Abstract
Backgroud
Heterotopic ossification (HO) is difficult to characterize and classify on simple radiographs. Therefore, we attempted to evaluate intraobserver and interobserver reliability of simple radiography and computed tomography (CT) for detecting and classifying HO after reverse shoulder arthroplasty (RSA). It was hypothesized that CT would provide more reliable results than simple radiography.
Methods
This retrospective study reviewed 30 patients who underwent RSA performed by a single surgeon. Patients were included if they had both postoperative simple radiographs and CT images taken immediately after surgery and at 1 year after surgery and if they had completed clinical assessment at least 1 year after surgery. We first evaluated the intraobserver and interobserver reliability for the detection of the presence of HO and Modified Brooker’s classification both on simple radiographs and CT scans with the use of Kappa statistics. Then, we analyzed the correlation of HO observed in simple radiographs and CT scans with clinical outcomes. All radiographic evaluations were performed by 2 independent reviewers in random orders with 3 weeks of intervals.
Results
The intraobserver reliability outcomes of both reviewers in simple radiography and CT were almost perfect or perfect for the detection of HO and classification. However, CT images improved the interobserver reliability for the detection of HO (kappa value for simple radiographs [KXR] = 0.6018 and kappa value for CT [KCT] = 0.8316) and classification (KXR = 0.5300 and KCT = 0.6964). At a mean follow-up of 25 months (range, 12–54 months), clinical scores were not significantly different according to the presence of HO based on simple radiographs. However, when CT images were used, the University of California, Los Angeles score and physical component score of short-form 36-item health survey were significantly lower in patients with HO than in patients without HO (27.0 vs. 30.4, p = 0.045 and 57.6 vs. 70.7, p = 0.034, respectively).
Conclusions
Both simple radiography and CT provided excellent intraobserver reliability for detecting and classifying HO after RSA. Compared to simple radiography, CT tended to improve interobserver reliability and defined the presence and severity of HO more clearly.
Keywords: Acetubulum, Fracture, Outcome, Complication, Prognostic factor
Heterotopic ossification (HO) can occur frequently in the orthopedic field, especially after arthroplasty. The reported incidence rate of HO is 5% to 30.9% after hip arthroplasty1,2,3) and 3.8% to 39% after knee arthroplasty.4,5,6) The reported incidence after shoulder arthroplasty ranges from 1.6% to 29.5%.7,8,9,10) The development of HO after arthroplasty has a clinical impact on the outcomes. Mild HO is generally asymptomatic, but higher-grade HO can lead to severe pain, limited mobility, functional impairment, and overall reduced quality of life.11) In 10% of hip arthroplasty3,12,13) and 2% to 8% of knee arthroplasty,4,5,6) severe pain and functional limitation due to HO occurred. However, this issue has not been frequently studied in shoulder arthroplasty.
HO is difficult to characterize and classify based on simple radiographs, especially when evaluating the HO in patients whose structures are overlapped by the complex metal components of arthroplasty. Computed tomography (CT) reliably demonstrates the bone structure and facilitates 3-dimensional (3D) evaluations using multiple slices in axial, coronal, and sagittal planes. Thus, it can reduce the potential ambiguity of image interpretation even in metal-implanted regions. A previous study on total ankle arthroplasty suggested that CT scans can be more accurate for diagnosing HO around the tibial and talar metal components.14) However, we are unaware of studies addressing the question of whether CT imaging can clearly improve the reliability of HO assessment after reverse shoulder arthroplasty (RSA).
In this study, we attempted to evaluate the intraobserver and interobserver reliability of simple radiography and CT for detecting and classifying HO after RSA. It was hypothesized that CT would provide more reliable results than simple radiography.
METHODS
The study was approved by the Institutional Review Board of Nowon Eulji Medical Center, Eulji University (IRB No. 2021-05-006). Informed consent was waived due to the retrospective nature of this study.
Patients
We retrospectively reviewed radiographic images and medical charts of 30 patients who underwent a primary RSA between March 2014 and February 2019. Patients were included if they (1) had postoperative shoulder joint simple radiographs and CT images immediately after surgery and at 1 year after surgery and (2) completed clinical assessment at least 1 year after surgery. Exclusion criteria were patients without postoperative simple radiographs and CT scans immediately or 1 year after surgery, patients who were lost to follow-up, and arthroplasties for revision or chronic infectious sequelae. The availability of immediate CT scans after surgery was chosen as one of the inclusion criteria. In simple radiograph and CT image analysis for HO, bone debris can be a concern, which often remains during the procedure of osteophyte removal or glenoid reaming, as well as procedures in patients with proximal humeral fractures. To distinguish it from a newly developed HO, immediate postoperative x-ray and CT images were used for comparison with final follow-up images. Only bone that had formed after surgery was considered to represent HO; bone debris or osteophytes present immediately after surgery were not considered HO.14)
The study cohort consisted of 7 men and 23 women with a mean age of 73 years (range, 59–84 years). The primary diagnosis was cuff tear arthropathy or irreparable rotator cuff tear in 19 patients, displaced proximal humeral fracture in 6, chronic shoulder dislocation with glenoid bone loss in 3, and osteoarthritis in 2. Four implant systems were used, including 16 Ascend Flex (Wright Medical), 6 Reverse Shoulder Prosthesis (DJO surgical), 5 Equinoxe reverse shoulder (Exactech), and 3 Aequalis Reversed shoulder prosthesis fracture stem (Wright Medical). The mean time to the immediate postoperative CT scan and the last follow-up CT scan was 2.3 days (range, 1–16 days) and 14 months (range, 12–16 months), respectively. The mean duration of follow-up for clinical assessment was 25 months (range, 12–54 months).
All surgeries were performed by a single surgeon (TKL) in a beach chair position under general anesthesia. Routine surgical procedures of RSA were performed according to the standard technique and the manufacturer’s guidelines of each system, such as deltopectoral approach for exposure, biceps tenodesis, subscapularis tenotomy, anatomic humeral neck cutting using the extramedullary guide, osteophyte removal, circumferential capsular release, glenoid preparation and reaming, baseplate positioning, glenosphere fixation, and humeral stem insertion. A cementless press-fit humeral stem was used in most cases, while cemented fixation was performed in proximal humeral fractures or some osteoporotic patients. The subscapularis was repaired whenever possible via No. 5 Ethibond sutures (Ethicon Inc.) in a transosseous fashion. A closed suction drain was routinely used to prevent hematoma formation. The postoperative rehabilitation protocol was also identical in all patients. An abduction brace was applied for 4 weeks postoperatively with elbow and finger exercises. A stretching exercise of the shoulder joint was started after the removal of the brace and strengthening exercises 3 months postoperatively. Patients were followed up at 1, 3, 6, and 12 months postoperatively and annually thereafter. HO prophylaxis such as low-dose radiation and pharmaceutical therapy was not used in this study.
Imaging
Simple radiographs were taken in a standard fashion, including true anteroposterior (Grashey’s view), axial, and supraspinatus outlet views preoperatively and during the follow-up. CT scans were performed with a dual-energy 64-channel multi-detector CT scanner (Discovery CT 750HD; GE Healthcare) employing 140 kVp, rotation time of 0.5 seconds, and collimation of 40 mm using gemstone spectral imaging, 250- to 250-mm fields of view, pitch 1.0, and 0.625-mm slice thickness acquisition detail. Images were reconstructed in the axial plane (2.5-mm slice thickness) and in the coronal and sagittal planes (2-mm slice thickness each). Additional 3D images were also acquired. Specifically, gemstone spectral imaging with metal artifact reduction algorithm, fast-kV switching between 80 kV and 140 kV, was used to reduce metal artifacts and improve image quality around the reverse prosthesis.15,16)
Radiographic Evaluation
Radiographic analysis was performed by 2 independent observers (experienced musculoskeletal subspecialty radiologist (YSC), observer 1, who was not involved in the care of patients; and the treating orthopedic surgeon (TKL), observer 2). First, all radiographic images of simple radiographs and CT scans immediately after surgery and at the follow-up period were extracted from the Picture Archiving Computerized System, saved in DICOM file format with patient information removed, and delivered to the 2 observers. They read these images using a DICOM viewer software (Radiant). All images were subjected to 2 rounds of evaluation at 3-week intervals in a blinded and randomized fashion. The observers performed a second reading using DICOM images rearranged in random order at 3 weeks after completion of the primary review. The presence or absence of HO was recorded, and the severity of HO was classified according to Modified Brooker’s classification. The Brooker’s classification was originally designed for the hip joint3,17) and modified to the shoulder joint for this study. This modified Brooker’s classification system divides the extent of HO formation into 4 grades: grade I, small separate small foci of bony islands within the soft tissues about the shoulder joint (Fig. 1); grade II, ossifications projecting from the proximal humerus or scapula with at least 1 cm between opposing bone surfaces; grade III, bone spurs originating from the proximal humerus or scapula with a separation distance of less than 1 cm; and grade IV, complete ossification bridging between the proximal humerus and scapula.
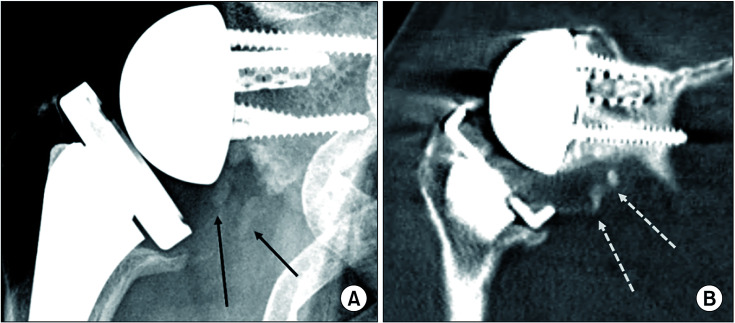
Fig. 1. A 73-year-old female patient underwent reverse shoulder arthroplasty due to cuff tear arthropathy of the right shoulder. (A) Anteroposterior radiograph at 1 year after surgery showing multiple radio-opaque lesions (black arrows) below the scapular neck (modified Brooker’s classification, grade I). (B) Coronal computed tomography scan showing the same lesions (white dotted arrows) as grade I, better visualizing densely ossified lesions.
Based on this review, we analyzed the intraobserver and interobserver reliability for the detection of HO and Modified Brooker’s classification both on simple radiographs and CT scans. After 2 rounds of reviews were complete, the 2 observers openly discussed the cases until a consensus was reached. Based on this consensus reading, we calculated the agreement for the diagnosis of HO and modified Brooker’s classification between simple radiographs and CT images and analyzed the correlation of HO in x-rays and CT scans with clinical outcomes.
Clinical Evaluation
Based on medical chart reviews, demographic data were collected, including age, sex, arm dominance, traumatic onset, diabetes mellitus, thyroid disease, demand of shoulder activity during job performance, and demand of shoulder activity during sports or hobby. Our routine clinical assessment after RSA includes the visual analog scale for pain, the American Shoulder and Elbow Surgeon (ASES)’s score, the University of California, Los Angeles (UCLA) score, short-form 36-item health survey (SF-36), and range of motion. An independent research assistant who was not involved in surgery evaluated all outcome measures and collected all data.
Statistical Analysis
The kappa statistics was used to estimate the intraobserver reliability for the detection of HO and modified Brooker’s classification (weighted kappa for multiple categories). Interpretation was performed according to the guidelines of Landis and Koch with values of 0.00 to 0.20 indicating slight agreement; 0.21 to 0.40, fair agreement; 0.41 to 0.60, moderate agreement; 0.61 to 0.80, substantial agreement; and ≥ 0.81, almost perfect agreement. Zero represents no agreement and 1.00, perfect agreement. Interobserver reliability between the 2 observers was calculated with the use of the multi-rater agreement coefficient measure (AC1 statistic) described by Gwet.18) The analysis of interobserver reliability was based on the first rounds of observations to prevent recall bias. Finally, clinical scores were compared using the student t-test or Mann-Whitney U-test for the continuous variables and chi-square test or Fisher exact test for the categorical variables to determine the difference according to the detection of HO by x-ray and CT. SAS version 9.4 (SAS Institute Inc.) was used for data analysis. The significance level was set at p = 0.05.
RESULTS
Detection of HO
The intraobserver reliability for the detection of HO by observer 1 was almost perfect (kappa value for simple radiographs [KXR1] = 0.9296) when using simple radiographs (Table 1). On the CT images, it was improved to perfect (kappa value for CT [KCT1] = 1.0000). The intraobserver reliability by observer 2 was also almost perfect both with simple radiographs (KXR2 = 0.8667) and CT images (KCT2 = 0.9268). Interobserver reliability for the detection of HO was only substantial on simple radiographs (KXR = 0.6018) but improved to almost perfect in CT images (KCT = 0.8316).
Table 1. Intraobserver and Interobserver Reliability Test Results of Simple Radiography and Computed Tomography.
| Variable | Observer | Simple radiography | Computed tomography | |||||
|---|---|---|---|---|---|---|---|---|
| Kappa | 95% CI | Category | Kappa | 95% CI | Category | |||
| Intraobserver reliability | ||||||||
| Heterotopic ossification | 1 | 0.9296 | 0.7942–1.0000 | Almost perfect | 1.0000 | 1.0000–1.0000 | Perfect | |
| 2 | 0.8667 | 0.6897–1.0000 | Almost perfect | 0.9268 | 0.7862–1.0000 | Almost perfect | ||
| Modified Brooker’s classification | 1 | 0.9652 | 0.8973–1.0000 | Almost perfect | 1.0000 | 1.0000–1.0000 | Perfect | |
| 2 | 0.8588 | 0.7230–1.0000 | Almost perfect | 0.9026 | 0.7639–1.0000 | Almost perfect | ||
| Interobserver reliability | ||||||||
| Heterotopic ossification | 1 and 2 | 0.6018 | 0.3108–0.8928 | Substantial | 0.8316 | 0.6377–1.0000 | Almost perfect | |
| Modified Brooker’s classification | 1 and 2 | 0.5300 | 0.3001–1.0000 | Moderate | 0.6964 | 0.4658–0.8969 | Substantial | |
Modified Brooker’s Classification
Similarly, intraobserver reliability for the classification of HO by observer 1 was almost perfect (KXR1 = 0.9652) when using simple radiographs (Table 1). On the CT images, it was improved to perfect (KCT1 = 1.0000). The intraobserver reliability by observer 2 was also almost perfect both with simple radiographs (KXR2 = 0.8588) and CT images (KCT2 = 0.9026). However, interobserver reliability for the classification was only moderate in simple radiographs (KXR = 0.5300) but improved to substantial in CT images (KCT = 0.6964).
Agreement between Simple Radiography and CT
Based on the consensus reading of simple radiographs, HO was found in 19 patients (63%) including 10 patients with modified Brooker’s classification grade I, 8 with grade II, and 1 with grade III. On the other hand, CT images demonstrated HO in 23 patients (77%) including 10 patients with grade I, 12 with grade II, and 1 with grade III. In terms of agreement between simple radiographs and CT scans, x-ray results showed substantial agreement (Kappa, 0.6891; 95% CI, 0.4196–0.9587) for the detection of HO with CT images, and this was the same for modified Brooker’s classification (Kappa 0.7241; 95% CI, 0.5144–0.9339). Six patients showed disagreement between simple radiographs and CT scans (Fig. 2). In 4 of them, HO was detected only on CT scans, including 2 with grade I and 2 with grade II, and HOs of the remaining 2 patients were upgraded to grade II on CT from grade I on simple radiographs. The lesions on their CT images were more radiodense and the extent of ossification on the axial and sagittal images was clearer. Therefore, CT scans tended to define HO more accurately and even more readily define higher-grade HO, compared to simple radiographs.
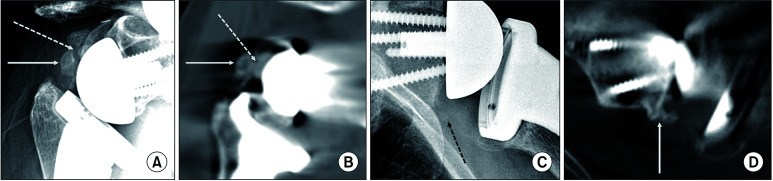
Fig. 2. Examples of discrepancy between simple radiography and computed tomography (CT). (A) The simple radiograph of a 67-year-old female patient at 1 year after surgery exhibited large heterotopic ossification between the glenosphere and proximal humerus (white arrow), which appeared as a bony island unconnected to the glenoid (white dotted arrow; modified Brooker’s classification, grade I). However, the CT image demonstrated the same ossifying lesion projecting from the posterior glenoid neck (B, white dotted arrow) as a grade II lesion. (C) The simple radiograph of a 67-year-old male patient showed a faint radio-opaque lesion below the scapular neck (black dotted arrow), but it was not clear due to the overlapped ribs. It was read as grade I by observer 1 and grade 0 by observer 2. However, the CT image clearly visualized the grade II heterotopic ossification projecting from the inferior glenoid neck (D, white arrow).
When clinical outcomes were correlated with the consensus reading results, all clinical scores were not significantly different according to the presence of HO in simple radiographs (Table 2). However, when used in CT results (Table 3), the UCLA score and physical component score of SF-36 were significantly lower in patients with HO than patients without HO (27.0 vs. 30.4, p = 0.0435 and 57.6 vs. 70.7, p = 0.0335, respectively).
Table 2. Clinical Outcomes According to the HO on Simple Radiographs.
| Variable | No HO (n = 11) | Yes HO (n = 19) | p-value | |
|---|---|---|---|---|
| Age (yr) | 73.9 ± 4.9 | 73.4 ± 6.9 | 0.857 | |
| Sex | ||||
| Male | 1 | 6 | 0.215 | |
| Female | 10 | 13 | ||
| Primary diagnosis | 0.445 | |||
| CTA or MRCT | 8 | 11 | ||
| Proximal humerus Fx | 2 | 4 | ||
| Chronic shoulder D/L with glenoid bone loss | 1 | 2 | ||
| Osteoarthritis | 0 | 2 | ||
| Implant system | 0.966 | |||
| Ascend Flex | 6 | 10 | ||
| DJO | 2 | 4 | ||
| Equinoxe Eactech | 2 | 3 | ||
| Aqualis Fx stem | 1 | 2 | ||
| Follow-up period (mo) | 23.2 ± 10.5 | 24.4 ± 13.7 | 0.790 | |
| VAS pain score | 2.2 ± 1.8 | 2.3 ± 1.8 | 0.893 | |
| ASES score | 87.6 ± 8.9 | 83.5 ± 13.4 | 0.382 | |
| UCLA score | 29.6 ± 3.7 | 26.7 ± 7.1 | 0.155 | |
| SF-36 MCS | 69.6 ± 15.1 | 69.8 ± 15.7 | 0.971 | |
| SF-36 PCS | 67.7 ± 16.9 | 56.5 ± 21.4 | 0.151 | |
| FF | 127.7 ± 24.3 | 132.8 ± 24.6 | 0.583 | |
| ER | 55.9 ± 16.8 | 48.1 ± 26.4 | 0.390 | |
| IR | 5.2 ± 2.6 | 4.4 ± 2.4 | 0.387 | |
Values are presented as mean ± standard deviation.
HO: heterotopic ossification, CTA: cuff tear arthropathy, MRCT: massive rotator cuff tear, Fx: fracture, D/L: dislocation, VAS: visual analog score, ASES: American shoulder and Elbow Surgeons, UCLA: University of California, Los Angeles score, SF-36 MCS: short-form 36-item mental component score, SF-36 PCS: short-form 36-item physical component score, FF: forward flexion, ER: external rotation, IR: internal rotation.
Table 3. Clinical Outcomes According to the HO on Computed Tomography.
| Variable | Heterotopic ossification on computed tomography | p-value | ||
|---|---|---|---|---|
| No HO (n = 7) | Yes HO (n = 23) | |||
| Age (yr) | 75.0 ± 5.0 | 73.2 ± 6.5 | 0.517 | |
| Sex | 1.000 | |||
| Male | 1 | 6 | ||
| Female | 6 | 17 | ||
| Primary diagnosis | 0.886 | |||
| CTA or MRCT | 4 | 15 | ||
| Proximal humerus Fx | 2 | 4 | ||
| Chronic shoulder D/L with glenoid bone loss | 1 | 2 | ||
| Osteoarthritis | 0 | 2 | ||
| Implant system | 0.962 | |||
| Ascend Flex | 4 | 12 | ||
| DJO | 1 | 5 | ||
| Equinoxe Eactech | 1 | 4 | ||
| Aqualis Fx stem | 1 | 2 | ||
| Follow-up period (mo) | 21.0 ± 5.1 | 24.9 ± 13.9 | 0.478 | |
| VAS pain score | 2.2 ± 2.0 | 2.3 ± 1.8 | 0.939 | |
| ASES score | 86.8 ± 9.1 | 84.5 ± 12.8 | 0.661 | |
| UCLA score | 30.4 ± 2.1 | 27.0 ± 6.8 | 0.045* | |
| SF-36 MCS | 72.0 ± 12.0 | 69.0 ± 16.2 | 0.661 | |
| SF-36 PCS | 70.7 ± 9.5 | 57.6 ± 21.8 | 0.034* | |
| FF | 118.5 ± 19.7 | 134.7 ± 24.5 | 0.123 | |
| ER | 54.2 ± 16.1 | 50.0 ± 25.3 | 0.678 | |
| IR | 4.2 ± 1.8 | 4.8 ± 2.7 | 0.604 | |
Values are presented as mean ± standard deviation.
HO: heterotopic ossification, CTA: cuff tear arthropathy, MRCT: massive rotator cuff tear, Fx: fracture, D/L: dislocation, VAS: visual analog score, ASES: American Shoulder and Elbow Surgeons, UCLA: University of California, Los Angeles score, SF-36 MCS: short-form 36-item mental component score, SF-36 PCS: short-form 36-item physical component score, FF: forward flexion, ER: external rotation, IR: internal rotation.
*Statistically significant.
DISCUSSION
The aim of this study was to evaluate the intraobserver and interobserver reliability of simple radiographs and CT for detecting and classifying HO after RSA. Our hypothesis was that CT would be more reliable than simple radiography. To address this issue, we conducted standardized, blinded, and randomized image readings by 2 independent observers and compared CT images taken immediately after surgery and at the final follow-up to minimize the likelihood of misdiagnosing the remaining small bony fragments as HO. With this study design, we demonstrated that the intraobserver reliability outcomes of both reviewers in simple radiography and CT were almost perfect or perfect for the detection of HO and classification. CT tended to improve the interobserver reliability for the detection of HO (KXR = 0.6018 and KCT = 0.8316) and classification (KXR = 0.5300 and KCT = 0.6964). While CT has been used in the evaluation of HO in other joints,19,20,21,22) to the best of our knowledge, no studies have assessed the reliability of simple radiography and CT for the evaluation of HO after RSA.
Several studies on HO after RSA have reported incidences between 1.6% and 29.5%.7,8,9,10) Recently, a systematic review of modern RSA focusing on complications showed 0.8% of incidence among 5,529 shoulders in 74 studies.23) This wide range of incidence rates might be related to a lack of standardized definition, diagnostic criteria, and imaging modality of choice for the evaluation of HO after shoulder arthroplasty.24) Furthermore, the intraobserver and interobserver reliability test results were rarely reported. Verhofste et al.9) reported a good agreement for HO evaluation in 132 patients who received an RSA. With the use of simple radiography, the average intraobserver reliability was high for the detection and grading of HO (K = 0.932 and K = 0.904, respectively) and interobserver reliability was also high (K = 0.861 and K = 0.727, respectively). Two observers, experienced senior shoulder surgeons, were involved in the HO evaluation. Those outcomes were similar to our study results of simple radiographs.
The utilization of CT for the evaluation of HO has several advantages. Compared to simple radiography, CT has superior spatial and contrast resolution, which enables the detection of subtle focal mineralization in the earlier stages of ossification.20,22) This may be attributed to the good outcomes of intraobserver and interobserver reliability tests by CT in our study. Typical CT findings of soft-tissue density of lower attenuation than muscle in an early stage of ossification,20) as well as multi-planar evaluation, may have also resulted in successfully detecting 2 cases of grade I HOs on CT, which were not found on simple radiographs, and 4 cases of higher classification grades. Therefore, our study may reinforce the previous knowledge that CT defines the presence and severity of HO more clearly than other techniques do.19,20,22) The discrepancy between simple radiography and CT for the HO evaluation was also reported on the hip joint. Mary Jiayi et al.21) presented specific problems in Brooker’s classification of the hip joint in comparison of simple radiography and CT, including superimposing structures, variations in radiographic techniques, and unaccountability for differences in volumetric measurements of HO and arthroplasty lengths. For similar reasons, simple radiography could underestimate the incidence and severity of HO after RSA in our study. Metal artifacts on CT images are problematic and might have influenced the outcomes of this study. However, we facilitated image interpretation using the specified CT protocols, which have been known to be useful in minimizing the potential artifacts generated by complex reverse arthroplasty.15) The potential disadvantages of CT are the time and cost, as well as the radiation exposure.
Notably, patients with CT imaging-based HO had significantly lower clinical scores than patients without CT imaging-based HO, but this correlation of HO with clinical outcome was not seen when simple radiographs were used. Inferior functional outcomes measured by the UCLA score and physical component scores of SF-36 may reflect an adverse effect of CT imaging-based HO on shoulder function and quality of life after RSA in our patients. Since there have been no studies on the association of CT imaging-based HO with the clinical outcomes after shoulder arthroplasty, our results cannot be compared with others. However, a similar finding was observed in a previous study evaluating HO after RSA based on simple radiographs. Only grade 2 HO was clinically relevant with a negative effect on the shoulder function.25) Those findings may challenge the opinion that most cases of HO that develop after RSA are benign.23) However, the effect of HO on the clinical outcome after RSA is still inconclusive because our study has a small number of cases and outcomes after RSA can be related to multiple factors, not solely to the development of HO. Instead, it can be only suggested that compared to simple radiographs, CT scans may be more useful for diagnosing clinically significant HO that might be associated with clinical scores after RSA. This issue deserves further investigation in the future with a sufficiently large number of patients and multivariate analysis.
This study has several limitations. First, the study cohort is inhomogeneous because mixed preoperative diagnoses were included, and different implant systems were used. It is unclear how HO depends on preoperative diagnosis such as cuff tear arthropathy or proximal humeral fracture, and the implant systems. Previously, a higher incidence of HO in 121 patients with cuff tear arthropathy (36.4%) than in other pathologies (10.7%–4.6%) was reported after hemi- or anatomic total shoulder arthroplasty.25) Therefore, studies with a more homogeneous group may help interpret the results. Second, the 2 observers (a musculoskeletal radiologist and an orthopedic surgeon) with different specialties reviewed the images. The evaluation of different observers can be always limited by differences in training, experience, and especially understanding of specific bone and soft-tissue images surrounding the complex metal implants of RSA. Although the study background, an appropriate description and examples of HO, and Brooker’s classification diagram taken from the original publication were shared and educated prior to the evaluation session, this issue likely affected the outcome of the interobserver reliability. The involvement of the treating surgeon as a reviewer could also make the outcome vulnerable to a detection bias. However, we conducted a blind and randomized review process to minimize this bias and thought that the orthopedic surgeon’s perspective evaluating the HO might be clinically relevant. Third, new bones projecting from the inferior scapular neck can be confused with scapular notching, which is common after RSA. Conversely, there is another opinion that grade 2 scapular notch based on the Nero-Sirveaux classification may be misinterpretation of a bony spur.9) Although it is recommended to evaluate the location, borders, and progression of new bones on radiographs to differentiate notching from HO,9) further studies on this issue are needed. Finally, this study lacks a gold standard for the diagnosis of HO such as surgical exploration. Therefore, it is not possible to further evaluate the diagnostic performances of simple radiographs and CT.
Both simple radiographs and CT images provided excellent intraobserver reliability for detecting and classifying HO after RSA. Compared to simple radiography, CT tended to improve interobserver reliability and defined the presence and severity of HO more clearly.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Rama KR, Vendittoli PA, Ganapathi M, Borgmann R, Roy A, Lavigne M. Heterotopic ossification after surface replacement arthroplasty and total hip arthroplasty: a randomized study. J Arthroplasty. 2009;24(2):256–262. doi: 10.1016/j.arth.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Fransen M, Neal B, Cameron ID, et al. Determinants of heterotopic ossification after total hip replacement surgery. Hip Int. 2009;19(1):41–46. doi: 10.1177/112070000901900108. [DOI] [PubMed] [Google Scholar]
- 3.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement: incidence and a method of classification. J Bone Joint Surg Am. 1973;55(8):1629–1632. [PubMed] [Google Scholar]
- 4.Toyoda T, Matsumoto H, Tsuji T, Kinouchi J, Fujikawa K. Heterotopic ossification after total knee arthroplasty. J Arthroplasty. 2003;18(6):760–764. doi: 10.1016/s0883-5403(03)00194-3. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa M, Ohashi T, Uchida A. Heterotopic ossification around distal femur after total knee arthroplasty. Arch Orthop Trauma Surg. 2002;122(5):274–278. doi: 10.1007/s00402-001-0377-0. [DOI] [PubMed] [Google Scholar]
- 6.Furia JP, Pellegrini VD., Jr Heterotopic ossification following primary total knee arthroplasty. J Arthroplasty. 1995;10(4):413–419. doi: 10.1016/s0883-5403(05)80139-1. [DOI] [PubMed] [Google Scholar]
- 7.Giuseffi SA, Streubel P, Sperling J, Sanchez-Sotelo J. Short-stem uncemented primary reverse shoulder arthroplasty: clinical and radiological outcomes. Bone Joint J. 2014;96(4):526–529. doi: 10.1302/0301-620X.96B3.32702. [DOI] [PubMed] [Google Scholar]
- 8.Ji JH, Jeong JY, Song HS, et al. Early clinical results of reverse total shoulder arthroplasty in the Korean population. J Shoulder Elbow Surg. 2013;22(8):1102–1107. doi: 10.1016/j.jse.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Verhofste B, Decock T, Van Tongel A, De Wilde L. Heterotopic ossification after reverse total shoulder arthroplasty. Bone Joint J. 2016;98(9):1215–1221. doi: 10.1302/0301-620X.98B9.37761. [DOI] [PubMed] [Google Scholar]
- 10.Werner BS, Abdelkawi AF, Boehm D, et al. Long-term analysis of revision reverse shoulder arthroplasty using cemented long stems. J Shoulder Elbow Surg. 2017;26(2):273–278. doi: 10.1016/j.jse.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Garland DE. A clinical perspective on common forms of acquired heterotopic ossification. Clin Orthop Relat Res. 1991;(263):13–29. [PubMed] [Google Scholar]
- 12.Charnley J. The long-term results of low-friction arthroplasty of the hip performed as a primary intervention. J Bone Joint Surg Br. 1972;54(1):61–76. [PubMed] [Google Scholar]
- 13.Ritter MA, Vaughan RB. Ectopic ossification after total hip arthroplasty: predisposing factors, frequency, and effect on results. J Bone Joint Surg Am. 1977;59(3):345–351. [PubMed] [Google Scholar]
- 14.Lee KB, Cho YJ, Park JK, Song EK, Yoon TR, Seon JK. Heterotopic ossification after primary total ankle arthroplasty. J Bone Joint Surg Am. 2011;93(8):751–758. doi: 10.2106/JBJS.J.00178. [DOI] [PubMed] [Google Scholar]
- 15.Lee DH, Choi YS, Potter HG, et al. Reverse total shoulder arthroplasty: an imaging overview. Skeletal Radiol. 2020;49(1):19–30. doi: 10.1007/s00256-019-03275-0. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Park C, Jeong HS, et al. The optimal combination of monochromatic and metal artifact reconstruction dual-energy CT to evaluate total knee replacement arthroplasty. Eur J Radiol. 2020;132:109254. doi: 10.1016/j.ejrad.2020.109254. [DOI] [PubMed] [Google Scholar]
- 17.Hug KT, Alton TB, Gee AO. Classifications in brief: Brooker classification of heterotopic ossification after total hip arthroplasty. Clin Orthop Relat Res. 2015;473(6):2154–2157. doi: 10.1007/s11999-014-4076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61(Pt 1):29–48. doi: 10.1348/000711006X126600. [DOI] [PubMed] [Google Scholar]
- 19.Bachman DR, Kamaci S, Thaveepunsan S, Park SE, Vasileiadis GI, O’Driscoll SW. Preoperative nerve imaging using computed tomography in patients with heterotopic ossification of the elbow. J Shoulder Elbow Surg. 2015;24(7):1149–1155. doi: 10.1016/j.jse.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 20.Bressler EL, Marn CS, Gore RM, Hendrix RW. Evaluation of ectopic bone by CT. AJR Am J Roentgenol. 1987;148(5):931–935. doi: 10.2214/ajr.148.5.931. [DOI] [PubMed] [Google Scholar]
- 21.Mary Jiayi T, Linda P, Michael P, et al. Potential discrepancy between plain films and CT scans in Brooker classification of heterotopic ossification. Br J Radiol. 2017;90(1080):20170263. doi: 10.1259/bjr.20170263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zagarella A, Impellizzeri E, Maiolino R, Attolini R, Castoldi MC. Pelvic heterotopic ossification: when CT comes to the aid of MR imaging. Insights Imaging. 2013;4(5):595–603. doi: 10.1007/s13244-013-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah SS, Roche AM, Sullivan SW, et al. The modern reverse shoulder arthroplasty and an updated systematic review for each complication: part II. JSES Int. 2020;5(1):121–137. doi: 10.1016/j.jseint.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperling JW, Cofield RH, Rowland CM. Heterotopic ossification after total shoulder arthroplasty. J Arthroplasty. 2000;15(2):179–182. doi: 10.1016/s0883-5403(00)90154-2. [DOI] [PubMed] [Google Scholar]
- 25.Boehm TD, Wallace WA, Neumann L. Heterotopic ossification after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1):6–10. doi: 10.1016/j.jse.2004.04.007. [DOI] [PubMed] [Google Scholar]