Abstract
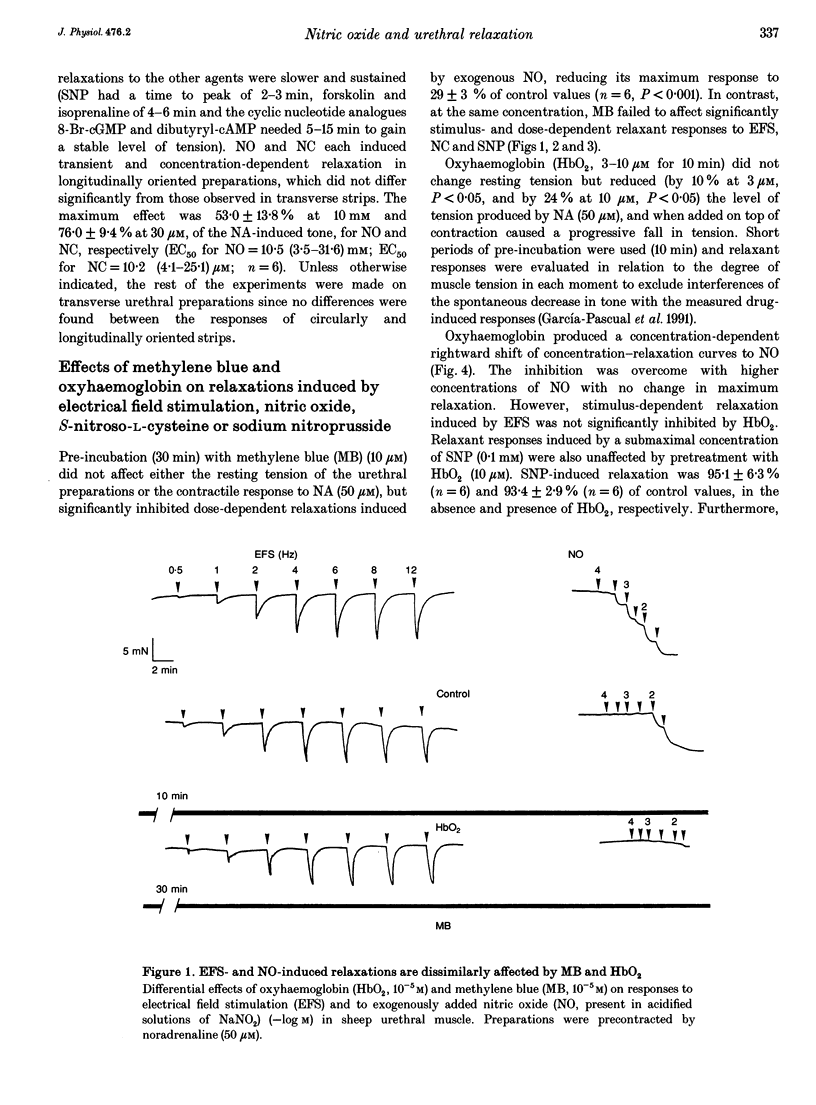
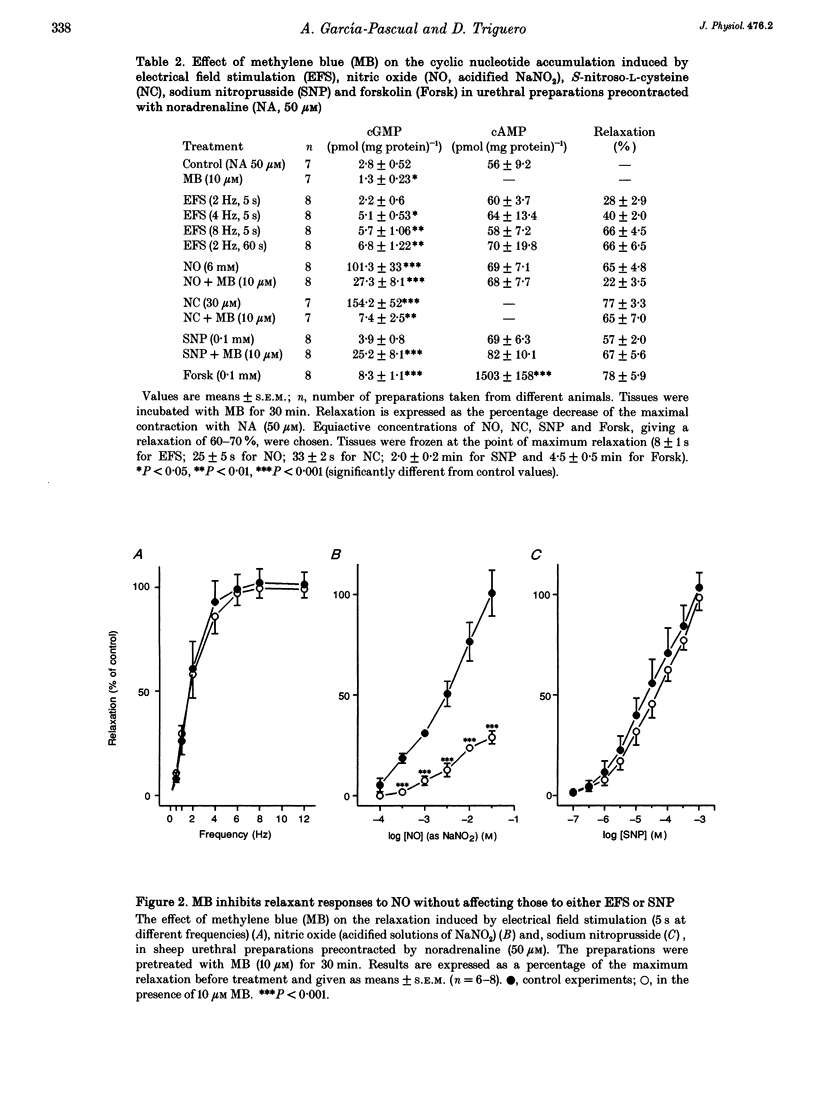
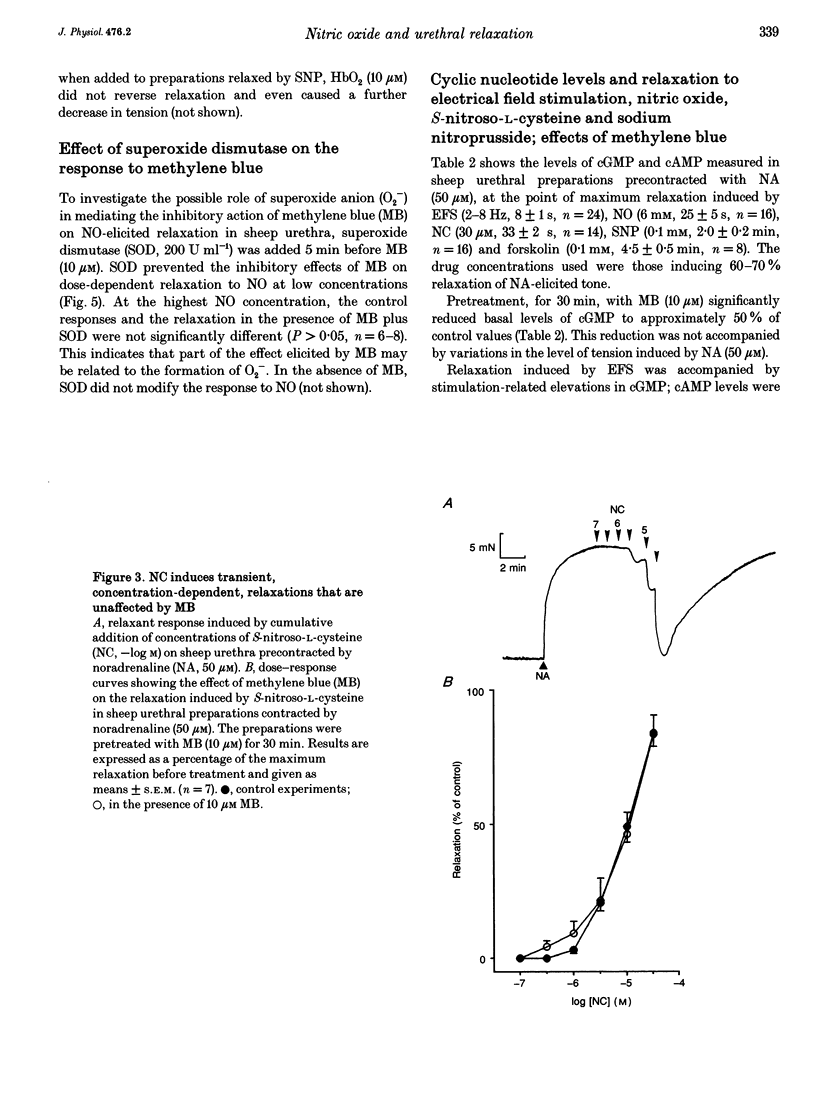
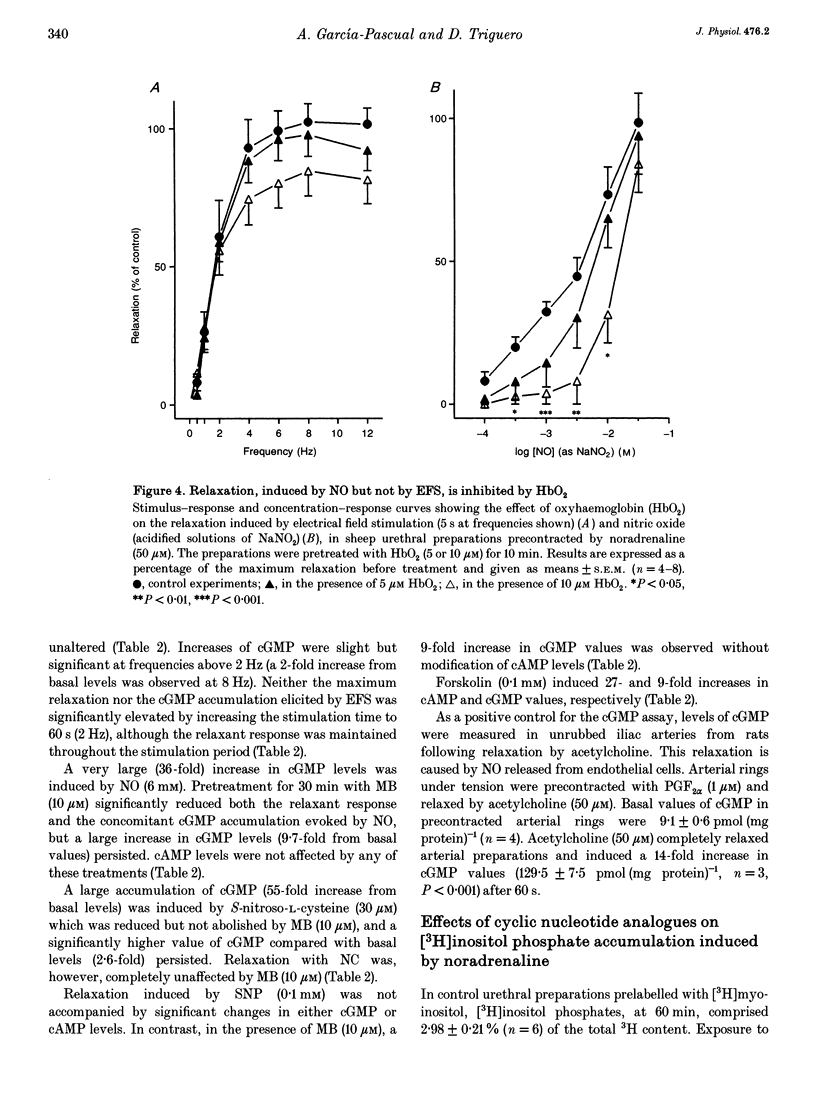
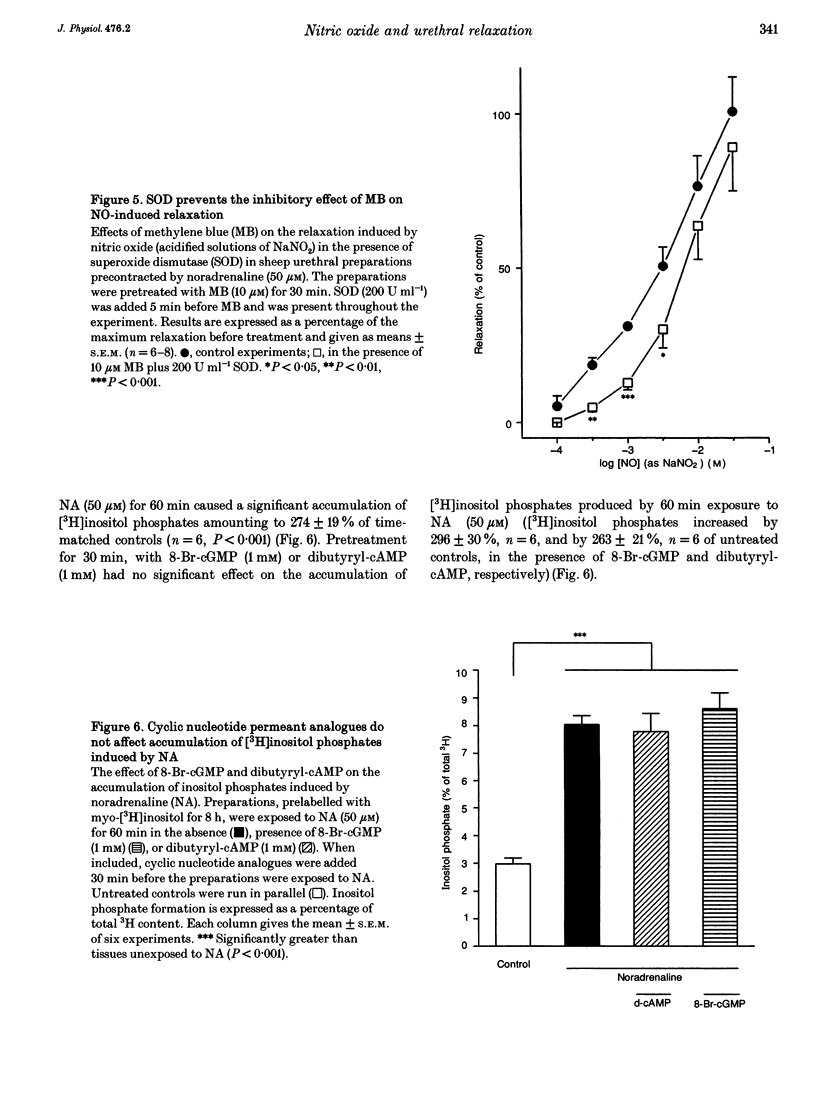
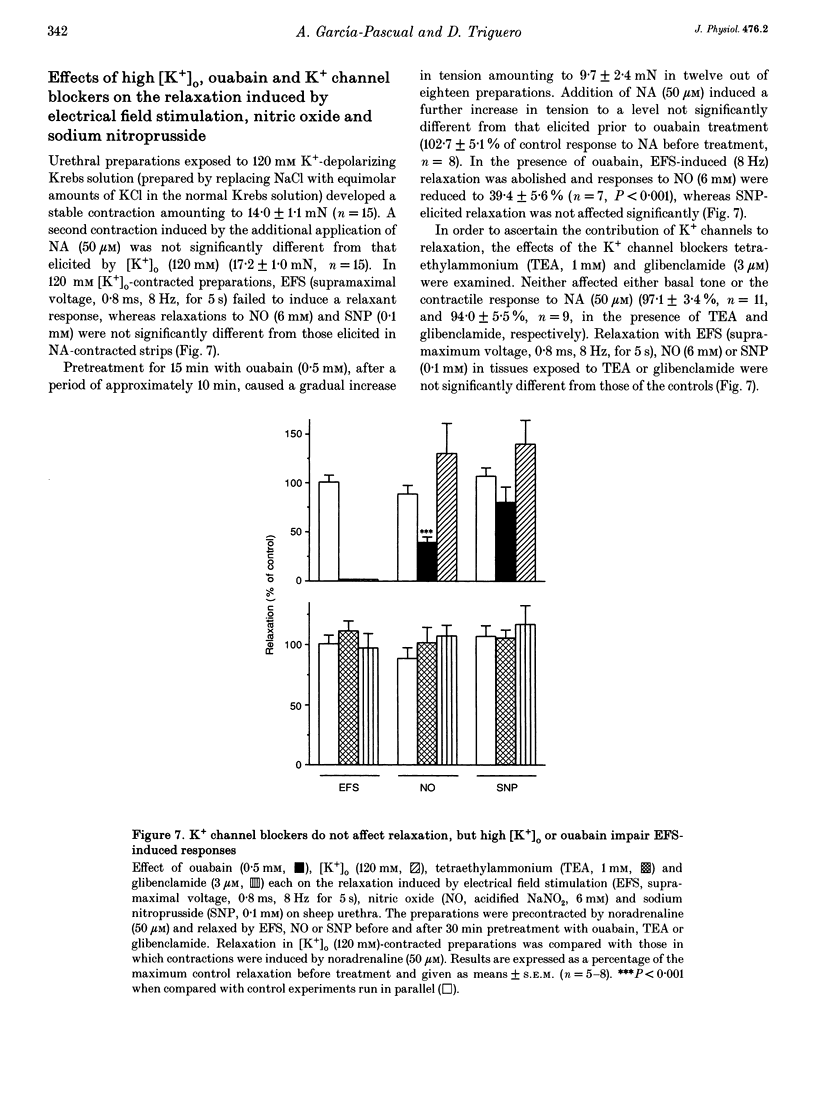
Isolated transverse and longitudinally oriented preparations of sheep urethra precontracted with noradrenaline responded to electrical field stimulation (EFS) with stimulus-dependent non-adrenergic, non-cholinergic (NANC) relaxations. Exogenous nitric oxide (NO) (acidified NaNO2), S-nitroso-L-cysteine (NC), sodium nitroprusside (SNP), 8-Br-cGMP, dibutyryl-cAMP, forskolin and isoprenaline each relaxed precontracted transverse urethral preparations in a concentration-dependent manner in order of protency: NC > forskolin > isoprenaline = SNP > NO > 8-Br-cGMP = dibutyryl-cAMP. Longitudinally oriented preparations responded to NO and NC with concentration-dependent relaxation, no different from that observed in transverse strips. Methylene blue (MB) and oxyhaemoglobin (HbO2) each shifted the concentration-response curve for NO to the right without affecting EFS-induced relaxation. Similarly, concentration-dependent responses to NC were not affected by MB. The inhibition of relaxation to NO by MB was prevented by superoxide dismutase, suggesting the inhibition was caused by extracellular generation of superoxide anions. EFS-induced relaxation was accompanied by elevation of cGMP. However, for the same level of relaxation, exogenous NO and NC induced 15- and 23-times higher increases in cGMP values, respectively, than EFS. cAMP levels were not affected by EFS- or NO-induced relaxation, although a large increase accompanied relaxation induced by forskolin. Forskolin also increased cGMP content. Pretreatment with MB reduced basal levels of cGMP and inhibited both relaxation and rise in cGMP levels induced by NO. SNP-elicited relaxant responses, in the presence of MB, were accompanied by an accumulation of cGMP; cAMP levels were unaffected. MB reduced cGMP levels induced by NC, while the relaxant response was unchanged. In urethral preparations prelabelled with [3H]myoinositol, exposure to NA caused an accumulation of [3H]inositol phosphates, which was unaffected by pretreatment with 8-Br-cGMP or dibutyryl-cAMP. EFS failed to induce a relaxant response in excess [K+]o-contracted preparations, while relaxation with exogenous NO was unaffected. Ouabain abolished EFS-induced relaxation and reduced responses to NO. Neither TEA nor glibenclamide affected relaxation to either EFS or NO. Relaxation elicited by SNP was not accompanied by any change in cGMP or cAMP levels, and was unaffected by MB, HbO2, K+ channel blockers (TEA and glibenclamide), ouabain or high [K+]o solution. This suggested that relaxation was caused by a mechanism independent of NO generation. A dense network of NADPH diaphorase-positive fibres associated with both the circular and longitudinal smooth muscle layers of sheep urethra was found.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlner J., Axelsson K. L. Nitrates. Mode of action at a cellular level. Drugs. 1987;33 (Suppl 4):32–38. doi: 10.2165/00003495-198700334-00007. [DOI] [PubMed] [Google Scholar]
- Andersson K. E., Garcia Pascual A., Forman A., Tøttrup A. Non-adrenergic, non-cholinergic nerve-mediated relaxation of rabbit urethra is caused by nitric oxide. Acta Physiol Scand. 1991 Jan;141(1):133–134. doi: 10.1111/j.1748-1716.1991.tb09056.x. [DOI] [PubMed] [Google Scholar]
- Andersson K. E., Garcia Pascual A., Persson K., Forman A., Tøttrup A. Electrically-induced, nerve-mediated relaxation of rabbit urethra involves nitric oxide. J Urol. 1992 Jan;147(1):253–259. doi: 10.1016/s0022-5347(17)37208-7. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokita S., Morgan W. R., Wheeler M. A., Yoshida M., Latifpour J., Weiss R. M. NG-nitro-L-arginine inhibits non-adrenergic, non-cholinergic relaxation in rabbit urethral smooth muscle. Life Sci. 1991;48(25):2429–2436. doi: 10.1016/0024-3205(91)90377-n. [DOI] [PubMed] [Google Scholar]
- Du C., Murray J., Bates J. N., Conklin J. L. Nitric oxide: mediator of NANC hyperpolarization of opossum esophageal smooth muscle. Am J Physiol. 1991 Dec;261(6 Pt 1):G1012–G1016. doi: 10.1152/ajpgi.1991.261.6.G1012. [DOI] [PubMed] [Google Scholar]
- Emilsson A., Sundler R. Differential activation of phosphatidylinositol deacylation and a pathway via diphosphoinositide in macrophages responding to zymosan and ionophore A23187. J Biol Chem. 1984 Mar 10;259(5):3111–3116. [PubMed] [Google Scholar]
- Garcia-Pascual A., Costa G., Garcia-Sacristan A., Andersson K. E. Relaxation of sheep urethral muscle induced by electrical stimulation of nerves: involvement of nitric oxide. Acta Physiol Scand. 1991 Apr;141(4):531–539. doi: 10.1111/j.1748-1716.1991.tb09114.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Pascual A., Persson K., Holmquist F., Andersson K. E. Endothelin-1-induced phosphoinositide hydrolysis and contraction in isolated rabbit detrusor and urethral smooth muscle. Gen Pharmacol. 1993 Jan;24(1):131–138. doi: 10.1016/0306-3623(93)90023-q. [DOI] [PubMed] [Google Scholar]
- Greenberg S. S., Wilcox D. E., Rubanyi G. M. Endothelium-derived relaxing factor released from canine femoral artery by acetylcholine cannot be identified as free nitric oxide by electron paramagnetic resonance spectroscopy. Circ Res. 1990 Dec;67(6):1446–1452. doi: 10.1161/01.res.67.6.1446. [DOI] [PubMed] [Google Scholar]
- Grous M., Ormsbee H., 3rd, Barnette M. Dimethylphenylpiperazinium (DMPP)-induced relaxation and elevation of cyclic GMP content in canine lower esophageal sphincter (LES). Biochem Pharmacol. 1990 Oct 15;40(8):1757–1762. doi: 10.1016/0006-2952(90)90352-l. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Zembowicz A., Salvemini D., Taylor G. W., Vane J. R. Modulation of the pharmacological actions of nitrovasodilators by methylene blue and pyocyanin. Br J Pharmacol. 1992 Aug;106(4):838–845. doi: 10.1111/j.1476-5381.1992.tb14422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuzé-Joubert I., Mennecier P., Simonet S., Laubie M., Verbeuren T. J. Effect of vasodilators, including nitric oxide, on the release of cGMP and cAMP in the isolated perfused rat kidney. Eur J Pharmacol. 1992 Sep 22;220(2-3):161–171. doi: 10.1016/0014-2999(92)90744-o. [DOI] [PubMed] [Google Scholar]
- Hu Z. W., Honda K., Murad F., Hoffman B. B. Prolonged exposure to catecholamines enhances sensitivity of smooth muscle relaxation induced by sodium nitroprusside and atriopeptin. J Pharmacol Exp Ther. 1992 Feb;260(2):756–761. [PubMed] [Google Scholar]
- Ignarro L. J., Harbison R. G., Wood K. S., Kadowitz P. J. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986 Jun;237(3):893–900. [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Ignarro L. J. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990 Nov;16(5):477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- Kanada A., Hata F., Suthamnatpong N., Maehara T., Ishii T., Takeuchi T., Yagasaki O. Key roles of nitric oxide and cyclic GMP in nonadrenergic and noncholinergic inhibition in rat ileum. Eur J Pharmacol. 1992 Jun 5;216(2):287–292. doi: 10.1016/0014-2999(92)90372-b. [DOI] [PubMed] [Google Scholar]
- Kowaluk E. A., Fung H. L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther. 1990 Dec;255(3):1256–1264. [PubMed] [Google Scholar]
- Kowaluk E. A., Seth P., Fung H. L. Metabolic activation of sodium nitroprusside to nitric oxide in vascular smooth muscle. J Pharmacol Exp Ther. 1992 Sep;262(3):916–922. [PubMed] [Google Scholar]
- Kukovetz W. R., Holzmann S. Tolerance and cross-tolerance between SIN-1 and nitric oxide in bovine coronary arteries. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S40–S46. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lincoln T. M. Cyclic GMP and mechanisms of vasodilation. Pharmacol Ther. 1989;41(3):479–502. doi: 10.1016/0163-7258(89)90127-7. [DOI] [PubMed] [Google Scholar]
- Marshall J. J., Wei E. P., Kontos H. A. Independent blockade of cerebral vasodilation from acetylcholine and nitric oxide. Am J Physiol. 1988 Oct;255(4 Pt 2):H847–H854. doi: 10.1152/ajpheart.1988.255.4.H847. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Mayer B., Brunner F., Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacol. 1993 Jan 26;45(2):367–374. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. II. The mechanism of the mediation of cytochrome c reduction by a variety of electron carriers. J Biol Chem. 1970 Mar 25;245(6):1374–1377. [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Nagao T., Vanhoutte P. M. Hyperpolarization as a mechanism for endothelium-dependent relaxations in the porcine coronary artery. J Physiol. 1992 Jan;445:355–367. doi: 10.1113/jphysiol.1992.sp018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Urakawa N. Na-Ca exchange and tension development in guinea-pig aorta. Naunyn Schmiedebergs Arch Pharmacol. 1979 Nov;309(2):171–178. doi: 10.1007/BF00501226. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Persson K., Andersson K. E. Nitric oxide and relaxation of pig lower urinary tract. Br J Pharmacol. 1992 Jun;106(2):416–422. doi: 10.1111/j.1476-5381.1992.tb14349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson K., Igawa Y., Mattiasson A., Andersson K. E. Effects of inhibition of the L-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br J Pharmacol. 1992 Sep;107(1):178–184. doi: 10.1111/j.1476-5381.1992.tb14483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M. Cyclic guanosine monophosphate inhibition of contraction may be mediated through inhibition of phosphatidylinositol hydrolysis in rat aorta. Circ Res. 1986 Mar;58(3):407–410. doi: 10.1161/01.res.58.3.407. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M. Inhibitory effects of cyclic AMP-elevating agents on norepinephrine-induced phosphatidylinositide hydrolysis and contraction in rat aorta. Gen Pharmacol. 1991;22(3):449–458. doi: 10.1016/0306-3623(91)90005-q. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Graham A. Role of nitric oxide in the autonomic innervation of smooth muscle. J Auton Pharmacol. 1992 Dec;12(6):445–456. doi: 10.1111/j.1474-8673.1992.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Tanagho E. A., Miller E. R. Initiation of voiding. Br J Urol. 1970 Apr;42(2):175–183. doi: 10.1111/j.1464-410x.1970.tb10019.x. [DOI] [PubMed] [Google Scholar]
- Thornbury K. D., Hollywood M. A., McHale N. G. Mediation by nitric oxide of neurogenic relaxation of the urinary bladder neck muscle in sheep. J Physiol. 1992;451:133–144. doi: 10.1113/jphysiol.1992.sp019157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M., Vanhoutte P. M., Miller V. M. Dissociation between endothelium-dependent relaxations and increases in cGMP in systemic veins. Am J Physiol. 1991 May;260(5 Pt 2):H1531–H1537. doi: 10.1152/ajpheart.1991.260.5.H1531. [DOI] [PubMed] [Google Scholar]
- Wolin M. S., Cherry P. D., Rodenburg J. M., Messina E. J., Kaley G. Methylene blue inhibits vasodilation of skeletal muscle arterioles to acetylcholine and nitric oxide via the extracellular generation of superoxide anion. J Pharmacol Exp Ther. 1990 Sep;254(3):872–876. [PubMed] [Google Scholar]




