Abstract
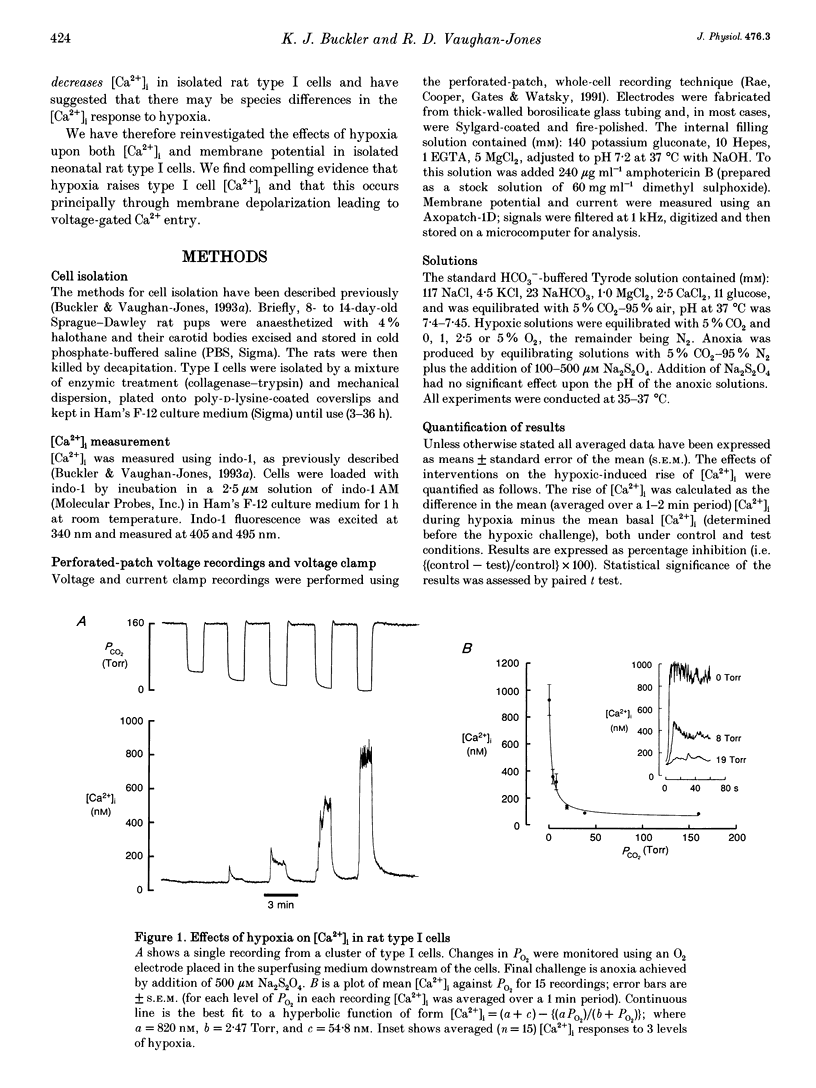
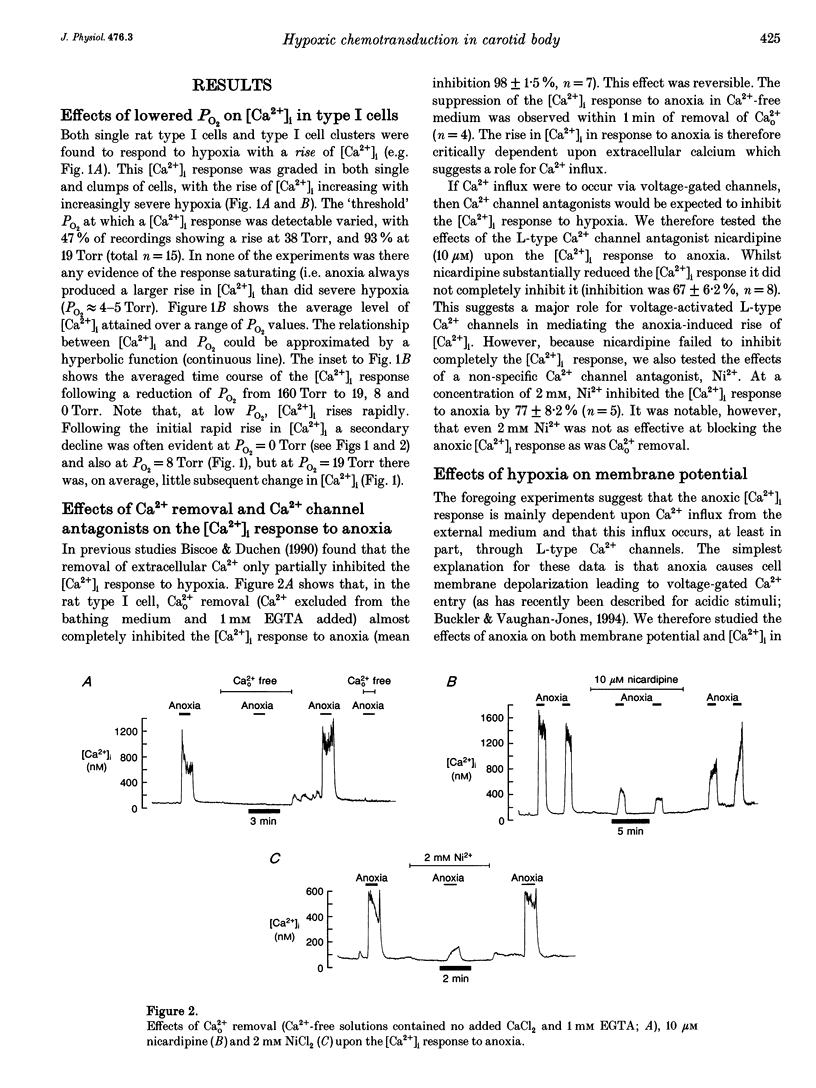
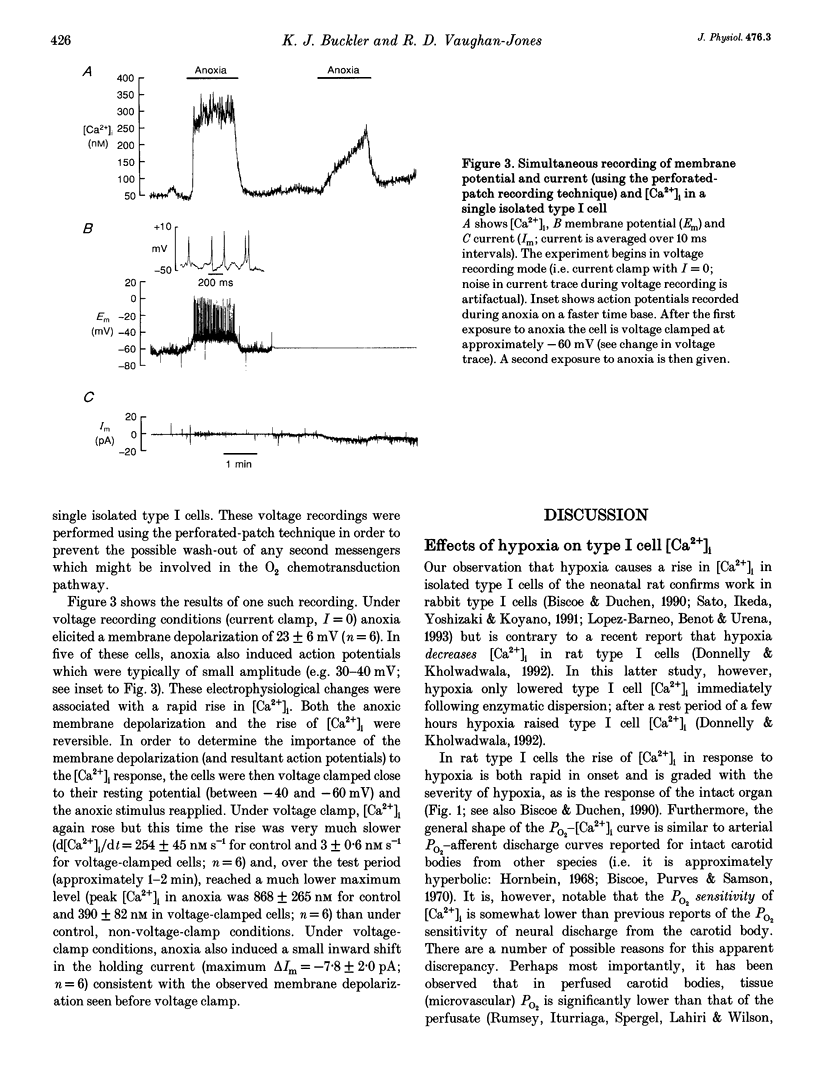
1. We have studied the effects of hypoxia on membrane potential and [Ca2+]i in enzymically isolated type I cells of the neonatal rat carotid body (the principal respiratory O2 chemosensor). Isolated cells were maintained in short term culture (3-36 h) before use. [Ca2+]i was measured using the Ca(2+)-sensitive fluoroprobe indo-1. Indo-1 was loaded into cells using the esterified form indo-1 AM. Membrane potential was measured (and clamped) in single isolated type I cells using the perforated-patch (amphotericin B) whole-cell recording technique. 2. Graded reductions in PO2 from 160 Torr to 38, 19, 8, 5 and 0 Torr induced a graded rise of [Ca2+]i in both single and clumps of type I cells. 3. The rise of [Ca2+]i in response to anoxia was 98% inhibited by removal of external Ca2+ (+1 mM EGTA), indicating the probable involvement of Ca2+ influx from the external medium in mediating the anoxic [Ca2+]i response. 4. The L-type Ca2+ channel antagonist nicardipine (10 microM) inhibited the anoxic [Ca2+]i response by 67%, and the non-selective Ca2+ channel antagonist Ni2+ (2 mM) inhibited the response by 77%. 5. Under voltage recording conditions, anoxia induced a reversible membrane depolarization (or receptor potential) accompanied, in many cases, by trains of action potentials. These electrical events were coincident with a rapid rise of [Ca2+]i. When cells were voltage clamped close to their resting potential (-40 to -60 mV), the [Ca2+]i response to anoxia was greatly reduced and its onset was much slower.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biscoe T. J., Duchen M. R. Responses of type I cells dissociated from the rabbit carotid body to hypoxia. J Physiol. 1990 Sep;428:39–59. doi: 10.1113/jphysiol.1990.sp018199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J., Sampson S. R. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970 May;208(1):121–131. doi: 10.1113/jphysiol.1970.sp009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of acidic stimuli on intracellular calcium in isolated type I cells of the neonatal rat carotid body. Pflugers Arch. 1993 Oct;425(1-2):22–27. doi: 10.1007/BF00374499. [DOI] [PubMed] [Google Scholar]
- Delpiano M. A., Hescheler J. Evidence for a PO2-sensitive K+ channel in the type-I cell of the rabbit carotid body. FEBS Lett. 1989 Jun 5;249(2):195–198. doi: 10.1016/0014-5793(89)80623-4. [DOI] [PubMed] [Google Scholar]
- Donnelly D. F., Kholwadwala D. Hypoxia decreases intracellular calcium in adult rat carotid body glomus cells. J Neurophysiol. 1992 Jun;67(6):1543–1551. doi: 10.1152/jn.1992.67.6.1543. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Biscoe T. J. Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. J Physiol. 1992 May;450:33–61. doi: 10.1113/jphysiol.1992.sp019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber L. A., McCleskey E. W. L-type calcium channels in type I cells of the rat carotid body. J Neurophysiol. 1993 Oct;70(4):1378–1384. doi: 10.1152/jn.1993.70.4.1378. [DOI] [PubMed] [Google Scholar]
- Ganfornina M. D., López-Barneo J. Potassium channel types in arterial chemoreceptor cells and their selective modulation by oxygen. J Gen Physiol. 1992 Sep;100(3):401–426. doi: 10.1085/jgp.100.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González C., Almaraz L., Obeso A., Rigual R. Oxygen and acid chemoreception in the carotid body chemoreceptors. Trends Neurosci. 1992 Apr;15(4):146–153. doi: 10.1016/0166-2236(92)90357-e. [DOI] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2(+)-activated K+ current. Neurosci Lett. 1990 Nov 13;119(2):253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rumsey W. L., Iturriaga R., Spergel D., Lahiri S., Wilson D. F. Optical measurements of the dependence of chemoreception on oxygen pressure in the cat carotid body. Am J Physiol. 1991 Oct;261(4 Pt 1):C614–C622. doi: 10.1152/ajpcell.1991.261.4.C614. [DOI] [PubMed] [Google Scholar]
- Sato M., Ikeda K., Yoshizaki K., Koyano H. Response of cytosolic calcium to anoxia and cyanide in cultured glomus cells of newborn rabbit carotid body. Brain Res. 1991 Jun 14;551(1-2):327–330. doi: 10.1016/0006-8993(91)90951-q. [DOI] [PubMed] [Google Scholar]
- Shirahata M., Fitzgerald R. S. Dependency of hypoxic chemotransduction in cat carotid body on voltage-gated calcium channels. J Appl Physiol (1985) 1991 Sep;71(3):1062–1069. doi: 10.1152/jappl.1991.71.3.1062. [DOI] [PubMed] [Google Scholar]
- Stea A., Nurse C. A. Whole-cell and perforated-patch recordings from O2-sensitive rat carotid body cells grown in short- and long-term culture. Pflugers Arch. 1991 Mar;418(1-2):93–101. doi: 10.1007/BF00370457. [DOI] [PubMed] [Google Scholar]


