Abstract
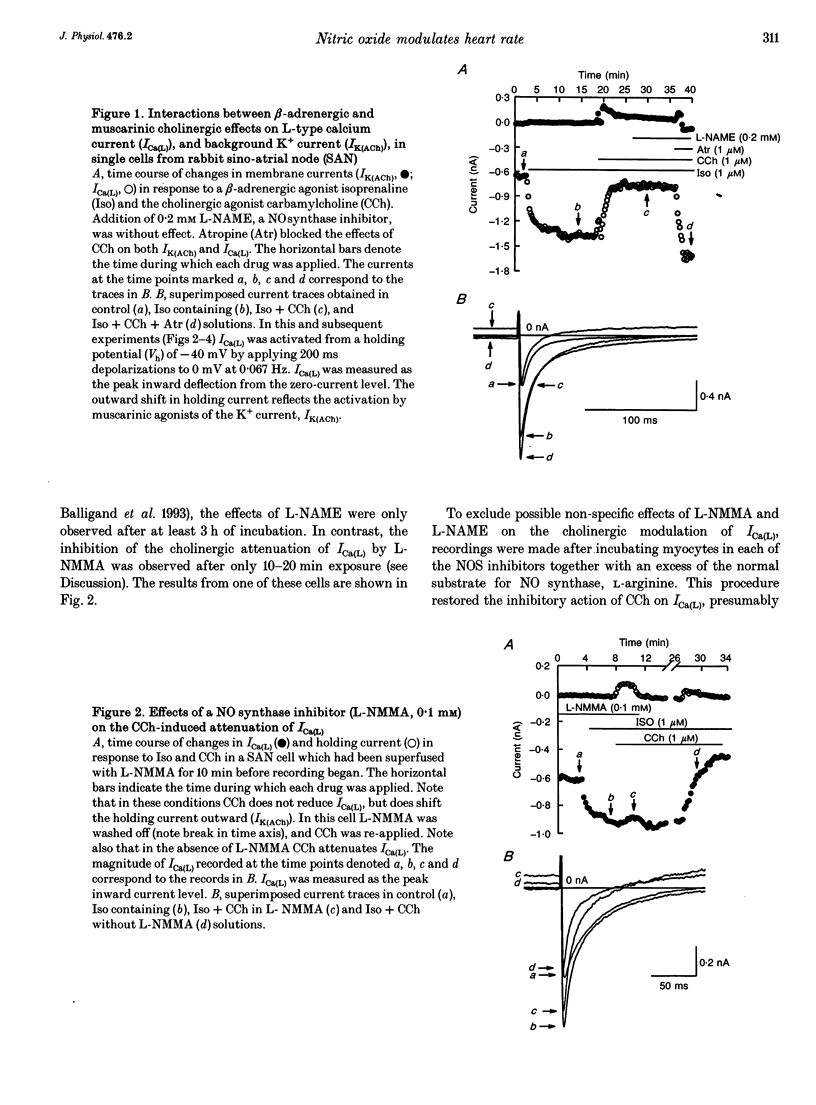
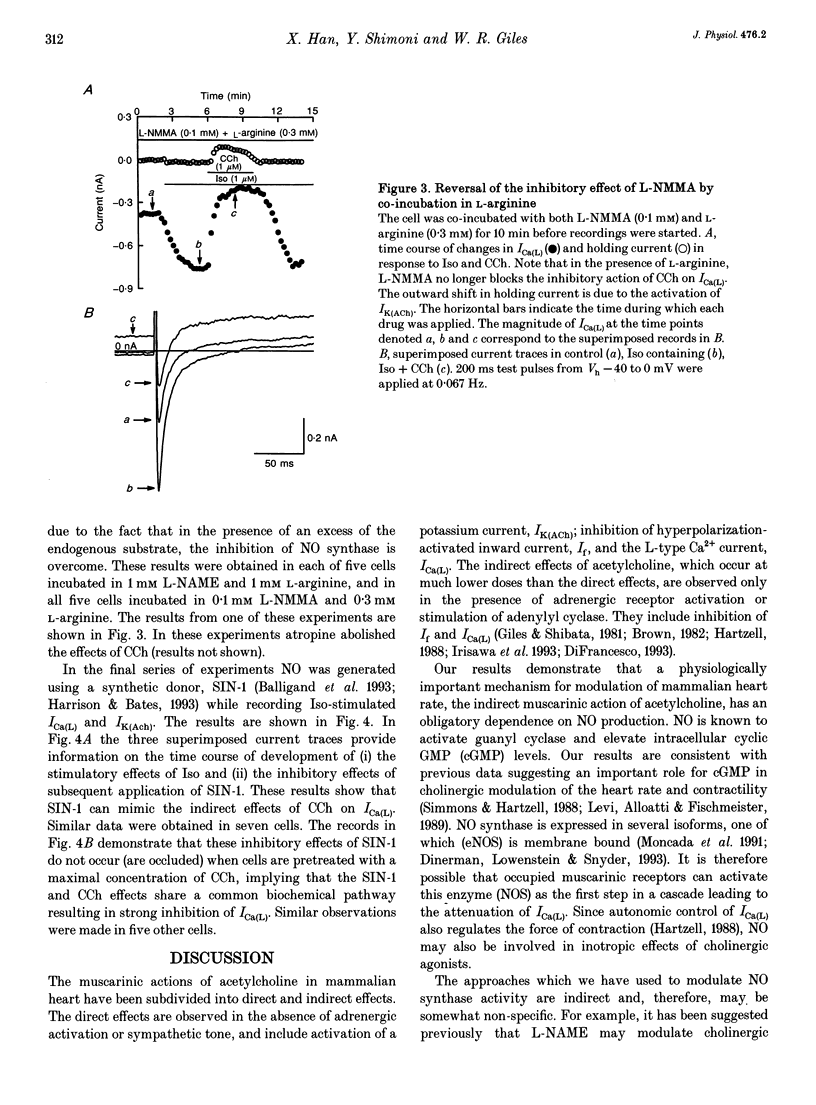
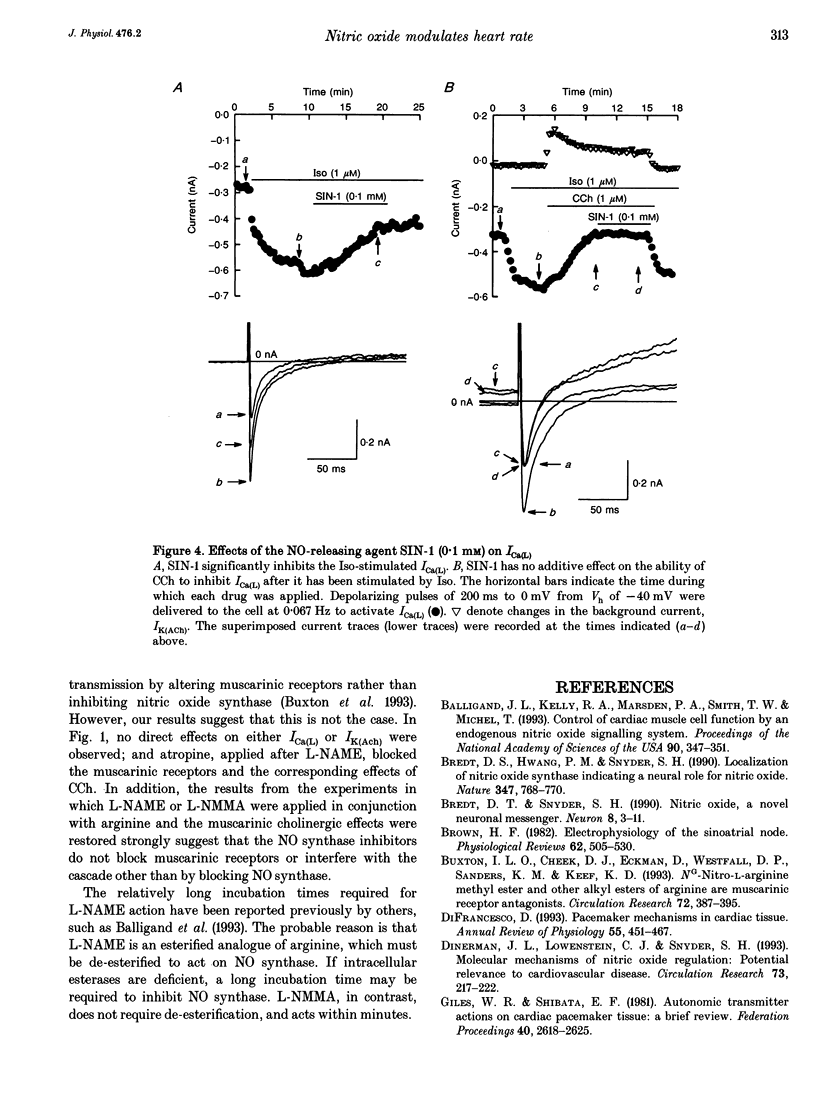
Cholinergic modulation of heart rate in isolated spontaneously beating single cells from the rabbit sino-atrial node was investigated by measuring transmembrane ionic currents using the nystatin-perforated patch whole-cell voltage-clamp technique. Carbamylcholine (CCh), a stable analogue of acetylcholine (ACh), significantly inhibited L-type calcium currents (Ica(L) which had been augmented by beta-adrenergic stimulation. In addition, CCh activated a potassium outward current (IK(ACh)). Both effects were blocked by atropine. The possible involvement of nitric oxide (NO) in these responses was evaluated by inhibiting NO synthesis. In the presence of NG-monomethyl-L-arginine (L-NMMA, 100 microM) or nitro-L-arginine methyl ester (L-NAME, 1 mM), two specific inhibitors of nitric oxide synthase (NOS), CCh no longer inhibited ICa(L). IK(ACh) could still be activated. Co-incubation of cells in L-NAME or in L-NMMA with arginine (the endogenous substrate of NOS) restored the CCh-induced attenuation of ICa(L), indicating that L-NAME or L-NMMA did not interfere directly with the muscarinic action of CCh on ICa(L). Effects of the NO-releasing agent molsidomine (SIN-1) on CCh-induced changes in ICa(L) were also investigated. After ICa(L) had been augmented by beta-adrenergic stimulation, SIN-1 (0.1 mM) inhibited ICa(L); however, SIN-1 had no further inhibitory effect after a maximal CCh concentration had been applied. These findings suggest that NO generation is an obligatory process in cholinergic inhibition of ICa(L) in mammalian cardiac pacemaker tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balligand J. L., Kelly R. A., Marsden P. A., Smith T. W., Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide, a novel neuronal messenger. Neuron. 1992 Jan;8(1):3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Brown H. F. Electrophysiology of the sinoatrial node. Physiol Rev. 1982 Apr;62(2):505–530. doi: 10.1152/physrev.1982.62.2.505. [DOI] [PubMed] [Google Scholar]
- Buxton I. L., Cheek D. J., Eckman D., Westfall D. P., Sanders K. M., Keef K. D. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993 Feb;72(2):387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- Dinerman J. L., Lowenstein C. J., Snyder S. H. Molecular mechanisms of nitric oxide regulation. Potential relevance to cardiovascular disease. Circ Res. 1993 Aug;73(2):217–222. doi: 10.1161/01.res.73.2.217. [DOI] [PubMed] [Google Scholar]
- Giles W., Shibata E. Autonomic transmitter actions on cardiac pacemaker tissue: a brief review. Fed Proc. 1981 Sep;40(11):2618–2624. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison D. G., Bates J. N. The nitrovasodilators. New ideas about old drugs. Circulation. 1993 May;87(5):1461–1467. doi: 10.1161/01.cir.87.5.1461. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H., Brown H. F., Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993 Jan;73(1):197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Klimaschewski L., Kummer W., Mayer B., Couraud J. Y., Preissler U., Philippin B., Heym C. Nitric oxide synthase in cardiac nerve fibers and neurons of rat and guinea pig heart. Circ Res. 1992 Dec;71(6):1533–1537. doi: 10.1161/01.res.71.6.1533. [DOI] [PubMed] [Google Scholar]
- Levi R. C., Alloatti G., Fischmeister R. Cyclic GMP regulates the Ca-channel current in guinea pig ventricular myocytes. Pflugers Arch. 1989 Apr;413(6):685–687. doi: 10.1007/BF00581823. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Méry P. F., Pavoine C., Belhassen L., Pecker F., Fischmeister R. Nitric oxide regulates cardiac Ca2+ current. Involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl cyclase activation. J Biol Chem. 1993 Dec 15;268(35):26286–26295. [PubMed] [Google Scholar]
- Petit-Jacques J., Bois P., Bescond J., Lenfant J. Mechanism of muscarinic control of the high-threshold calcium current in rabbit sino-atrial node myocytes. Pflugers Arch. 1993 Apr;423(1-2):21–27. doi: 10.1007/BF00374956. [DOI] [PubMed] [Google Scholar]
- Simmons M. A., Hartzell H. C. Role of phosphodiesterase in regulation of calcium current in isolated cardiac myocytes. Mol Pharmacol. 1988 Jun;33(6):664–671. [PubMed] [Google Scholar]
- van Ginneken A. C., Giles W. Voltage clamp measurements of the hyperpolarization-activated inward current I(f) in single cells from rabbit sino-atrial node. J Physiol. 1991 Mar;434:57–83. doi: 10.1113/jphysiol.1991.sp018459. [DOI] [PMC free article] [PubMed] [Google Scholar]


