGF-βR1i) in cardiac fibroblasts treated with PFsup resulted in significant decrease in fibrotic activity in vitro. The experiment was done on 10 distinct biological replicates. The Student t test was done for statistical purposes and a P value of <0.05 was considered to be significant. Data are presented as mean ± SD. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001. (G) RNA-seq was performed on fibroblasts exposed to TGF-βR1i, identifying upstream genes that were down-regulated compared with fibroblasts that were treated with only PFsup. Abbreviations as in Figure 1.
Evaluation of potential upstream mediators of DEGs in the PFsup stimulated human cardiac derived fibroblasts identified TGF-β1 as the top ligand (Figure 4D) with numerous up-regulated downstream targets in its network (Figure 4E). It should be noted that Figure 4D shows the top and bottom 5 ligands, based on the z-score, respectively. The remainder were truncated (as depicted by the dashed green line). The full list is provided in Supplemental Figure 1, with the highest z-scores in orange (left) and lowest z-scores in blue (right).
Given these findings, along with its presence in PFsup, we queried whether inhibiting TGF-β can attenuate PFsup-mediated fibrotic activity in vitro. The TGF-β signalling pathway was first interrogated at the receptor level. Inhibition of TGF-β receptor 1 (TGF-βR1i) in cardiac fibroblasts treated with PFsup resulted in significant decrease in myofibroblast related-genes (ACTA2, POSTN) (Figure 4F) and fibrotic activity in vitro (Figure 4G). These results were further corroborated with the use of a TGF-β-blocking antibody in the same experiment setup, which also resulted in reduced fibrotic activity in vitro (Supplemental Figure 2A).
PFsup likely drives fibrotic activity via the classical canonical SMAD pathway and inhibiting FGF does not blunt PFsup-mediated fibrotic activity in vitro
Western blotting confirmed the downstream PFsup-mediated activation of the SMAD proteins, where total SMAD3 expression levels were significantly increased in fibroblasts treated with PFsup (Supplemental Figure 2B). Activated SMAD3 levels did not significantly change upon exposure to PFsup (data not shown). To explore whether noncanonical pathways were involved, the addition of rapamycin was used to inhibit of mammalian target of rapamycin. Rapamycin-mediated mammalian target of rapamycin inhibition did not impact the fibroblast activity by PFsup stimulation (Supplemental Figure 2C). Wnt1 was also identified as a potential upstream mediator by the RNA sequencing analysis and the Wnt pathway is associated with fibrotic activity and there is a strong crosstalk with TGF-β signalling.30 The addition of Notum, a carboxylesterase known to inhibit Wnt , did not decrease PFsup-induced fibrotic activity in vitro (Supplemental Figure 2D). FGF-2 was also found in PFsup and has been noted to counteract TGF-β in fibroblasts. Given its importance in fibroblast activity, we determined whether PFsup-mediated fibrotic activity was at all dampened via FGF. An FGF receptor inhibitor (FGFR1i) was added to cardiac fibroblasts treated with PFsup. However, this addition did not result in a significant attenuation of fibrotic activity in vitro (Supplemental Figure 2E). These observations suggest that PFsup can activate TGF-β through the classical canonical SMAD-dependent pathway in cardiac fibroblasts.
BMP4 can contribute to PFsup-mediated fibrotic activity
Our multiplex analysis found that the concentration of TGF-β1 in PFsup was in the picogram/mL range. However, when cardiac fibroblasts were treated with that concentration of exogenous recombinant human TGF-β1 (rhTGF-β1), no significant fibrotic activity was noted in vitro (Supplemental Figure 3A), suggesting that PFsup likely sensitizes fibroblasts through other mediators to respond to lower TGF-β levels present in the PF. Upstream regulators were analyzed to filter the impact of TGF-β and identify alternative mediator-driven priming pathways. Potential upstream regulators were identified based on genes that were not different between PFsup exposed samples in the presence or absence of the TGF-βR1i, but were equally up-regulated relative to media control samples. This streamlined analysis revealed 8 plasma membrane proteins, including BMP receptor-1 (BMPR1) (Supplemental Figure 3B). BMPR1 network analysis with integration of DEGs uncovered multiple input including BMP4 (Supplemental Figure 3C). Given these observations, using multiplex analysis, we next confirmed the presence of BMP4 in PFsup of patients undergoing CABG (Supplemental Figure 3D). We treated cardiac fibroblasts with exogenous recombinant BMP4 and noted significant fibrotic activity in vitro at the same concentration range as was found in PFsup (Supplemental Figure 3E). Finally, when dorsomorphin, a known inhibitor of BMP , was added to fibroblasts treated with PFsup a significant decrease in fibrotic activity was noted in vitro (Supplemental Figure 3F). Taken together, these observations provide evidence supporting the role BMP plays in PFsup-mediated fibrotic activity.
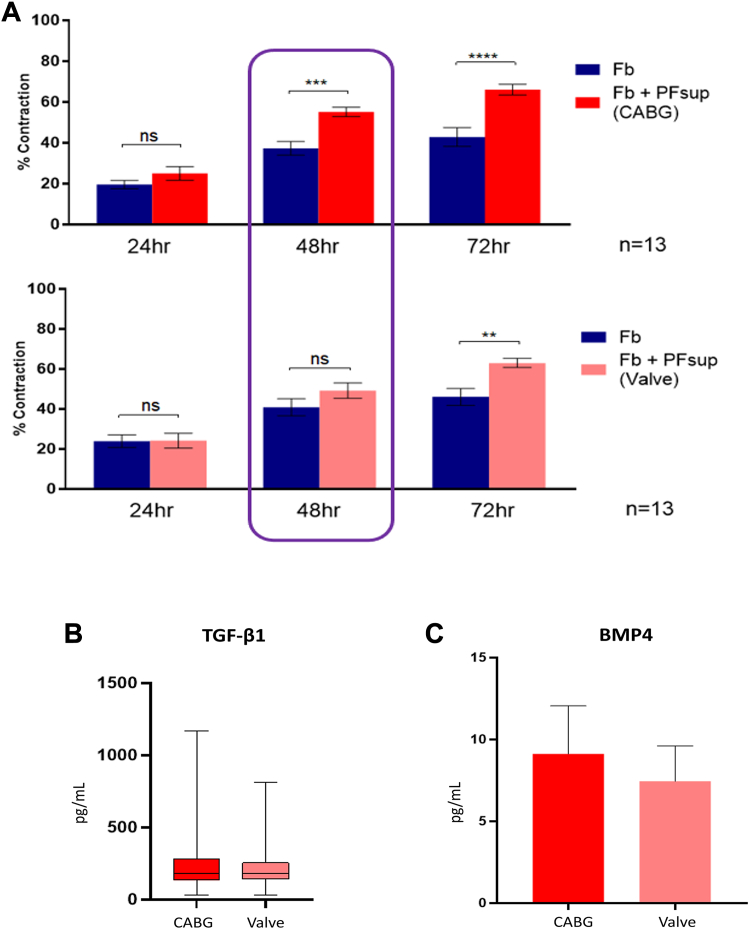
There are kinetic difference in the PFsup-mediated fibrotic activity of CABG vs valve surgery patients in vitro
We exposed cardiac derived fibroblasts to PFsup obtained from 13 CABG patients and 13 valve surgery patients and noted a difference in the kinetics for fibrotic activity in vitro (Figure 5A). Patient characteristics are summarized in Table 2. At 48 hours, PFsup from CABG patients was able to induce fibrotic activity, whereas PFsup from valve surgery patients resulted in significant fibrotic activity at 72 hours (Figure 5A). To delineate the mechanism driving this observation and given our finding on TGF-β1 and BMP, we queried whether the concentration of these effector molecules is different in PFsup of CABG patients compared with valve surgery patients, but did not note a significant difference (Figures 5B and 5C). These observations suggest 2 important phenomena. First, CAD can affect the profibrotic potential of human PFsup (kinetics). Second, although TGF-β1 and BMP4 can drive PFsup-mediated fibrotic activity in vitro, there are likely other factors present in PFsup that contribute to its fibrotic capacity.
Figure 5.
The Kinetics of Pericardial Fluid-Driven Cardiac Fibrosis In Vitro
(A) Human cardiac fibroblasts were treated with PFsup obtained from CABG patients (n = 13) and valve surgery patients (n = 13), where a difference in the kinetics of fibrotic activity was noted in vitro. A Student t test was used for statistical purposes where P < 0.05 was taken to be significant. The PFsup concentration of TGF-β1 (B) and bone morphogenic protein (BMP)-4 (C) was not significantly different in CABG vs valve surgery patients. (A and C) Data are presented as mean ± SD, ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001. (B) Data are presented as box plots. Other abbreviations as in Figures 1 and 4.
Table 2.
Baseline Patient Demographics for 13 CABG and 13 Valve Surgery Patients, Where the In Vitro Profibrotic Potential of PFsup Was Compared
| CABG (n = 13) | Valve Surgery (n = 13) | |
|---|---|---|
| Mean age (y) | 69.1 ± 12.6 | 67.8 ± 9.4 |
| Sex | ||
| Male | 11 | 6 |
| Female | 2 | 7 |
| Hypertension | 12 | 9 |
| Dyslipidemia | 13 | 11 |
| Diabetes | ||
| Type I | 0 | 0 |
| Type II | 3 | 1 |
| Insulin-dependent | 0 | 0 |
| Smoking | ||
| Active | 2 | 1 |
| Remote | 7 | 6 |
| Atrial fibrillation | ||
| Paroxysmal | 0 | 0 |
| Permanent | 1 | 4 |
| Severe LV dysfunction | 2 | 0 |
| Chronic kidney disease | ||
| eGFR <45 mL/min | 2 | 2 |
| Dialysis dependent | 0 | 0 |
| Chronic obstructive lung disease | 4 | 1 |
| Peripheral arterial disease | 0 | 2 |
| Cerebrovascular disease | 1 | 0 |
| Rheumatoid disease | 0 | 0 |
| Autoimmune disease | 0 | 0 |
| Immunosuppressed | 0 | 0 |
| Active infective endocarditis | 0 | 0 |
| Preoperative medication use | ||
| Steroids | 0 | 0 |
| Aspirin | 13 | 9 |
| NSAIDs | 0 | 0 |
| Anti-inflammatory | 0 | 0 |
| Antibiotics | 0 | 0 |
Values are mean ± SD or n.
Abbreviations as in Table 1.
Discussion
The pericardial space is a rich reservoir of biologically active factors,3 and, given its proximity to the heart, it can have an important diagnostic and therapeutic role. Moreover, inflammation and immune-mediated processes continue to be implicated in the pathogenesis of various cardiovascular diseases, such as atherosclerosis, CAD, and valvular heart diseases.31, 32, 33, 34 However, little attention has been given to this space and its content, because clinicians and scientists have focused mainly on circulating markers to diagnose cardiovascular diseases. In the present study, we comprehensively characterize the soluble mediator profile of the acellular component, or supernatant sup, of PF in patients undergoing CABG and valve surgery. In doing so, we quantify precisely the concentration and anticipated ranges of cytokines, chemokines, growth factors, and MMPs.
Although more extensive research is needed to elucidate the exact mechanisms, these mediators can have important clinical implications, such as potentially contributing to postoperative atrial fibrillation4 and ischemia/reperfusion injury.35 For example, IL-23, one of the cytokines we found in PFsup, is proinflammatory and elevated in myocardial tissue exposed to ischemia/reperfusion injury.35 Thrombopoietin, another immune mediator we found in PFsup, has been shown to enhance platelet activation and monocyte-platelet interaction in patients with unstable angina.36 Furthermore, immune markers and inflammatory pathways are believed to drive the formation of postsurgical pericardial adhesions, which also have significant clinical implications, especially for patients undergoing repeat heart surgery.37
TGF, and TGF-β1 in particular, has been shown to play a critical role in fibrosis.38 Importantly, TGF-β1 is associated with collagen secretion and activation in myocardial fibroblasts, which play a pivotal role in developing cardiac fibrosis and heart failure.39, 40, 41, 42 Our study shows that PFsup can influence cardiac fibroblast activity in vitro through the TGF-β pathway. Whether this specific pathway can contribute to clinical sequalae remains to be further explored. If proven to be essential in driving pathologies, such as CAD, or postoperative complications, including postoperative atrial fibrillation and postsurgical pericardial adhesions, we may be able to target the TGF-β pathway locally and precisely to improve outcomes while minimizing the risks of systemic side effects. Ongoing work in our laboratory is focused on creating a large animal model of postsurgical pericardial adhesions so we can evaluate whether blocking TGF-β using receptor inhibitors or monoclonal antibodies can decrease the number and severity of adhesions.
BMPs are a group of evolutionarily conserved secretory proteins that play an important role in growth and development.43 Except for BMP-1, all BMPs belong to the TGF-β superfamily, and are associated strongly with different cardiovascular diseases, including atherosclerosis.44 Our study shows that PFsup can drive fibrotic activity in vitro through BMP-4 and BMP. Our findings can have important clinical implications. Previous studies have determined that the level of BMP-4 was increased in the aortic wall in an atherosclerosis mouse model45; it could accelerate foam cell formation and atherosclerosis through SMAD ,46 and it can induce endothelial inflammation and endothelial dysfunction in vivo and in vitro.47,48 Evidence also supports the effects of BMP-4 in mediating cardiac hypertrophy, apoptosis, and fibrosis in experimentally pathological cardiac hypertrophy.49 Although more research is warranted, our work taken together with these studies, suggests that BMP-4 plays an atherogenic and proinflammatory role in early atherosclerosis. It will be important to determine whether blocking BMP and inhibiting BMP-4 in the pericardial space can translate into a decrease in the progression of atherosclerosis or the development of heart failure.
Collectively, our findings in this study underscore several important concepts, which may have clinical implications. Human PF is composed of mediators involved in inflammation and tissue remodeling. These profiles and levels differ considerably from those observed in the blood, highlighting the PT has its own unique environment. Moreover, for the first time, we show that tissues and cells present in the pericardial space can contribute to these local factors in human PF. Importantly, we uncover that human PFsup can potentiate cardiac fibrotic activity in vitro. Given that TGF-β can drive fibrotic processes, our findings may have clinical applications where the administration of inhibitors into the pericardial space could potentially attenuate cardiac fibrosis and its adverse sequalae. Finally, and perhaps most significantly, for the first time we found that CAD can affect the kinetics of PFsup-mediated fibrotic activity in vitro. Although more investigations are warranted, this finding emphasizes the notion that CAD can alter the local environment; in this study, such an alteration manifests in a more expeditious fibrotic capacity in vitro. It will be essential to determine whether any of the secreted factors can translocate to the heart to mitigate adverse injury-induced cardiac remodeling.
Clinical Implications
Many studies have reported on how cardiovascular diseases affect systemic inflammatory mediators. To date, little attention has been given to the local immune microenvironment surrounding the heart. In the present study, we comprehensively characterize the inflammatory profile of human PF. Of clinical relevance, we show that human PF contains mediators that can drive profibrotic processes in vitro via the TGF-β pathway. Finally, we show that the PF of patients with CAD has an increased profibrotic capacity compared with PF collected from patients without CAD. Although more mechanistic and translational work is needed, the clinical implications of this study are that: 1) CAD can affect the inflammatory mediator profile of PF; 2) the acellular component of PF can drive profibrotic processes; and 3) these fibrotic processes are mediated via the TGF-β pathway.
Study Limitations
Although our study provides novel insight into the mediator profiles of human PF and implicates some of its contents in driving fibrotic activity in vitro, it does have a few limitations. First, given that this was a pilot study, we could not deduce whether any specific immune mediators present in PFsup can be used as diagnostic or prognostic biomarkers for cardiovascular disease. Second, in this study, we use a rat tail collagen macrogel model as a surrogate for fibrosis. Ongoing work in our group aims at evaluating whether our observations are recapitulated by other in vitro assays and, more clinically relevant, by imaging modalities powered to assessing cardiac fibrosis, such as cardiac magnetic resonance. Third, although we diligently elucidate the mechanism by which 2 PFsup-mediated pathways drive fibrotic activity in vitro, we are presently unable to comment on whether manipulating these pathways has any effects in vivo or clinically. Finally, although not the objective of this study, because patients were not followed long term, we are not able to comment on whether the inflammatory profile of native PF can be used to predict postsurgical outcomes in patients undergoing cardiac surgery.
Conclusions
In addition to providing a homeostatic niche, given its proximity to the heart, PF can be an important indicator of cardiac function and disease. Although initially considered static, mounting evidence suggests that PF and the pericardial space are dynamic and undergo changes in response to myocardial injury and surgical interventions. Herein, for the first time, we comprehensively characterize the inflammatory profile of human PF. We also show that PF can drive cardiac fibroblast activity in vitro through the TGF-β pathway. Ongoing work is focused on determining whether specific pericardial inflammatory markers contribute to cardiovascular pathologies, including atrial fibrillation and cardiomyopathies.
Perspectives.
COMPETENCEY IN MEDICAL KNOWLEDGE: This study provides insight into the inflammatory profile of the human PF, offers clues on the sources of these local markers, shows that different diseases can affect the composition of the mediator profile, and suggests that human PF can drive profibrotic processes, where the TGF-β pathway is implicated.
TRANSLATIONAL OUTLOOK: The next steps to the present work are to: 1) identify which specific pericardial mediator can drive profibrotic processes in vitro; 2) determine whether that mediator can impact fibrosis in vivo using a high fidelity large animal model that can accurately evaluate cardiac fibrosis; 3) aim to use agents that can target the implicated mediator in the pericardial space and investigate whether cardiac fibrosis can be attenuated; and 4) once the safety and efficacy of local, targeted therapy has been shown in preclinical studies, develop a pilot clinical trial that can assess the clinical usefulness and benefit of the identified agent.
Funding Support and Author Disclosures
Dr Fatehi Hassanabad was supported by a Canadian Institutes of Health Research–Vanier Canada Graduate Scholarship, a Killam Foundation Doctoral Award, an Alberta Innovates: Health Solutions Doctoral Scholarship, and a Kertland Family Doctoral Award. A Canadian Institutes of Health Research Grant supports Drs Dundas and Fedak. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the cardiac surgeons at the Libin Cardiovascular Institute. They also thank the operating room staff at the Libin Cardiovascular Institute and the staff in the preoperative unit at the Foothills Medical Center.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures, please see the online version of this paper.
Appendix
References
- 1.Stewart R.H., Rohn D.A., Allen S.J., Laine G.A. Basic determinants of epicardial transudation. Am J Physiol. 1997;273:H1408–H1414. doi: 10.1152/ajpheart.1997.273.3.H1408. [DOI] [PubMed] [Google Scholar]
- 2.Trindade F., Vitorino R., Leite-Moreira A., Falcão-Pires I. Pericardial fluid: an underrated molecular library of heart conditions and a potential vehicle for cardiac therapy. Basic Res Cardiol. 2019;114:10. doi: 10.1007/s00395-019-0716-3. [DOI] [PubMed] [Google Scholar]
- 3.Fatehi Hassanabad A., Zarzycki A., Deniset J.F., Fedak P.W. An overview of human pericardial space and pericardial fluid. Cardiovasc Pathol. 2021;53 doi: 10.1016/j.carpath.2021.107346. [DOI] [PubMed] [Google Scholar]
- 4.Hassanabad A.F., Deniset J.F., Fedak P.W.M. Pericardial inflammatory mediators that can drive postoperative atrial fibrillation in cardiac surgery patients. Can J Cardiol. 2023;39:1090–1102. doi: 10.1016/j.cjca.2023.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Yuan Z., Boulanger B., Flessner M., Johnston M. Relationship between pericardial pressure and lymphatic pericardial fluid transport in sheep. Microvasc Res. 2000;60:28–36. doi: 10.1006/mvre.2000.2239. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan A., Dennis A.S.C., Rathod K., et al. Pericardial fluid analysis in diagnosis and prognosis of patients who underwent pericardiocentesis. Am J Cardiol. 2023;198:79–87. doi: 10.1016/j.amjcard.2023.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Jain C.C., Reddy Y.N.V. Pericardial effusions: perspective of the acute cardiac care physician. Eur Heart J Acute Cardiovasc Care. 2023;12:467–474. doi: 10.1093/ehjacc/zuad050. [DOI] [PubMed] [Google Scholar]
- 8.Ismail T.F. Acute pericarditis: update on diagnosis and management. Clin Med (Lond) 2020;20:48–51. doi: 10.7861/clinmed.cme.20.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deniset J.F., Belke D., Lee W.Y., et al. Gata6(+) pericardial cavity macrophages relocate to the injured heart and prevent cardiac fibrosis. Immunity. 2019;51:131–140.e5. doi: 10.1016/j.immuni.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin H., Liu K., Huang X., et al. Genetic lineage tracing of pericardial cavity macrophages in the injured heart. Circ Res. 2022;130:1682–1697. doi: 10.1161/CIRCRESAHA.122.320567. [DOI] [PubMed] [Google Scholar]
- 11.Perezgrovas-Olaria R., Chadow D., Lau C., et al. Characteristics of postoperative atrial fibrillation and the effect of posterior pericardiotomy. Ann Thorac Surg. 2022;116:615–622. doi: 10.1016/j.athoracsur.2022.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudino M., Di Franco A., Rong L.Q., et al. Pericardial Effusion provoking atrial fibrillation after cardiac surgery: JACC review topic of the week. J Am Coll Cardiol. 2022;79:2529–2539. doi: 10.1016/j.jacc.2022.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Lau C., Soletti G.J., Olaria R.P., Myers P., Girardi L.N., Gaudino M. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery. Multimed Man Cardiothorac Surg. 2021;2021 doi: 10.1510/mmcts.2021.083. [DOI] [PubMed] [Google Scholar]
- 14.Gaudino M., Sanna T., Ballman K.V., et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: an adaptive, single-centre, single-blind, randomised, controlled trial. Lancet. 2021;398:2075–2083. doi: 10.1016/S0140-6736(21)02490-9. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y., Du Z., Fang M., et al. Metabolic signatures in pericardial fluid and serum are associated with new-onset atrial fibrillation after isolated coronary artery bypass grafting. Transl Res. 2023;256:30–40. doi: 10.1016/j.trsl.2023.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y., Yang Y., Yang X., Hua K. Myeloperoxidase levels in pericardial fluid is independently associated with postoperative atrial fibrillation after isolated coronary artery bypass surgery. J Clin Med. 2022;11:7018. doi: 10.3390/jcm11237018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fatehi Hassanabad A., Fedak P.W.M., Deniset J.F. Acute ischemia alters human pericardial fluid immune cell composition. JACC Basic Transl Sci. 2021;6:765–767. doi: 10.1016/j.jacbts.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fatehi Hassanabad A., Schoettler F.I., Kent W.D.T., et al. Comprehensive characterization of the postoperative pericardial inflammatory response: Potential implications for clinical outcomes. JTCVS Open. 2022;12:118–136. doi: 10.1016/j.xjon.2022.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butts B., Goeddel L.A., George D.J., et al. Increased inflammation in pericardial fluid persists 48 hours after cardiac surgery. Circulation. 2017;136:2284–2286. doi: 10.1161/CIRCULATIONAHA.117.029589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fatehi Hassanabad A., Schoettler F.I., Kent W.D.T., et al. Cardiac surgery elicits pericardial inflammatory responses that are distinct compared with postcardiopulmonary bypass systemic inflammation. JTCVS Open. 2023;16:389–400. doi: 10.1016/j.xjon.2023.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goeddel L.A., Zaky A., Aban I., et al. Feasibility study of intraoperative pericardial fluid biomarkers and length of stay after cardiac surgery. JTCVS Tech. 2023;19:86–92. doi: 10.1016/j.xjtc.2023.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bray N.L., Pimentel H., Melsted P., Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 23.Pimentel H., Bray N.L., Puente S., Melsted P., Pachter L. Differential analysis of RNA-seq incorporating quantification uncertainty. Nat Methods. 2017;14:687–690. doi: 10.1038/nmeth.4324. [DOI] [PubMed] [Google Scholar]
- 24.Teng G., Svystonyuk D., Mewhort H.E., et al. Tetrandrine reverses human cardiac myofibroblast activation and myocardial fibrosis. Am J Physiol Heart Circ Physiol. 2015;308:H1564–H1574. doi: 10.1152/ajpheart.00126.2015. [DOI] [PubMed] [Google Scholar]
- 25.Svystonyuk D.A., Ngu J.M., Mewhort H.E., et al. Fibroblast growth factor-2 regulates human cardiac myofibroblast-mediated extracellular matrix remodeling. J Transl Med. 2015;13:147. doi: 10.1186/s12967-015-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mewhort H.E., Lipon B.D., Svystonyuk D.A., et al. Monocytes increase human cardiac myofibroblast-mediated extracellular matrix remodeling through TGF-β1. Am J Physiol Heart Circ Physiol. 2016;310:H716–H724. doi: 10.1152/ajpheart.00309.2015. [DOI] [PubMed] [Google Scholar]
- 27.Lijnen P., Petrov V., Rumilla K., Fagard R. Transforming growth factor-beta 1 promotes contraction of collagen gel by cardiac fibroblasts through their differentiation into myofibroblasts. Methods Find Exp Clin Pharmacol. 2003;25:79–86. doi: 10.1358/mf.2003.25.2.723680. [DOI] [PubMed] [Google Scholar]
- 28.Kang S., Verma S., Hassanabad A.F., et al. Direct effects of empagliflozin on extracellular matrix remodelling in human cardiac myofibroblasts: novel translational clues to explain EMPA-REG OUTCOME Results. Can J Cardiol. 2020;36:543–553. doi: 10.1016/j.cjca.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 29.Mewhort H.E.M., Svystonyuk D.A., Turnbull J.D., et al. Bioactive extracellular matrix scaffold promotes adaptive cardiac remodeling and repair. JACC Basic Transl Sci. 2017;2:450–464. doi: 10.1016/j.jacbts.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akhmetshina A., Palumbo K., Dees C., et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henein M.Y., Vancheri S., Longo G., Vancheri F. The role of inflammation in cardiovascular disease. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232112906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberale L., Badimon L., Montecucco F., et al. Inflammation, aging, and cardiovascular disease. J Am Coll Cardiol. 2022;79:837–847. doi: 10.1016/j.jacc.2021.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liberale L., Montecucco F., Schwarz L., Lüscher T.F., Camici G.G. Inflammation and cardiovascular diseases: lessons from seminal clinical trials. Cardiovasc Res. 2021;117:411–422. doi: 10.1093/cvr/cvaa211. [DOI] [PubMed] [Google Scholar]
- 34.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 35.Liao Y., Hu X., Guo X., Zhang B., Xu W., Jiang H. Promoting effects of IL-23 on myocardial ischemia and reperfusion are associated with increased expression of IL-17A and upregulation of the JAK2-STAT3 pathway. Mol Med Rep. 2017;16:9309–9316. doi: 10.3892/mmr.2017.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lupia E., Goffi A., Bosco O., Montrucchio G. Thrombopoietin as biomarker and mediator of cardiovascular damage in critical diseases. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/390892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fatehi Hassanabad A., Zarzycki A.N., Jeon K., Deniset J.F., Fedak P.W.M. Post-operative adhesions: a comprehensive review of mechanisms. Biomedicines. 2021;9:867. doi: 10.3390/biomedicines9080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng X-m, Nikolic-Paterson D.J., Lan H.Y. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 39.Ko T., Nomura S., Yamada S., et al. Cardiac fibroblasts regulate the development of heart failure via Htra3-TGF-β-IGFBP7 axis. Nature Commun. 2022;13:3275. doi: 10.1038/s41467-022-30630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yousefi F., Shabaninejad Z., Vakili S., et al. TGF-β and WNT pathways in cardiac fibrosis: non-coding RNAs come into focus. Cell Commun Signal. 2020;18:87. doi: 10.1186/s12964-020-00555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saadat S., Noureddini M., Mahjoubin-Tehran M., et al. Pivotal role of TGF-β/Smad in cardiac fibrosis: non-coding RNAs as effectual players. Front Cardiovasc Med. 2020;7 doi: 10.3389/fcvm.2020.588347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khalil H., Kanisicak O., Prasad V., et al. Fibroblast-specific TGF-β-Smad2/3 underlies cardiac fibrosis. J Clin Invest. 2017;127:3770–3783. doi: 10.1172/JCI94753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urist M.R. Bone: formation by autoinduction. Science (New York, NY) 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 44.Ye D., Liu Y., Pan H., et al. Insights into bone morphogenetic proteins in cardiovascular diseases. Front Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1125642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao Y., Watson A.D., Ji S., Boström K.I. Heat shock protein 70 enhances vascular bone morphogenetic protein-4 by binding matrix Gla protein. Circ Res. 2009;105:575–584. doi: 10.1161/CIRCRESAHA.109.202333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng J., Gao J., Li Y., et al. BMP4 enhances foam cell formation by BMPR-2/Smad1/5/8. Int J Mol Sci. 2014;15:5536–5552. doi: 10.3390/ijms15045536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Souilhol C., Gauci I., Feng S., et al. Homeobox B9 integrates bone morphogenic protein 4 with inflammation at atheroprone sites. Cardiovasc Res. 2020;116:1300–1310. doi: 10.1093/cvr/cvz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S., Lee E.S., Lee S.W., et al. Site-specific impairment of perivascular adipose tissue on advanced atherosclerotic plaques using multimodal nonlinear optical imaging. Proc Natl Acad Sci U S A. 2019;116:17765–17774. doi: 10.1073/pnas.1902007116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun B., Huo R., Sheng Y., et al. Bone morphogenetic protein-4 mediates cardiac hypertrophy, apoptosis, and fibrosis in experimentally pathological cardiac hypertrophy. Hypertension. 2013;61:352–360. doi: 10.1161/HYPERTENSIONAHA.111.00562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.