Graphical abstract
Keywords: Ventricular septal defect, Postinfarction ventricular septal rupture, Multiple angles, Multiple views, Sweep
Highlights
-
•
Intraoperative TEE aided in diagnosing VSD.
-
•
TTE and TEE help in evaluating PI-VSR better.
-
•
Multiview and multiangle echocardiography is useful in detecting dual shunts.
-
•
Careful sweeps through the entire ventricular septum is also important.
Introduction
Postinfarction ventricular septal rupture (PI-VSR) often presents as cardiogenic shock with newly formed left-to-right communication. In such cases, mechanical circulatory support can provide hemodynamic stabilization during the perioperative period.1,2
Many studies have been conducted on the efficacy of percutaneous circulatory support (pCS) devices.3, 4, 5 For example, Impella (Abiomed, Danvers, MA, USA) is a novel transaortic suction device designed to unload the left ventricle (LV) and increase antegrade flow to facilitate systemic blood supply. However, the use of pCS devices may lead to right-to-left shunting, resulting in systemic desaturation.1,6,7 Therefore, clinicians must remain alert about hidden shunts.
Here, we report a case where an undiagnosed ventricular septal defect (VSD) was found during PI-VSR closure with a pCS device. This case highlights the importance of echocardiography to exclude multiple shunts.
Case Presentation
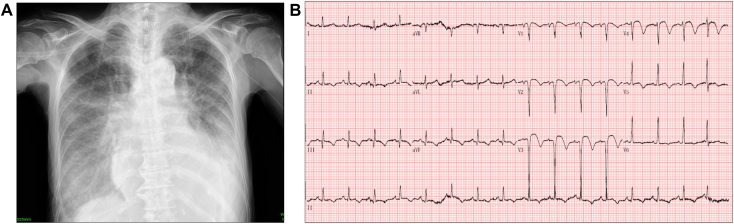
An 80-year-old woman with no significant medical history presented to a community hospital with a chief complaint of a 3-day history of dyspnea. The patient was in good health and had never been to a hospital or undergone physical examination before. Chest x-ray revealed cardiomegaly and pulmonary atelectasis along with bilateral pleural effusion and pulmonary congestion (Figure 1A). Electrocardiography revealed V1 to V4 ST-segment elevations with q-waves, consistent with a late presentation of myocardial infarction (MI; Figure 1B). Troponin and creatine kinase levels were slightly elevated. Physical examination revealed a systolic murmur best heard at the apex. Transthoracic echocardiography (TTE) revealed a PI-VSR at the apex with an aneurysm and thinning, presenting with left-to-right shunting. Left ventricular (LV) systolic function was preserved, with an LV ejection fraction of 79%. Despite normal global LV systolic function, there was abnormal regional motion of the basal and midanteroseptum, midinferoseptum, and apical septum. Doppler evaluation of the tricuspid valve indicated a maximum velocity of 3.7 m/sec and an estimated right ventricular pressure of 65 mm Hg.
Figure 1.
(A) Chest x-ray obtained at presentation, posteroanterior display, demonstrates cardiomegaly, pulmonary atelectasis, pulmonary congestion, and bilateral pleural effusion. (B) Twelve-lead electrocardiogram at presentation demonstrates sinus tachycardia with anteroseptal ST-segment elevation and associated q-waves.
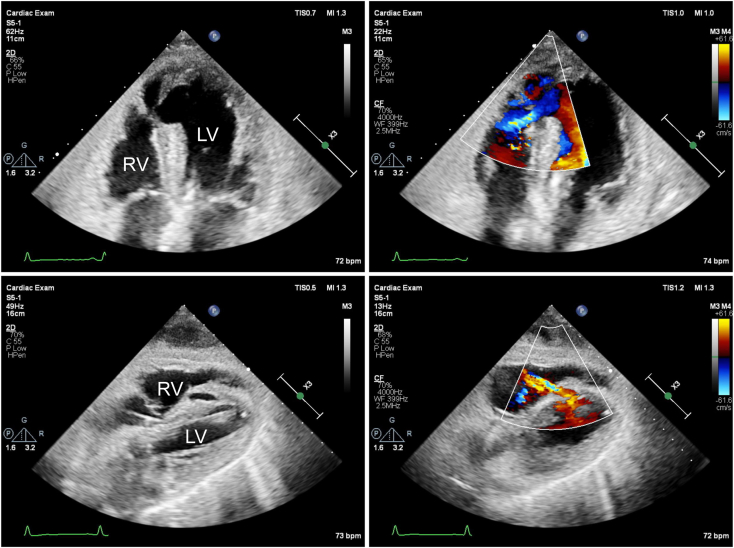
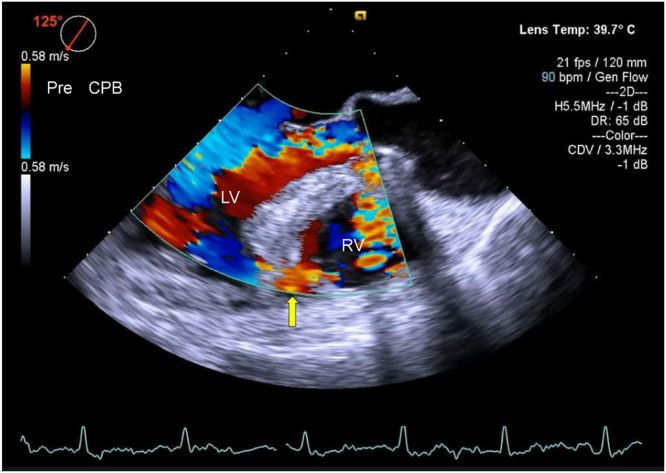
The patient was subsequently transferred to our hospital for surgery. The blood pressure was 90/58 mm Hg, and SpO2 was 95% with 5 L of mask oxygen. Coronary angiography showed 99% occlusion of the proximal left anterior descending artery, which was not treated because of subacute MI. A pCS device was inserted as a bridge to surgery in an effort to stabilize cardiogenic shock. A second TTE from the apical 4-chamber view showed a septal aneurysm protruding into the right ventricle, along with a small defect in the thin septum of the aneurysm. Color-flow Doppler evaluation from the apical view showed left-to-right shunting from the defect, whereas that from the subcostal view showed left-to-right shunting through a thick ventricular septum (Figure 2; Videos 1-3). General anesthesia was induced, and transesophageal echocardiography (TEE) was performed. Intraoperative color-flow Doppler evaluation revealed a VSD with left-to-right shunting (Figure 3, Video 4). A cardiopulmonary bypass (CPB) was established, and the LV apex was found to be reddish black. Left ventriculotomy was performed, and the small apical defect with the aneurysm was closed directly. The necrotic tissue around the PI-VSR was soft, fragile, and showed ischemic changes. Upon separation from the CPB and temporary discontinuation of pCS, color-flow Doppler evaluation of the ventricular septum revealed a defect with left-to-right shunting (Figure 4, Video 5) accompanied by a palpable shunt flow and persistently high pulmonary arterial pressure. As a residual shunt was suspected, cardiac arrest was induced again to inspect the suture site. Although the site was found to be tightly stitched, a second defect was identified in the mid-to-apical anteroseptum. The second defect was located anteriorly to the first and was more distant from the apex. Intraoperative findings showed that the ventricular septum around the second defect was not fragile and revealed no ischemic changes. The second defect was surrounded by healthy myocardial tissue of the ventricular septum, which was clearly different from the tissue around the first defect (Figure 5). Thereafter, the second defect was also directly closed. Subsequent color-flow Doppler evaluation of the ventricle revealed no left-to-right shunting.
Figure 2.
Two-dimensional TTE, apical (top) and subcostal (bottom) 4-chamber views without (left) and with (right) color-flow Doppler, demonstrates a mid-distal septal aneurysm protruding into the RV with a left-to-right shunt from the PI-VSR (top) located within the aneurysm and the VSD (bottom) located within the thick ventricular septum. RV, Right ventricle.
Figure 3.
Two-dimensional TEE, midesophageal long-axis (125°) systolic view, demonstrates a VSD with left-to-right shunting through the thick anteroseptal wall. This defect was initially considered to be the same PI-VSR identified earlier in the exam (yellow arrow). RV, Right ventricle.
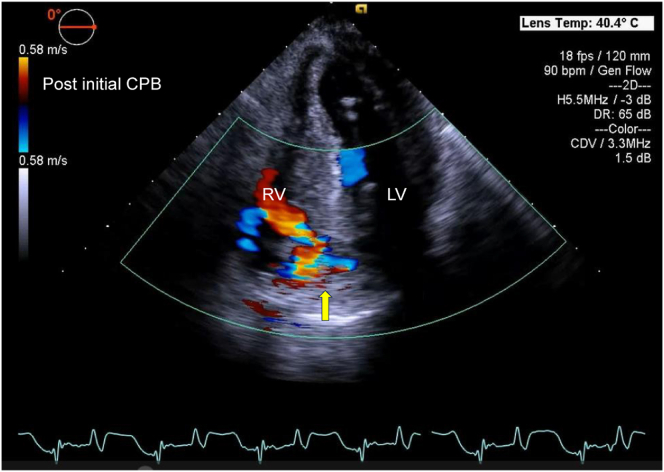
Figure 4.
Two-dimensional TEE, deep transgastric 4-chamber (0°) systolic view with color-flow Doppler, demonstrates the VSD with left-to-right shunting (yellow arrow). RV, Right ventricle.
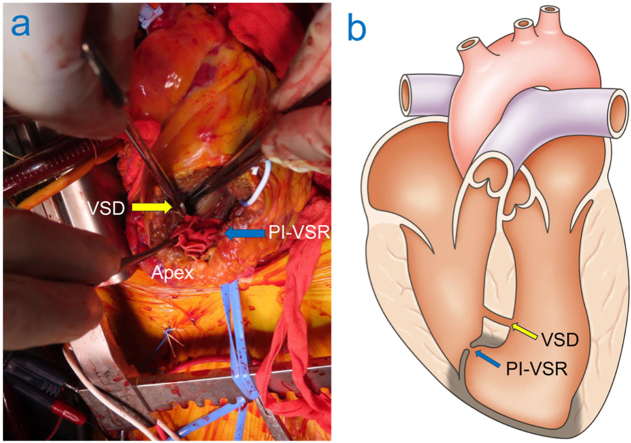
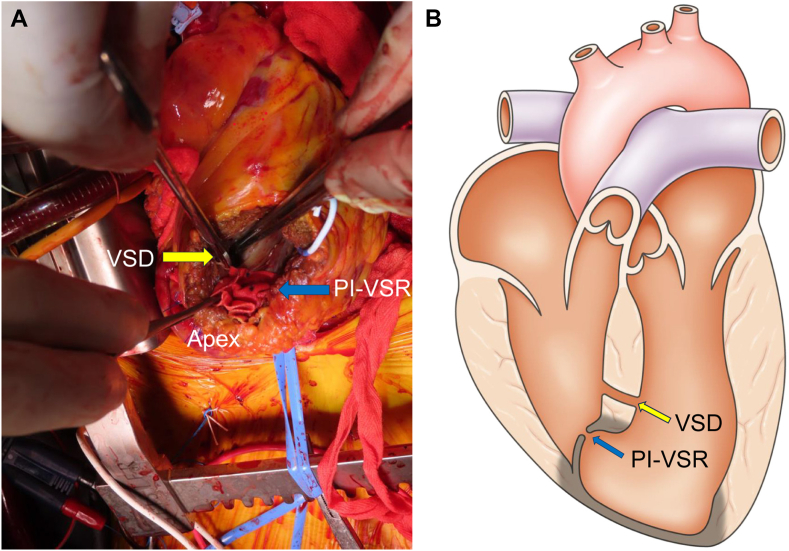
Figure 5.
(A) Intraoperative surgical view demonstrates a muscular VSD located anteriorly in the mid-to-apical anteroseptum and the separate PI-VSR, which is tightly stitched (indicating a successful appearing surgical patch repair). The VSD demonstrates a communication through healthy appearing, normal myocardium without ischemic changes. (B) Schematic diagram demonstrates the simultaneous presence of PI-VSR and muscular VSD located 20 mm apart with the PI-VSR located within the aneurysm and the VSD located within the muscular septum. The infarcted myocardium is demonstrated as being darker than the noninfarcted myocardial tissue.
Discussion
Here we report a rare case wherein we detected an undiagnosed VSD hidden behind a PI-VSR; TEE was helpful in diagnosing this VSD. In the current era of coronary revascularization, PI-VSR has emerged as a rare complication of MI.1 Undiagnosed VSDs are also uncommon in adults.8 Previous case reports have highlighted cases where clinicians mistakenly identified VSD as PI-VSR and vice versa.9,10 However, our case is unique because of the simultaneous presence of PI-VSR and VSD. In some cases, multiple VSRs may occur after MI. Sometimes, multiple muscular VSDs also occur simultaneously. However, in our case, intraoperative findings revealed that the PI-VSR was situated in the septal apical region, while the muscular VSD was located in the midanteroseptal region, 20 mm apart. The PI-VSR was situated in the pseudoaneurysm, while the VSD was located in the muscular tissue, suggesting that they were distinct defects. Careful retrospective examination of the preoperative imaging findings revealed that TTE from a different angle could have demonstrated evidence of 2 separate defects. However, the 2 defects were considered to be the same defect according to preoperative echocardiography until the second cardiac arrest was performed.
Transesophageal echocardiography played a notable role in diagnosing the VSD. After the initial CPB, intraoperative TEE revealed an undiagnosed shunt, which influenced the decision regarding operative management. The detection of an additional shunt based on TEE findings prompted the decision to implement CPB again. Contrary to our expectations, a VSD was initially misidentified as a PI-VSR before initial CPB. Two factors may have contributed to the intraoperative misidentification of a VSD as a PI-VSR using TEE. First, in this case, the defect's small size and location at the extreme apex of the ventricular septum made it challenging to identify a PI-VSR using TEE. Second, because the left anterior descending artery generally perfuses the midanteroseptal region, a defect in this region would be anatomically associated with the presence of a PI-VSR. Therefore, when we found a defect in the midanteroseptal region, we considered it to be a PI-VSR. We jumped to conclusions based on a single-view image taken from a single angle. We did not consider the possibility of a second shunt, which led us to discontinue further investigation. Thus, our study demonstrates that clinicians should continue to assess the entire septum in detail even after detecting a defect, because the PI-VSR may be serpentine and complex with more than a single shunt. The ventricular septum should be observed from multiple angles and views.
Assessing a shunt when weaning from CPB is difficult because many factors, such as the use of inotropes, CPBs, assist devices, and sedative drugs, affect shunt blood flow. This assessment becomes even more challenging when pCS is involved in PI-VSR management. This is because, while weaning from CPB, in addition to the dramatic changes of the endocardial pressure, pCS reduces the interventricular pressure gradient,2 potentially leading to the oversight or underestimation of the shunt. Decreased interventricular gradient is beneficial for perioperative PI-VSR management; however, it makes color-flow Doppler evaluation difficult for all echocardiographers. Taken together, temporary discontinuation of pCS during weaning from CPB should be recommended. It is advisable to perform repeated color-flow Doppler evaluation subsequently to identify hidden shunts, including residual and other shunts. Palpable shunt flow, persistently high pulmonary artery pressure, and increased oxygen saturation provide diagnostic clues for hidden shunts.
Our case demonstrated that multiangle, multiview, and multimodal echocardiography can successfully overcome these challenges during the perioperative period. It is important to understand the strengths of echocardiography and choose the most appropriate approach based on the specific situation.
One strength is that multiangle and multiview echocardiography provides valuable additional information. Upon careful retrospective review of the patient's preoperative imaging studies, the apical 4- and 3-chamber views showed the PI-VSR but not the VSD, whereas the subcostal view showed the VSD but not the PI-VSR. The two-dimensional image obtained through TTE is a slice through the three-dimensional heart, viewed as a cross-sectional image. Therefore, a single view may not capture all defects. To overcome this limitation, a combination of apical 4- and 3-chamber views and subcostal views should be employed. Moreover, color-flow Doppler imaging should be performed because it exhibits highly sensitive shunt flow detection and facilitates the assessment of shunt magnitude and direction. Finally, it would be worthwhile to encourage careful sweeps through the entire ventricular septum using both two-dimensional and color-flow Doppler imaging from multiple angles. Clinicians must try to track the entire septum without any gaps. These techniques may be helpful in identifying dual defects.
Second, multimodal echocardiography is effective. The apical view on TTE serves as an ideal window for detecting apical rupture. This view offers distinct advantages over TEE, because the proximity of the apex is sufficient to provide high-resolution images. Extremely apical rupture may be difficult to identify using TEE,11 given the distance of the probe from the apex. However, TTE is difficult to perform intraoperatively; therefore, epicardial echocardiography may be considered as an alternative for assessing the apex region.
Conclusion
We found an undiagnosed VSD hidden behind a PI-VSR in this case wherein echocardiography played a crucial role throughout the patient's treatment course. Multiangle, multiview, and multimodal echocardiography may help clinicians evaluate multiple shunts better.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent Statement
Complete written informed consent was obtained from the patient (or appropriate parent, guardian, or power of attorney) for the publication of this study and accompanying images.
Funding Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Disclosure Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank sonographer Shoko Shimizu for taking detailed TTE images.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2024.06.001.
Supplementary Data
Two-dimensional TTE, apical 4-chamber view with color-flow Doppler, demonstrates a middistal septal aneurysm protruding into the RV with a left-to-right shunt from the PI-VSR located within the aneurysm.
Two-dimensional TTE, apical 3-chamber view with color-flow Doppler, demonstrates the PI-VSR; aortic regurgitation is also demonstrated.
Two-dimensional TTE, subcostal 4-chamber view with color-flow Doppler, demonstrates the VSD located within the thick ventricular septum (initially misinterpreted as the same shunt originating from the PI-VSR).
Two-dimensional TEE, midesophageal long-axis (125°) view with color-flow Doppler, demonstrates a VSD with left-to-right shunting through the thick anteroseptal wall (initially misinterpreted as the same shunt originating from the PI-VSR); aortic regurgitation is also demonstrated.
Two-dimensional TEE, deep transgastric 4-chamber (0°) view with color-flow Doppler, demonstrates the VSD with left-to-right shunting.
References
- 1.Ronco D., Matteucci M., Ravaux J.M., et al. Mechanical circulatory support as a bridge to definitive treatment in post-infarction ventricular septal rupture. JACC Cardiovasc Interv. 2021;14:1053–1066. doi: 10.1016/j.jcin.2021.02.046. [DOI] [PubMed] [Google Scholar]
- 2.Pahuja M., Schrage B., Westermann D., Basir M.B., Garan A.R., Burkhoff D. Hemodynamic effects of mechanical circulatory support devices in ventricular septal defect. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.119.005981. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz Duque E., Hohenwarter M.R., Isom N.R., Singhal A.K. Impella support for surgical ventricular septal defect repair. ASAIO J. 2023;69:e278–e283. doi: 10.1097/MAT.0000000000001873. [DOI] [PubMed] [Google Scholar]
- 4.Jalli S., Spinelli K.J., Kirker E.B., Venkataraman A., Abraham J. Impella as a bridge-to-closure in post-infarction ventricular septal defect: a case series. Eur Heart J Case Rep. 2023;7 doi: 10.1093/ehjcr/ytad500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Via G., Buson S., Tavazzi G., et al. Early cardiac unloading with ImpellaCP™ in acute myocardial infarction with ventricular septal defect. ESC Heart Fail. 2020;7:708–713. doi: 10.1002/ehf2.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda K., Yoshioka I., Saiki Y. Emergence of right to left shunt via postmyocardial infarction ventricular septal defect under Impella support. Artif Organs. 2021;45:316–317. doi: 10.1111/aor.13824. [DOI] [PubMed] [Google Scholar]
- 7.Hiraoka A., Saku K., Nishikawa T., Sunagawa K. A case report of unexpected right-to-left shunt under mechanical support for post-infarction ventricular septal defect: evaluation with haemodynamic simulator. Eur Heart J Case Rep. 2021;5 doi: 10.1093/ehjcr/ytab209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warnes C.A., Liberthson R., Danielson G.K., et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170–1175. doi: 10.1016/s0735-1097(01)01272-4. [DOI] [PubMed] [Google Scholar]
- 9.Violaris A.G., Angelini G.D. Congenital ventricular septal defect presenting as rupture of the ventricular septum subsequent to myocardial infarction. Int J Cardiol. 1992;34:97–99. doi: 10.1016/0167-5273(92)90087-j. [DOI] [PubMed] [Google Scholar]
- 10.Davarpasand T., Mohseni-Badalabadi R., Sadeghian M., Mortazavi S.H., Lalvand A. Concomitant ventricular septal rupture and interventricular septal aneurysm in neglected inferior myocardial infarction misdiagnosed with congenital ventricular septal defect: a case report. Clin Case Rep. 2021;9 doi: 10.1002/ccr3.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price S., Via G., Sloth E., Guarracino F., Breitkreutz R., Catena E., et al. Echocardiography practice, training and accreditation in the intensive care: document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS) Cardiovasc Ultrasound. 2008;6:49. doi: 10.1186/1476-7120-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TTE, apical 4-chamber view with color-flow Doppler, demonstrates a middistal septal aneurysm protruding into the RV with a left-to-right shunt from the PI-VSR located within the aneurysm.
Two-dimensional TTE, apical 3-chamber view with color-flow Doppler, demonstrates the PI-VSR; aortic regurgitation is also demonstrated.
Two-dimensional TTE, subcostal 4-chamber view with color-flow Doppler, demonstrates the VSD located within the thick ventricular septum (initially misinterpreted as the same shunt originating from the PI-VSR).
Two-dimensional TEE, midesophageal long-axis (125°) view with color-flow Doppler, demonstrates a VSD with left-to-right shunting through the thick anteroseptal wall (initially misinterpreted as the same shunt originating from the PI-VSR); aortic regurgitation is also demonstrated.
Two-dimensional TEE, deep transgastric 4-chamber (0°) view with color-flow Doppler, demonstrates the VSD with left-to-right shunting.