Abstract
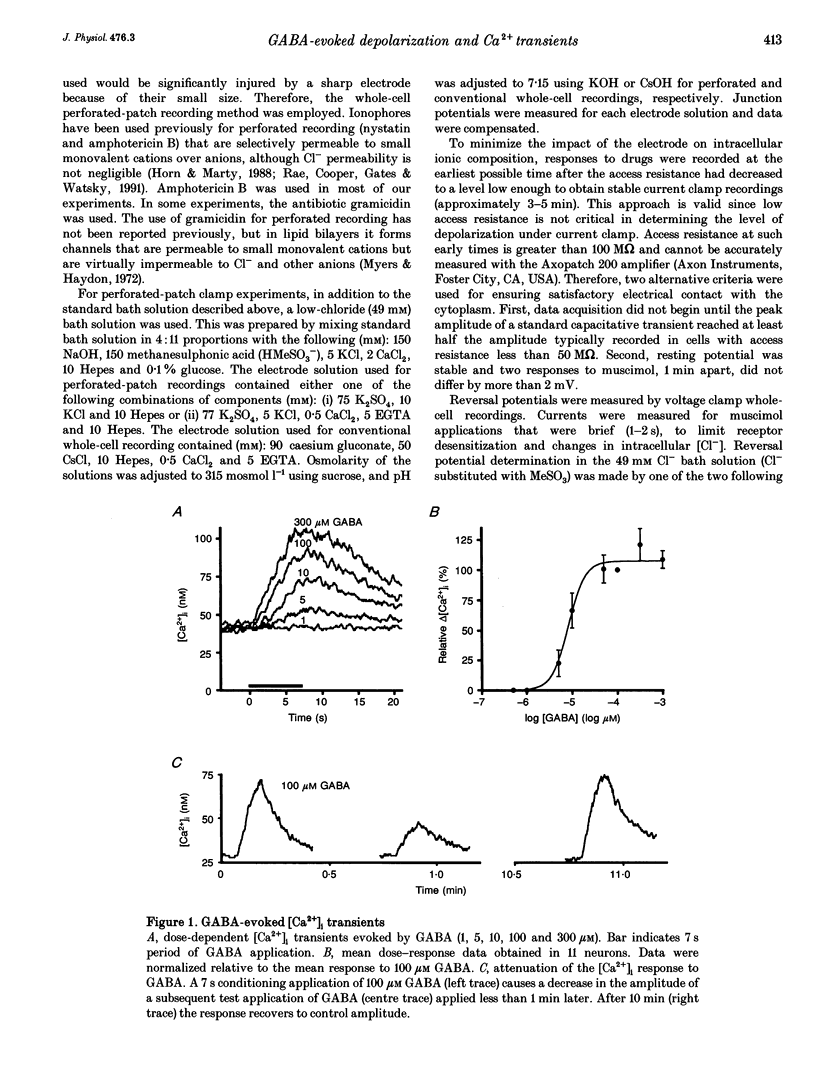
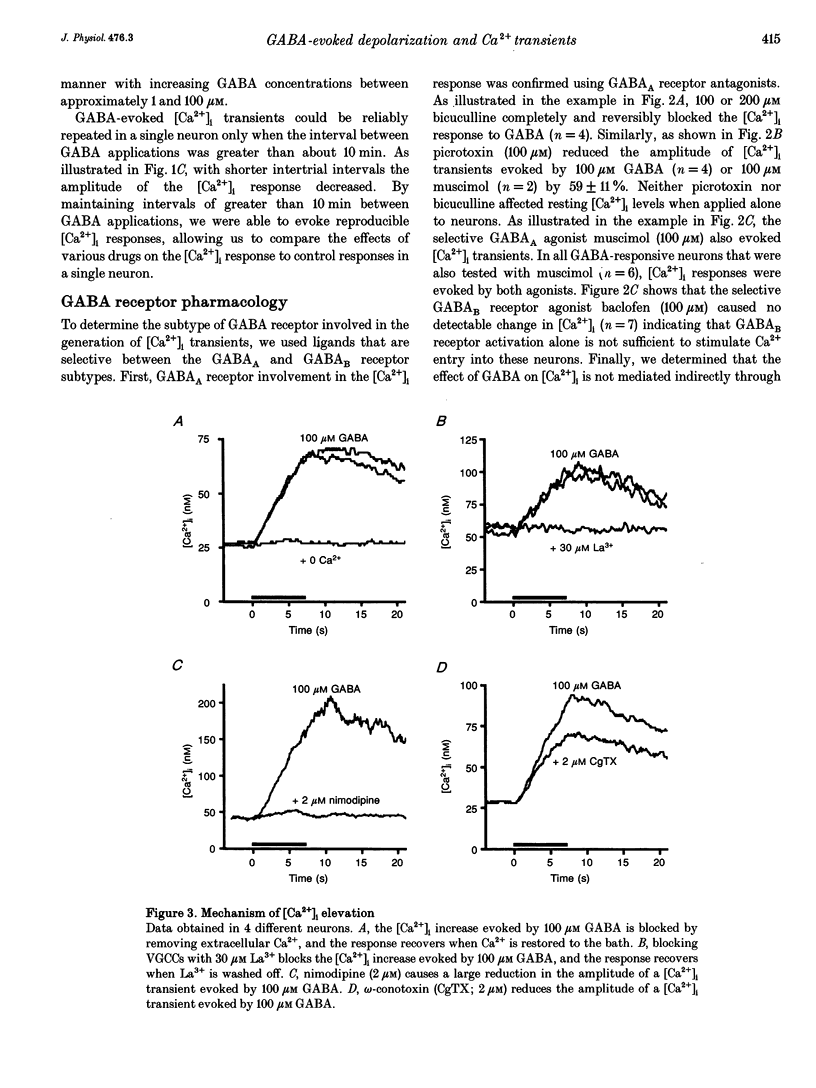
1. The mechanisms and effects of GABA- and glycine-evoked depolarization were studied in cultured rat dorsal horn neurons using indo-1 recordings of [Ca2+]i and patch clamp recordings in conventional whole-cell or perforated-patch mode. 2. Application of GABA to unclamped neurons caused [Ca2+]i increases that were dose dependent and exhibited GABAA receptor pharmacology. Calcium entered the neurons via high-threshold voltage-gated calcium channels (conotoxin and nimodipine sensitive). 3. In perforated-patch recordings employing cation-selective ionophores, GABAA receptor activation depolarized 123 of 132 cells to membrane potentials as depolarized as -33 mV (mean -50 mV in all 132 cells, +12 mV above resting potential). The ionic basis of the depolarization was determined by extracellular ion substitution; increased anionic conductance could account fully for the results. 4. Glycine, acting at a strychnine-sensitive receptor, also caused Ca2+ entry into these neurons through voltage-gated Ca2+ channels. Glycine and GABA both evoked [Ca2+]i responses in the same cells and the responses were highly correlated in amplitude. Glycine also depolarized all five cells tested with perforated recording. Each of the five cells was also depolarized by muscimol to a value similar to that obtained for glycine. 5. Both the depolarization and the increases in [Ca2+]i caused by GABA and glycine could potentially play a role in processes of development and differentiation and sensory transmission in the spinal cord dorsal horn.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. GABA-mediated biphasic inhibitory responses in hippocampus. Nature. 1979 Sep 27;281(5729):315–317. doi: 10.1038/281315a0. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Dingledine R., Gjerstad L., Langmoen I. A., Laursen A. M. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980 Aug;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A. The pharmacology and ionic dependency of amino acid responses in the frog spinal cord. J Physiol. 1973 Jan;228(2):259–277. doi: 10.1113/jphysiol.1973.sp010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Amino acid pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:331–354. doi: 10.1113/jphysiol.1978.sp012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhage B., Hansen G. H., Meier E., Schousboe A. Effects of inhibitors of protein synthesis and intracellular transport on the gamma-aminobutyric acid agonist-induced functional differentiation of cultured cerebellar granule cells. J Neurochem. 1990 Oct;55(4):1107–1113. doi: 10.1111/j.1471-4159.1990.tb03112.x. [DOI] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988 Mar;11(3):112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E., Gaiarsa J. L., Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991 Dec;14(12):515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Cherubini E., Rovira C., Gaiarsa J. L., Corradetti R., Ben Ari Y. GABA mediated excitation in immature rat CA3 hippocampal neurons. Int J Dev Neurosci. 1990;8(4):481–490. doi: 10.1016/0736-5748(90)90080-l. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Tseng H. Y., Hockberger P. E. Depolarization- and transmitter-induced changes in intracellular Ca2+ of rat cerebellar granule cells in explant cultures. J Neurosci. 1987 May;7(5):1384–1400. doi: 10.1523/JNEUROSCI.07-05-01384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdö S., Michler A., Wolff J. R. GABA accelerates excitotoxic cell death in cortical cultures: protection by blockers of GABA-gated chloride channels. Brain Res. 1991 Mar 1;542(2):254–258. doi: 10.1016/0006-8993(91)91575-l. [DOI] [PubMed] [Google Scholar]
- Feigenspan A., Wässle H., Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature. 1993 Jan 14;361(6408):159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Franklin J. L., Johnson E. M., Jr Suppression of programmed neuronal death by sustained elevation of cytoplasmic calcium. Trends Neurosci. 1992 Dec;15(12):501–508. doi: 10.1016/0166-2236(92)90103-f. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Nakamura J., Shinnick-Gallagher P. The effects of temperature, pH and Cl-pump inhibitors on GABA responses recorded from cat dorsal root ganglia. Brain Res. 1983 May 16;267(2):249–259. doi: 10.1016/0006-8993(83)90877-6. [DOI] [PubMed] [Google Scholar]
- Hansen G. H., Belhage B., Schousboe A. First direct electron microscopic visualization of a tight spatial coupling between GABAA-receptors and voltage-sensitive calcium channels. Neurosci Lett. 1992 Mar 16;137(1):14–18. doi: 10.1016/0304-3940(92)90287-h. [DOI] [PubMed] [Google Scholar]
- Hoppe D., Kettenmann H. GABA triggers a Cl- efflux from cultured mouse oligodendrocytes. Neurosci Lett. 1989 Feb 27;97(3):334–339. doi: 10.1016/0304-3940(89)90620-4. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. Y. Calcium channels in isolated rat dorsal horn neurones, including labelled spinothalamic and trigeminothalamic cells. J Physiol. 1989 Apr;411:161–177. doi: 10.1113/jphysiol.1989.sp017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K., Voipio J., Paalasmaa P., Pasternack M., Deisz R. A. The role of bicarbonate in GABAA receptor-mediated IPSPs of rat neocortical neurones. J Physiol. 1993 May;464:273–289. doi: 10.1113/jphysiol.1993.sp019634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito R. R. Molecular biology of the anion exchanger gene family. Int Rev Cytol. 1990;123:177–199. doi: 10.1016/s0074-7696(08)60674-9. [DOI] [PubMed] [Google Scholar]
- Lambert N. A., Borroni A. M., Grover L. M., Teyler T. J. Hyperpolarizing and depolarizing GABAA receptor-mediated dendritic inhibition in area CA1 of the rat hippocampus. J Neurophysiol. 1991 Nov;66(5):1538–1548. doi: 10.1152/jn.1991.66.5.1538. [DOI] [PubMed] [Google Scholar]
- Ma W., Behar T., Barker J. L. Transient expression of GABA immunoreactivity in the developing rat spinal cord. J Comp Neurol. 1992 Nov 8;325(2):271–290. doi: 10.1002/cne.903250210. [DOI] [PubMed] [Google Scholar]
- Michelson H. B., Wong R. K. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991 Sep 20;253(5026):1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Deisz R. A., Dodt H. U., Lux H. D. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986 Jun 13;232(4756):1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Morris M. E., Di Costanzo G. A., Fox S., Werman R. Depolarizing action of GABA (gamma-aminobutyric acid) on myelinated fibers of peripheral nerves. Brain Res. 1983 Nov 14;278(1-2):117–126. doi: 10.1016/0006-8993(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Mueller A. L., Taube J. S., Schwartzkroin P. A. Development of hyperpolarizing inhibitory postsynaptic potentials and hyperpolarizing response to gamma-aminobutyric acid in rabbit hippocampus studied in vitro. J Neurosci. 1984 Mar;4(3):860–867. doi: 10.1523/JNEUROSCI.04-03-00860.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers V. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972 Aug 9;274(2):313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The blockade of GABA mediated responses in the frog spinal cord by ammonium ions and furosemide. J Physiol. 1978 Oct;283:121–132. doi: 10.1113/jphysiol.1978.sp012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., Minota S., Karczmar A. G. Primary afferent neurones: the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974 Mar;13(3):215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Obata K., Oide M., Tanaka H. Excitatory and inhibitory actions of GABA and glycine on embryonic chick spinal neurons in culture. Brain Res. 1978 Apr 7;144(1):179–184. doi: 10.1016/0006-8993(78)90447-x. [DOI] [PubMed] [Google Scholar]
- Qian H., Dowling J. E. Novel GABA responses from rod-driven retinal horizontal cells. Nature. 1993 Jan 14;361(6408):162–164. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Reichling D. B., MacDermott A. B. Brief calcium transients evoked by glutamate receptor agonists in rat dorsal horn neurons: fast kinetics and mechanisms. J Physiol. 1993 Sep;469:67–88. doi: 10.1113/jphysiol.1993.sp019805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling D. B., MacDermott A. B. Lanthanum actions on excitatory amino acid-gated currents and voltage-gated calcium currents in rat dorsal horn neurons. J Physiol. 1991 Sep;441:199–218. doi: 10.1113/jphysiol.1991.sp018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. GABA induces a unique rise of [Ca]i in cultured rat hippocampal neurons. Hippocampus. 1993 Apr;3(2):229–238. doi: 10.1002/hipo.450030214. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Killisch I., Sprengel R., Sontheimer H., Köhler M., Schofield P. R., Seeburg P. H. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989 Sep;3(3):327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Staley K. J., Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol. 1992 Jul;68(1):197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Draguhn A., Ymer S., Seeburg P. H., Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990 Jun;4(6):919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Wu W. L., Ziskind-Conhaim L., Sweet M. A. Early development of glycine- and GABA-mediated synapses in rat spinal cord. J Neurosci. 1992 Oct;12(10):3935–3945. doi: 10.1523/JNEUROSCI.12-10-03935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh T. L. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989 Apr;37(1):111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- Yuste R., Katz L. C. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991 Mar;6(3):333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- Zhang J. H., Sato M., Tohyama M. Different postnatal development profiles of neurons containing distinct GABAA receptor beta subunit mRNAs (beta 1, beta 2, and beta 3) in the rat forebrain. J Comp Neurol. 1991 Jun 22;308(4):586–613. doi: 10.1002/cne.903080407. [DOI] [PubMed] [Google Scholar]


