Abstract
Backgroud
Although symptomatic local recurrence (SLR) of spinal metastasis is relatively common after aggressive surgery, there have been few studies on SLR according to the extent of tumor removal. This study aimed to evaluate the incidence of SLR after surgery in spinal metastasis and analyze the risk factors of SLR.
Methods
This study included patients with spinal metastasis to all 3 vertebral columns. SLR was defined as the occurrence of new symptoms, confirmed by radiologic regrowth of tumor. The extent of tumor removal was classified into 3 types (corpectomy, separation surgery, and only posterior column removal). The Kaplan-Meier method was used to analyze the SLR rate after surgery. The presumed risk factors of SLR were evaluated using log-rank test and Cox regression analysis.
Results
This study included 102 patients with a mean follow-up period of 17.7 months (range, 3–84 months). After surgical treatment, SLR was confirmed in 35 patients (34.3%). Kaplan-Meier analysis predicted that the incidence of SLR was 4.4% at 6 months, 21.5% at 12 months, 34.0% at 18 months, and 42.7% at 24 months. In the univariate analysis, the primary malignancy site, number of vertebral metastases, and surgery for progressed tumor after previous radiation therapy were significant (p = 0.042, p = 0.048, and p = 0.008, respectively). No significant differences were observed in the extent of tumor removal (p = 0.536). In the multivariate analysis, the significant risk factors of SLR included only previous radiation therapy (p = 0.012). The risk of SLR was 2.8 times higher in patients who received surgery for progressed tumor after previous radiation therapy than in those without it.
Conclusions
The SLR of spinal metastasis was predicted in 21.5% of patients at 1 year after surgical treatment. The extent of tumor removal did not seem to affect SLR. Surgery for progressed tumor after previous radiation therapy was confirmed as the only substantial risk factor. Therefore, the tumor's response to preoperative radiation therapy is the most important factor in determining SLR.
Keywords: Neoplasm metastasis, Surgical decompression, Tumor volume, Local neoplasm recurrence, Risk factors
In the past 20 years, the life expectancy of cancer patients has been increasing with the development of systemic therapy.1,2) As the spine is the most common site of metastasis in the musculoskeletal system, 70% of patients with cancer experience spinal metastases during their cancer course.3,4) Surgical treatment should be considered when patients experience neurological symptoms or spinal column instability due to metastatic tumors.5,6,7) The recently reported postoperative survival improvement for spinal metastases emphasizes the need for long-term outcome considerations after surgery.8) Considering the long-term outcomes of surgery for spinal metastasis, surgeons must consider the extent to which the metastatic tumor should be removed. To reduce symptomatic local recurrence (SLR), it is better to remove as many tumors as possible. However, when a large metastasized vertebral body is removed, excessive bleeding occurs, making the surgery more difficult and time-consuming.9) In our experience, even after such aggressive surgery, SLR of spinal metastases is still relatively common.
Owing to recent advances in radiation delivery technology, ablative radiation doses can be delivered safely and effectively to metastatic spinal tumors.10,11) The role of local control of metastatic tumors through radiation therapy is being emphasized.12,13,14) Systemic performance is often lowered by chemotherapy in patients with cancer; therefore, less invasive surgery focusing on symptom relief with appropriate radiation therapy could be a better option.9) However, especially when there is a large tumor burden that invades all 3 columns of the vertebra, there is concern that removing small amount of tumor will lead to rapid SLR. Therefore, a study on the relationship between the extent of the tumor removed in spinal metastasis surgery and SLR will provide important information for choosing a surgical method. However, there have only been a few studies on SLR after surgical treatment of spinal metastasis involving all 3 columns. This study aimed to evaluate the incidence of SLR after surgery in patients with spinal metastasis with involvement of all 3 columns and analyze the risk factors of SLR, including the extent of tumor removal.
METHODS
The study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2022-03-084). After the requirement for informed consent was waived by the IRB, we reviewed patients who underwent surgical treatment. Patient information was retrieved from a prospective spinal metastasis database at our institution.
The indications for surgical treatment were carefully determined through a multilateral, inter-department conference based on the following criteria: (1) refractory pain despite conservative treatment and (2) neurological deterioration or the potential for neurological deficits with spinal column instability. Recent patients from 2017 to 2022 were identified considering the period after the introduction of modern radiation therapy technique. This study included patients with Tomita classification types 3 to 5 with involvement of all 3 columns of single vertebra within the thoracolumbar spine, where the method of surgical treatment for the extent of tumor removal can be considered diverse. In addition, patients who were treated subsequently with adjuvant therapy at our department of medical oncology or radiation oncology and were able to be followed up for at least 3 months were included. Cases of instrument fixation for stabilization without removal of the tumor were excluded.
Postoperative 3-dimentional conformal radiation therapy was considered for the segments included in the surgical bed with 30 Gy in 10 fractions at 2 weeks or later after surgery. For patients who underwent preoperative radiation therapy, postoperative radiation therapy was administered in very limited cases, contingent upon factors such as the preoperative radiation dose, fractions, and tumor types, primarily due to tumor progression within the radiation therapy field. The regimen of previous radiation therapy before surgical treatment was determined according to the primary tumors whose radiation sensitivities are diverse and the radiation oncologist’s purpose of radiation therapy. For tumors such as hepatocellular carcinoma and colorectal cancer, which are radiation resistant and usually form soft-tissue mass, high-dose fractionated irradiation with 40–50 Gy in 10 fractions or stereotactic body radiotherapy with 20–24 Gy in 1–3 fractions was applied using intensity-modulated radiotherapy technique. For oligometastatic tumors, high-dose fractionated irradiation or stereotactic body radiotherapy with similar dose scheme and technique was performed. Otherwise, radiation therapies with palliative dose of 20–25 Gy in 5 fractions or 30 Gy in 10 fractions were done according to the radiation oncologist’s decision. Systemic treatment was chosen according to the primary tumor and patient’s status.
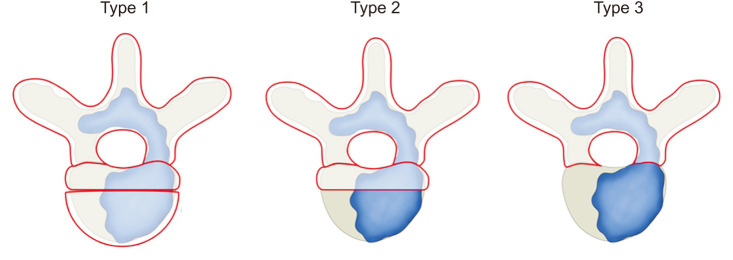
The occurrence of SLR was determined from patients’ electronic medical records and radiologic findings. SLR was defined as the occurrence of new symptoms, such as neurological pain or deficits during follow-up after surgical treatment. In addition. the regrowth of the tumor at the surgical site was confirmed by magnetic resonance imaging. Cases in which new symptoms were due to a tumor at another site other than the previous surgical site were excluded.15) The potential risk factors for SLR were identified. The extent of tumor removal was categorized into 3 types: corpectomy, separation surgery, and only posterior column removal (Fig. 1). These classifications were based on descriptions of tumor removal extent and surgical methods documented in the surgical records. Type 1 entailed complete removal of anteriorly invading tumors within the vertebral body, utilizing cages or cement for anterior support. Type 2 included partial removal of the posterior column and pedicle, along with the removal of tumors around the spinal cord in a separation surgery manner. Only partial removal of the anterior column, without anterior support, was performed. Type 3 comprised cases where only tumors in the posterior column were removed, typically through posterior decompression surgery. As the patients had a large tumor burden with involvement of all 3 columns, preoperative embolization and posterior fixation were performed in all cases. We confirmed the location of spinal metastasis and primary malignancy site. Primary malignancy burdens were determined according to the total number of other bones or vertebral or major organ metastases. The Eastern Cooperative Oncology Group status was used to determine the general performance status of the patients. The neurological deficits due to cord compression, pathologic fracture, and the degree of canal involvement were evaluated using Bilsky grade at the time of surgical treatment. In addition, intraoperative bleeding and postoperative complications were investigated.
Fig. 1. The extent of tumor removal classified into 3 types: type 1 was corpectomy, type 2 was separation surgery, and type 3 was only posterior column removal.
Statistical Analysis
The incidence and SLR after surgical treatment of spinal metastasis were calculated using life-table methods and Kaplan-Meier survivorship analysis. Differences in continuous variables between groups were analyzed using the Student t-test, whereas categorical variables were examined using the chi-square test. In the risk factor analysis, a log-rank test was used for univariate analysis, and the Cox proportional hazards model was also used for multivariate analysis to calibrate confounding variables. Statistical analysis was performed using the SPSS software version 22.0.0 (IBM Corp.). Statistical significance was set at p < 0.05.
RESULTS
Among the 163 patients who underwent surgical treatment of spinal metastasis, 48 patients without the involvement of all 3 columns and 13 patients with only instrument fixation were excluded. Finally, 102 patients were selected, and the mean follow-up period was 17.7 ± 16.4 months (range, 3–75 months). During the follow-up period, 59 patients died and the median survival time was 14.0 ± 1.8 months (95% CI, 10.5–17.5 months). Male patients comprised of 63.7% of the total patients, and the mean age at the time of spine surgery was 60.5 ± 9.0 years (range, 37.0–83.0 years). The most common site of metastasis was the thoracic spine in 70.6% of cases, followed by the lumbar. Seven primary malignancies were observed in 86.3% of the entire cohort, namely, the lung, liver, kidney, prostate, thyroid, colorectal, and breast, in decreasing order of frequency. In terms of the extent of tumor removal, corpectomy was performed in 27 patients, separation surgery in 35 patients, and only posterior column removal in 40 patients. Surgery for progression tumor after previous radiation therapy was performed in 63 patients and chemotherapy was performed in 72 patients. Postoperative radiation therapy was performed in 61 patients. Systemic treatments were performed in 72 and 33 patients before and after surgical treatment, respectively (Table 1).
Table 1. Baseline Demographics.
| Characteristics | Value (N = 102) | |
|---|---|---|
| Male | 65 (63.7) | |
| Age at spine surgery (yr) | 60.5 ± 9.0 (37.0–83.0) | |
| Follow-up period (mo) | 17.7 ± 16.4 | |
| Median survival time (mo) | 14.0 ± 1.8 | |
| Location | ||
| Thoracic | 72 (70.6) | |
| Lumbar | 30 (29.4) | |
| Primary site of cancer | ||
| Lung | 30 (29.4) | |
| Liver | 14 (13.7) | |
| Kidney | 13 (12.7) | |
| Prostate | 10 (9.8) | |
| Thyroid | 9 (8.8) | |
| Colorectal | 6 (5.9) | |
| Breast | 6 (5.9) | |
| Others | 14 (13.7) | |
| The extent of tumor removal | ||
| Corpectomy | 27 (26.5) | |
| Separation surgery | 35 (34.3) | |
| Only posterior column removal | 40 (39.2) | |
| Adjuvant therapy | ||
| Previous CTx | 72 (70.6) | |
| Previous RTx | 63 (61.8) | |
| Postoperative CTx | 33 (32.4) | |
| Postoperative RTx | 61 (59.8) | |
Values are presented as number (%), mean ± SD (range), or mean ± SD.
CTx: chemotherapy, RTx: radiotherapy, SD: standard deviation.
The Incidence of Local Recurrence
After surgical treatment, SLR was confirmed in 35 of the 102 patients (34.3%). According to the primary malignancy site, 9 of 30 patients (30.0%) showed SLR in the lung, 5 of 14 (40.0%) in the liver, 8 of 13 (53.8%) in the kidney, 0 of 10 (0%) in the prostate, 6 of 9 (66.7%) in the thyroid, 0 of 6 (0%) in the colorectal, 2 of 6 (33.3%) in the breast, and 5 of 14 (35.7%) in others. The Kaplan-Meier analysis predicted that the incidence of SLR was 4.4% at 6 months, 21.5% at 12 months, 34.0% at 18 months, and 42.7% at 24 months (Fig. 2, Table 2).
Fig. 2. The Kaplan-Meier survival curve shows symptomatic local recurrence after surgery for metastatic spinal tumor.
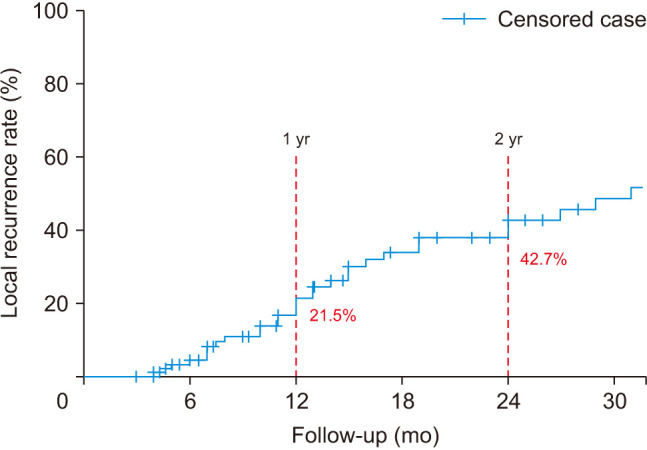
Table 2. The Incidence of Symptomatic Local Recurrence after Surgery for Metastatic Spinal Tumor Involving All 3 Columns of the Vertebral Body.
| Follow-up period | Recurrence rate (%, 95% CI) | |
|---|---|---|
| 6 mo | 4.4 (2.2–6.6) | |
| 12 mo | 21.5 (16.7–26.3) | |
| 18 mo | 34.0 (28.0–40.0) | |
| 24 mo | 42.7 (36.1–49.3) | |
The Risk Factor of Local Recurrence
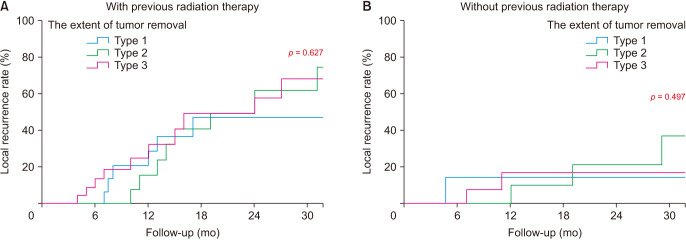
In the univariate analysis, the significant risk factors of SLR included the primary malignancy site, number of vertebral metastases, and surgery for progressed tumor after previous radiation therapy (p = 0.042, p = 0.048, and p = 0.008, respectively). No significant difference was found in the extent of tumor removal according to the surgical method (p = 0.536) (Table 3). In the multivariate analysis, including presumed risk factors (p < 0.01), the significant risk factors of SLR included only surgery for progressed tumor after previous radiation therapy (p = 0.012). The risk of SLR was 2.76 times higher in patients who received surgery for progressed tumor after previous radiation therapy than in those without it (Table 4). The extent of tumor removal did not affect SLR, regardless of the surgery performed for progressed tumor after radiation therapy (Fig. 3).
Table 3. Univariate Analysis of the Risk Factors Affecting Symptomatic Local Recurrence.
| Variable | Recurrence group (n = 35) | No recurrence group (n = 67) | p-value | ||
|---|---|---|---|---|---|
| Patient-related factor | |||||
| Age (yr) | ≥ 60 | 16 | 35 | 0.610 | |
| < 60 | 19 | 32 | |||
| Sex | Male | 22 | 43 | 0.859 | |
| Female | 13 | 24 | |||
| Performance status (ECOG) | PS 0 to 2 | 25 | 47 | 0.377 | |
| PS 3 to 4 | 10 | 20 | |||
| Neurological symptom (Frankel classification) | C | 16 | 39 | 0.918 | |
| D | 19 | 28 | |||
| Primary malignancy site factor* | 0.042 | ||||
| Slow: breast, prostate, thyroid | 10 | 44 | |||
| Moderate: kidney, uterus, colorectal | 11 | 10 | |||
| Rapid: lung, bladder, liver, stomach, sarcoma | 14 | 13 | |||
| Primary malignancy burden | |||||
| Major internal organ metastasis | Metastasis | 20 | 31 | 0.060 | |
| No metastasis | 15 | 36 | |||
| Number of vertebral metastasis | ≥ 3 | 15 | 33 | 0.048 | |
| 2 | 13 | 11 | |||
| 1 | 7 | 23 | |||
| Number of other bone metastasis | ≥ 3 | 8 | 13 | 0.236 | |
| 1–2 | 8 | 21 | |||
| 0 | 19 | 33 | |||
| Surgery-related factor | |||||
| The extent of tumor removal† | Type 1 | 8 | 19 | 0.536 | |
| Type 2 | 15 | 20 | |||
| Type 3 | 12 | 28 | |||
| Bilsky grade | Grade 2 | 8 | 17 | 0.831 | |
| Grade 3 | 27 | 50 | |||
| Pathologic fracture | Yes : no | 28 : 7 | 53 : 14 | 0.915 | |
| Intraoperative bleeding (mL) | 2,030.5 | 1,598.8 | - | ||
| Adjuvant therapy | 0.151 | ||||
| Previous CTx | Yes : no | 7 : 28 | 16 : 51 | 0.997 | |
| Previous RTx | Yes : no | 27 : 8 | 36 : 31 | 0.008 | |
| Postoperative CTx | Yes : no | 13 : 22 | 20 : 47 | 0.162 | |
| Postoperative RTx | Yes : no | 23 : 12 | 18 : 49 | 0.750 | |
| Postoperative complication | 6 | 11 | 0.926 | ||
| Wound dehiscence | 4 | 7 | |||
| Infection | 1 | 2 | |||
| Epidural hematoma | 0 | 1 | |||
| Screw malposition | 1 | 1 | |||
ECOG: eastern cooperative oncology group, CTx: chemotherapy, RTx: radiotherapy.
*Primary malignancy site factor: primary malignancy growth rate using the Tomita scoring system. †The extent of tumor removal: Type 1, corpectomy; Type 2, separation surgery; and Type 3, only posterior column removal.
Table 4. Multivariate Analysis of the Risk Factors Affecting Symptomatic Local Recurrence.
| Variable | HR | 95% CI | p-value | |
|---|---|---|---|---|
| Primary malignancy site | ||||
| Group A (slow) | 1 | Reference | ||
| Group B (moderate) | 1.69 | 0.69–1.69 | 0.253 | |
| Group C | 2.27 | 0.98–5.24 | 0.056 | |
| Number of vertebral metastasis | ||||
| ≥ 3 | 1 | Reference | ||
| 2 | 0.93 | 0.42–2.06 | 0.848 | |
| 1 | 0.43 | 0.16–1.17 | 0.099 | |
| Metastasis to major organs | 1.56 | 0.76–3.17 | 0.223 | |
| Previous radiation therapy | 2.76 | 1.25–6.08 | 0.012 | |
HR: hazard ratio.
Fig. 3. (A, B) The influence of the extent of tumor removal for symptomatic local recurrence depending on previous radiation therapy.
DISCUSSION
Remarkable advancements in cancer screening and surgical and medical management have improved the survival outcomes of cancer patients.16,17) As a result, management of metastatic disease in the spine is becoming increasingly important. The surgical treatment of metastatic spinal tumors is mostly performed on patients with a life expectancy of 3 months or more, who suffer neurological symptoms or pain with spinal column instability due to the metastatic tumor.5,18,19) For metastatic spinal tumors in which the vertebral body is affected by the epidural mass with cord compression, the epidural mass is removed to alleviate neurological symptoms; however, when the patient shows a poor condition or experiences heavy bleeding, the removal of the tumor in the anterior column is often challenging. However, even after aggressive tumor resection in the anterior column is performed to reduce SLR, recurrence frequently occurs.
According to previous studies, the rate of local recurrence following surgical treatment of spinal metastases ranges from 17.5 to 32.3%.20,21,22) In the current study, the rate of SLR after surgical treatment in 102 patients with metastatic tumors affecting all 3 columns of the vertebral body was 34.4% (32 patients) during follow-up period. The high rate of recurrence is presumed to be due to most patients in this study having a high tumor burden in vertebral body metastasis. However, survival analysis is more suitable to evaluate its rate because patients with cancer show rapid mortality before local recurrence during the follow-up period. The SLR predicted by the survival analysis in this study was 21.5% in the first year and 42.7% in the second year.
Lau et al.23) investigated the risk factors of postoperative local recurrence in metastatic spine tumors and reported less extensive spinal disease and radiotherapy as risk factors, which was attributed to the positive effect of such factors in increasing patient survival. Patients with local recurrence had a longer survival period, which was suggested to increase the chance of local recurrence. However, because the analysis of local recurrence should reflect the concept of time, survival analysis was performed to investigate risk factors in our study. The risk factors identified in the univariate analysis were primary malignancy site, number of vertebral metastasis, and previous radiation therapy. The first 2 factors accounted for the potential increase in the risk of local progression for the primary cancer type showing a more aggressive progression and high tumor burden. Notably, in the multivariate analysis, previous radiation therapy was verified as the most important risk factor for local progression, highlighting the role of local control in radiation therapy. In the case of surgery performed due to symptoms occurring after previous radiation therapy, the tumor is likely to be resistant to radiotherapy, making it difficult to select the effective postoperative radiation dose.24) In our study, preoperative radiation therapy had been administered in 61.8% of cases and the fact that postoperative radiotherapy was administered in only 59.8% of the cases reflects this outcome. Additionally, the administration of postoperative radiotherapy did not emerge as a significant factor influencing local recurrence. Instead, the significance of previous radiotherapy as an influential factor in our analysis reflects the importance of radiation therapy. This is because subsequent surgical intervention despite prior radiotherapy indicates a limited response to radiotherapy and has also been demonstrated to lead to a high recurrence rate even after surgery. Surgery after radiotherapy may also result in an adhesive mass around the cord. A mass in the anterior epidural space with extension to a nearby segment can cause cord compression at a slight progression and lead to recurring symptoms. After analyzing the recurrence risk factors even after en bloc spondylectomy, where tumor resection is the most aggressive, previous radiotherapy was shown to be a risk factor, which was accounted for by the adhesion mass due to contamination in the epidural space.25) Similarly, for metastatic spinal tumors, even a small residual mass after resection may cause sudden progression depending on the therapeutic response regarding the primary cancer and systemic state of the patient. Thus, if the metastatic tumor burden in the vertebral body is high and if a high level of tumor cell leakage or seeding is expected for the surrounding tissue during tumor resection, less invasive surgery could be a more reasonable and a cost-effective option with a focus on symptomatic relief in consideration of the patient’s systemic state.
With recent advancements in radiotherapy, the trend regarding the surgical methods for spinal metastasis has changed. The rate of such surgeries involving extensive debulking as corpectomy has decreased compared to its frequent use in the past, and the rate of minimally invasive surgery such as separation surgery to remove only the mass surrounding the cord has increased.12,13) Although there is a concern of local recurrence in the case of a large residual tumor, no significant effect of the extent of tumor removal on symptomatic recurrence was found in this study. In line with this finding, Bate et al.26) compared 48 patients with stereotactic body radiation therapy only and 21 patients with stereotactic body radiation therapy after the debulking of the epidural and anterior columns and reported a lack of clear effect of additional resection on the improvement of local control. In contrast, Pennington et al.22) compared 85 patients with anterior debulking and 12 patients with epidural mass resection only and reported a difference in local recurrence. In the group with epidural mass resection only, 5 patients (41.7%) showed recurrence, while 12 patients (14.1%) showed recurrence in the group with anterior debulking, indicating a significantly higher rate of recurrence in the former group. However, in the survival analysis with the concept of time, the statistical difference in the rate of local recurrence was only significant for patients who demonstrated a survival of more than 2 years after surgery. Thus, less invasive surgery such as decompression alone was stated to be as effective as anterior column resection for patients with a life expectancy of less than 2 years.22) Similarly, in the current study, tumor resection up to the anterior column was shown to have no further benefits in lowering the rate of recurrence when all 3 columns of the vertebra were affected. As a result, aggressive tumor resection is limited and could be considered in cases with long life expectancy or small tumor burden with low risk of contamination during tumor removal.
This study has several limitations. Above all, as this was a retrospective study on patients at a single center, the study may have inevitable bias. Specifically, the classification of tumor removal into 3 types relied on descriptions from surgical records, which inherently introduces retrospective limitations. To improve objectivity, the degree of anterior debulking was categorized based on an objective surgical method, namely the presence of anterior support. Furthermore, the inclusion of various primary cancer types and the variability in combined therapy according to individual patient cases add complexity. However, the determination of surgical treatment and combined therapy was based on predefined criteria established through multidisciplinary discussions. Additionally, potential factors influencing recurrence were adjusted for through multivariate analysis. Lastly, routine radiography including magnetic resonance imaging was not performed to check for local progression. Given that only cases of symptomatic recurrence were investigated in this study, there might be an underestimation when compared to instances of actual local progression without accompanying symptoms. Further prospective studies should be conducted to minimize these limitations. The significance of this study lies in its role as a starting point for providing supporting evidence for a less invasive approach, primarily in metastatic spinal tumors, while considering the patient’s systemic state and metastatic tumor burden.
For metastatic spinal tumors affecting all 3 columns of the vertebral body, the predicted SLR after surgical treatment was 21.5% in the first year and 42.7% in the second year. Anterior debulking to maximize the extent of tumor removal did not influence the SLR. Only surgery for progressed tumor after radiation therapy has been shown to be a critical risk factor of SLR. Therefore, the tumor’s response to preoperative radiation therapy is the most important factor in determining the SLR.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Rothrock RJ, Barzilai O, Reiner AS, et al. Survival trends after surgery for spinal metastatic tumors: 20-year cancer center experience. Neurosurgery. 2021;88(2):402–412. doi: 10.1093/neuros/nyaa380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klimo P, Jr, Thompson CJ, Kestle JR, Schmidt MH. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7(1):64–76. doi: 10.1215/S1152851704000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amelot A, Terrier LM, Mazeron JJ, et al. Timeline metastatic progression: in the wake of the « seed and soil » theory. Med Oncol. 2017;34(11):185. doi: 10.1007/s12032-017-1045-8. [DOI] [PubMed] [Google Scholar]
- 4.Perrin RG, Laxton AW. Metastatic spine disease: epidemiology, pathophysiology, and evaluation of patients. Neurosurg Clin N Am. 2004;15(4):365–373. doi: 10.1016/j.nec.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Chang SY, Mok S, Park SC, Kim H, Chang BS. Treatment strategy for metastatic spinal tumors: a narrative review. Asian Spine J. 2020;14(4):513–525. doi: 10.31616/asj.2020.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciftdemir M, Kaya M, Selcuk E, Yalniz E. Tumors of the spine. World J Orthop. 2016;7(2):109–116. doi: 10.5312/wjo.v7.i2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavalparit P, Wilartratsami S, Santipas B, Ittichaiwong P, Veerakanjana K, Luksanapruksa P. Development of machine-learning models to predict ambulation outcomes following spinal metastasis surgery. Asian Spine J. 2023;17(6):1013–1023. doi: 10.31616/asj.2023.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Truong VT, Al-Shakfa F, Roberge D, et al. Assessing the performance of prognostic scores in patients with spinal metastases from lung cancer undergoing non-surgical treatment. Asian Spine J. 2023;17(4):739–749. doi: 10.31616/asj.2022.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinojosa-Gonzalez DE, Roblesgil-Medrano A, Villarreal-Espinosa JB, et al. Minimally invasive versus open surgery for spinal metastasis: a systematic review and meta-analysis. Asian Spine J. 2022;16(4):583–597. doi: 10.31616/asj.2020.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007;32(2):193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs IC, Kamnerdsupaphon P, Ryu MR, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82(2):185–190. doi: 10.1016/j.radonc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Laufer I, Bilsky MH. Advances in the treatment of metastatic spine tumors: the future is not what it used to be. J Neurosurg Spine. 2019;30(3):299–307. doi: 10.3171/2018.11.SPINE18709. [DOI] [PubMed] [Google Scholar]
- 13.Moussazadeh N, Laufer I, Yamada Y, Bilsky MH. Separation surgery for spinal metastases: effect of spinal radiosurgery on surgical treatment goals. Cancer Control. 2014;21(2):168–174. doi: 10.1177/107327481402100210. [DOI] [PubMed] [Google Scholar]
- 14.Hong SH, Chang BS, Kim H, Kang DH, Chang SY. An updated review on the treatment strategy for spinal metastasis from the spine surgeon’s perspective. Asian Spine J. 2022;16(5):799–811. doi: 10.31616/asj.2022.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knoll P, Lenschow M, Lenz M, et al. Local recurrence and development of spinal cord syndrome during follow-up after surgical treatment of metastatic spine disease. Cancers (Basel) 2023;15(19):4749. doi: 10.3390/cancers15194749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;2017:PO.17.00011. doi: 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 18.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 19.Choi D, Crockard A, Bunger C, et al. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J. 2010;19(2):215–222. doi: 10.1007/s00586-009-1252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aizenberg MR, Fox BD, Suki D, McCutcheon IE, Rao G, Rhines LD. Surgical management of unknown primary tumors metastatic to the spine. J Neurosurg Spine. 2012;16(1):86–92. doi: 10.3171/2011.9.SPINE11422. [DOI] [PubMed] [Google Scholar]
- 21.Chataigner H, Onimus M. Surgery in spinal metastasis without spinal cord compression: indications and strategy related to the risk of recurrence. Eur Spine J. 2000;9(6):523–527. doi: 10.1007/s005860000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennington Z, Pairojboriboon S, Chen X, et al. Utility of expanded anterior column resection versus decompression-alone for local control in the management of carcinomatous vertebral column metastases undergoing adjuvant stereotactic radiotherapy. Spine J. 2022;22(5):835–846. doi: 10.1016/j.spinee.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Lau D, Than KD, La Marca F, Park P. Independent predictors for local recurrence following surgery for spinal metastasis. Acta Neurochir (Wien) 2014;156(2):277–282. doi: 10.1007/s00701-013-1973-9. [DOI] [PubMed] [Google Scholar]
- 24.Supe SS, Ganesh KM, Naveen T, Jacob S, Sankar BN. Spinal cord response to altered fractionation and re-irradiation: radiobiological considerations and role of bioeffect models. J Cancer Res Ther. 2006;2(3):105–118. doi: 10.4103/0973-1482.27597. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi T, Murakami H, Demura S, et al. Risk factors for local recurrence after total en bloc spondylectomy for metastatic spinal tumors: a retrospective study. J Orthop Sci. 2018;23(3):459–463. doi: 10.1016/j.jos.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Bate BG, Khan NR, Kimball BY, Gabrick K, Weaver J. Stereotactic radiosurgery for spinal metastases with or without separation surgery. J Neurosurg Spine. 2015;22(4):409–415. doi: 10.3171/2014.10.SPINE14252. [DOI] [PubMed] [Google Scholar]