Abstract
Percutaneous balloon ganglyolysis (PBG) for trigeminal neuralgia (TN) is an inexpensive and minimally invasive treatment modality that is effective and safe. While there are reports of its efficacy, there is still a lack of evidence of which patients are at a higher risk of treatment failures and needing retreatment. We performed a retrospective study at a major academic institution from 2012 to 2023, including TN patients who underwent PBG procedures to evaluate predictors of retreatment. Patients without imaging available from the PBG were excluded. Fifty-two patients who underwent 83 procedures in total were included in the analysis. All patients had typical TN and were primarily female (59.6%), with a median age of 61.5 years. Immediately after PBG, 42.3% had pain resolution, and 57.7% had improved but persistent pain. 30.8% underwent retreatment with PBG in a median of 32 months. From multiple factors assessed, TN disease duration ≤ 6 months and trigeminal nerve enhancement on pre-operative MRI were identified as significant retreatment predictors on univariate analysis. However, after performing logistic regression, only TN disease duration ≤ 6 months remained significant OR 3.99 (95% CI 1.59–10.0; p = 0.003). This was further confirmed in a Kaplan-Meier survival analysis, which showed that patients with TN duration ≤ 6 months require retreatment earlier (22 vs. 41 months; p = 0.01). Retreatment after PBG occurs roughly in a third of patients, and TN disease duration of ≤ 6 months is an important predictor in this study. Further studies should be performed to confirm these findings, which may impact treatment considerations in the future.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10143-024-03099-0.
Keywords: Percutaneous balloon ganglyolysis, Trigeminal neuralgia, Retreatment
Introduction
Trigeminal Neuralgia (TN) is an uncommon but excruciating neurological condition that typically presents as severe, unilateral, stimulus-evoked, stabbing facial pain [1, 2]. Its incidence is roughly 4–13 per 100,000 individuals, with middle-aged females significantly more affected than males [2, 3]. The condition has been attributed to demyelination of the trigeminal nerve (CNV) at the root entry zone adjacent to the pons, either due to vascular compression or idiopathic causes [4]. Medical management with carbamazepine is considered first-line therapy. Baclofen, tricyclic antidepressants, lamotrigine, and gabapentin are also employed as second and third-line medication choices [2, 4–7]. Procedural intervention is usually considered for individuals whose pain is refractory to medical management or for whom the side effects of these drugs are too debilitating. [4, 7]. Options for intervention include non-ablative procedures such as microvascular decompression (MVD), in which a retrosigmoid craniotomy is performed to approach the CNV root entry zone and gently dissect and relieve the compression effect exerted by a vessel by separating it from the nerve through the placement of a Teflon sponge. Other options include ablative procedures that are less invasive, such as stereotactic radiosurgery (SRS), glycerol rhizotomy, radiofrequency rhizotomy, and percutaneous balloon ganglyolysis. In choosing a specific modality, the surgeon and patient consider a variety of factors, including pre-operative imaging (nerve compression/enhancement), patient comorbidities and functional status, previously applied interventions, and most importantly, the patient’s preferences.
Percutaneous balloon ganglyolysis (initially named percutaneous micro-compression) is an effective surgical intervention in the treatment of TN [3, 4, 8–12]. During this procedure, the surgeon inserts a 14-gauge Tuohy needle through the foramen ovale to reach the Meckel’s cave. Once inside the Gasserian ganglion, the balloon is inflated, inflicting compression injury. This compression is hypothesized to damage the thick myelinated fibers that trigger pain through ephaptic contact with nociceptive fibers while leaving the thin unmyelinated fibers unharmed [1, 3, 6, 13].
PBG has also been shown to be an effective choice for those who experience recurrence following the failure of a different treatment modality [14]. It is an inexpensive and minimally invasive option with minimal risks associated [8]. While there are many reports on its efficacy, there is a lack of understanding of what patient factors contribute to treatment failure and subsequent need for retreatment [13, 15]. This study assesses retreatment predictors for typical TN patients treated with PBG.
Materials and methods
Study design
A retrospective study of patients with typical TN treated with PBG between 2012 and 2023 was performed at a single academic institution. This study defines typical trigeminal neuralgia as unilateral pain in the distribution of the trigeminal nerve that cannot be attributed to a space occupying lesion, multiple sclerosis, or any other medical diagnosis according to the internationally accepted guidelines for the diagnosis of classic trigeminal neuralgia [16]. Patients without fluoroscopic imaging available from the PBG (n = 2), those with a diagnosis of multiple sclerosis (n = 4), and postherpetic neuralgia (n = 1) were excluded. Fifty-two patients who underwent 83 procedures in total (35 retreatments) were included in the analysis. PBG was chosen as the treatment modality for these cases per the senior attending neurosurgeon criteria (blinded for review) after considering a variety of factors, which include risk/benefit discussion, recovery time, age, comorbidities, ability to tolerate a procedure, surgeon experience, prior failed treatment modality, and most importantly patient preference. Furthermore, all patients were treated following this surgeon’s specific protocol. Achievement of the pear-shaped balloon after inflating was confirmed in all cases, and the balloon was inflated for 3 intervals of 30 s in each procedure. Following institution review board approval, the need for patient consent was waived due to the study’s retrospective nature.
Variables and outcomes
Medical charts and neuroimaging reviews were used to collect all relevant variables for analysis. These included patient demographics, pre-operative MRI findings, PBG procedure details, complications, outcomes, retreatment, and the results of radiographic studies. Functional outcomes in the immediate post-procedure period were evaluated according to the documentation on the first post-operative check and were characterized as resolution, persistent but improved, or persistent and unchanged. Pain outcomes at subsequent outpatient follow-up appointments were graded using the Barrow Neurological Institute (BNI) scale to assess facial pain in conjunction with oral medication treatment response or changes [17]. TN disease duration was determined from the patient’s self-reported symptom onset to the day of the procedure. Foramen ovale morphology on fluoroscopy was classified according to the Elnashar et al.. classification [18]. Median annual household income was determined by using each patient’s zip code to find the median income of the areas they reside in through policymap.com, a mapping and analytics platform provided by [blinded for review].
Statistical analysis
Categorical variables are reported as proportions. Continuous variables are reported as mean ± standard deviation (SD) or median [Interquartile range (IQR)] as appropriate according to the distribution of the data. A univariate analysis was used to determine which factors were associated with retreatment. After that, a logistic regression, including factors identified in univariate comparisons, was run to evaluate retreatment risk. The Kaplan-Meier (KM) estimator analysis was used to evaluate time to retreatment. Comparative KM curves were developed to determine the effect of retreatment predictors over time. The log-rank test evaluated statistically significant differences in KM curve distributions. All statistical analyses were performed using the Stata statistical software package 14.0 (StataCorp., College Station, TX).
Results
Baseline patient characteristics
Most patients were female (59.6%), with a median age of 61.5 years (IQR 53–73). Regarding race, 43.5% were Caucasian, 30.4% Black, 19.6% Hispanic, and 6.5% Asian. Additionally, 60.8% had never smoked, 19.6% were former smokers, and 19.6% were current smokers at the time of procedure. The median household income of all the patients was $73,868 (62.068–92.826).
The right-sided trigeminal nerve distribution was affected in 61.5% of the patients, and the pain was present most often in the V2-V3 distribution (42.6%) and least often (10.6%) in V1 and V2 (Table 1). The median TN disease duration from onset to PBG treatment was 36 months. 23.1% of the sample had received prior treatment for TN on the same side. The prior treatments included microvascular decompression (3.8%), radiosurgery (3.8%), and previous balloon ganglyolysis performed before 2012 (7.6%). Some patients had multiple prior treatments performed. Two patients (3.8%) received microvascular decompression, glycerol rhizotomy, and radiosurgery before PBG, while another 2 (3.8%) received microvascular decompression and radiosurgery before PBG.
Table 1.
Trigeminal neuralgia characteristics
| Number of patients | n = 52 |
|---|---|
| Side of TN | |
| Left | 22 (38.5%) |
| Right | 32 (61.5%) |
| Affected dermatomes* | |
| V2 | 6 (12.8%) |
| V3 | 8 (17.0%) |
| V1 and V2 | 5 (10.6%) |
| V2 and V3 | 20 (42.6%) |
| V1, V2 and V3 | 8 (17.0%) |
| Duration of TN (Months) | 36 (11–78) |
| Previous treatments | 12 (23.1%) |
| Microvascular decompression | 2 (16.7%) |
| Radiosurgery | 2 (16.7%) |
| Previous balloon ganglyolysis** | 4 (33.3%) |
| Microvascular decompression, glycerol rhizotomy and radiosurgery | 2 (16.7%) |
| Microvascular decompression and radiosurgery | 2 (16.7%) |
*Missing data: 5
**Patients had prior balloon ganglyolysis performed before 2012 and had treatment failure
Radiological data
Pre-operative MRI was performed on 45 patients. A single individual had a non-MRI compatible ICD and thus did not undergo preop MRI. Imaging could not be accessed in 5 of the 52 patients. For one individual, we could not confirm the presence or absence of an MRI. A vascular loop near CNV was found in 48.9% but with visible compressive effects only in 22.9%. CNV enhancement was seen in 7.1% and was interpreted as evidence of inflammation or scarring from an unknown process unrelated to prior treatments. CNV thinning was present in 10.8%, ipsilateral Meckel’s cave narrowing in 8.1%, and congenital absence of Meckel’s cave in 2.7% of patients (Table 2).We categorized the foramen ovale morphologies on the affected side and found that the oval type was the most prevalent (59.6%; Table 3). Elongated and crescent were equally prevalent at 12.8%. The three least common were round (10.6%), almond (2.1%), and cordate (2.1%). The median maximum diameter of the foramen ovale in the fluoroscopy AP view was 9.35 mm (8.44–10.6), while the median width and height were 9.35 mm (8.44–10.6) and 4.5 mm (3.1–5.2), respectively.
Table 2.
Pre-operative MRI data
| Number of patients | n = 52 |
|---|---|
| Pre-operative MRI available† | n = 45 |
| Presence of vascular loop (artery or vein at the affected side near CNV | 22 (48.9%) |
| Vascular loop compressive to CNV* | 8 (22.9%) |
| Congenital absence of Meckel’s cave | 1 (2.7%) |
| CNV thinning on ipsilateral TN side | 4 (10.8%) |
| Previous Meckel’s cave narrowing ipsilateral to TN side | 3 (8.1%) |
| CNV enhancement on pre-operative MRI*** | 3 (7.1%) |
† 7 patients did not have pre-operative MRI available to review. Five individuals had confirmed MRI, but the imaging was inaccessible to analyze. One individual did not undergo an MRI because they had a non-MRI-compatible ICD. For 1 individual, we could not confirm if a pre-operative MRI was performed
*Missing data: 10 (T2 DRIVE was not available for review)
**Missing data: 3 (MRI brain with contrast was not available)
Table 3.
Procedure and Hospital Course characteristics
| Number of patients | n = 52 |
|---|---|
| Foramen ovale morphology on fluoroscopy* | |
| Oval | 28 (59.6%) |
| Crescent | 6 (12.8%) |
| Cordate | 1 (2.1%) |
| Round | 5 (10.6%) |
| Almond | 1 (2.1%) |
| Elongated | 6 (12.8%) |
| Foramen ovale size (mm) ** | |
| Maximum diameter | 9.25 (8.44–10.5) |
| Width | 9.25 (8.44–10.5) |
| Height | 4.5 (3.9–5.1) |
| Procedure duration (Minutes) | 71 (60–96) |
| Complications | 2 (3.8%) |
| Facial numbness post-procedure*** | 39 (76.5%) |
| mastication difficulties post-procedure *** | 4 (7.8%) |
| TN pain control on post-operative check | |
| Resolution | 22 (42.3%) |
| Persistent but improved | 30 (57.7%) |
| Persistent and unchanged | 0 (0.0%) |
*Missing data: 6
**Missing data: 7
***Missing data: 1
Procedure and hospital course
The median procedure length was 71 min (60–96). A Tuohy needle was inserted into the foramen ovale under fluoroscopic guidance. Upon entering the foramen ovale a decreased sense of resistance was noted and patients typically developed transient self-resolving bradycardia. All included cases achieved the classic pear-like balloon shape which was confirmed using lateral fluoroscopic views. The balloon was kept inflated for 3 intervals of 30 s as is the surgeon’s practice. The complication rate was 3.8%. One individual experienced a post operative episode of bradycardia that resolved spontaneously, while another individual developed a transient CNVI palsy that also resolved without medical intervention. Facial numbness post-procedure was very common (76.5%), while mastication difficulty was only present post-op in 7.8% of individuals and was transient. On post-operative check 4 h post procedure, 42.3% of patients reported complete resolution of pain, while 57.7% reported persistent but improved pain. No one reported an unchanged pain level on the post-operative check (Table 3).
Outcomes
The median time to first outpatient post-operative follow-up was one month (1–2). At this follow-up, 26.1% of patients reported excellent pain relief, 56.5% good pain relief, 13.0% fair relief, and 4.3% poor pain relief (Table 4). The median time to the last follow-up was 5.5 months (1–30.5 months). At this follow-up, 16.7% reported excellent pain control, 39.6% reported good pain control, 12.5% reported fair pain control, and 31.2% (n = 15) reported poor pain control. In total, 30.8% (n = 16) of individuals needed retreatment. Of these individuals, the median number of months to retreatment was 32 (12.5–41.5). Of those who received retreatment, 25% reported excellent pain control on their last follow-up visit, 50% reported good pain control, 12.5% reported fair pain control, and 12.5% reported poor pain control.
Table 4.
Outcomes
| Outcomes | n = 52 |
|---|---|
| Clinical follow-up | n = 47 |
| Months to first follow-up* | 1 (1–2) |
| Pain outcome on first follow-up* | |
| Excellent (No pain,no medications) | 12 (26.1%) |
| Good (no pain with medication) | 26 (56.5%) |
| Fair (pain controlled with medication) | 6 (13.0%) |
| Poor (some pain not adequately controlled with medication) | 2 (4.3%) |
| Very poor (Severe pain or no pain relief) | 0 (0.0%) |
| Months to last follow-up | 5.5 (1–30.5) |
| Pain outcome on last follow-up | |
| Excellent (No pain,no medications) | 8 (16.7%) |
| Good (no pain with medication) | 19 (39.6%) |
| Fair (pain controlled with medication) | 6 (12.5%) |
| Poor (some pain not adequately controlled with medication) | 15 (31.2) |
| Very poor (Severe pain or no pain relief) | 0 (0.0%) |
| Pain outcome on last follow-up | |
| Excellent | 4 (25.0%) |
| Good | 8 (50.0%) |
| Fair | 2 (12.5%) |
| Poor | 2 (12.5%) |
| Retreatment | 16 (30.8%) |
| Months to retreatment | 32 (12.5–41.5) |
*Missing data: 1
Retreatment predictors
We performed univariate analyses between the group of individuals who required retreatment and those who did not to determine predictors of need for retreatment (Table 5). Gender (p = 0.74), age (p = 0.47), smoking status (p = 0.65), median household income (p = 0.79), facial numbness post-procedure (p = 0.87), resolution of TN pain in post-operative check (p = 0.64), foramen ovale morphology (p = 0.21), previous treatment (p = 0.62) were all found to be not related.
Table 5.
Comparison according to Retreatment
| Variables | No retreatment 36 (69.2%) |
Retreated 16 (30.8%) |
p-Value |
|---|---|---|---|
| Female gender | 22 (61.1%) | 9 (56.2%) | 0.74 |
| Age | 60.5 (53.5–73.5) | 62 (51–64.5) | 0.47 |
| Never smoker | 22 (62.9%) | 9 (56.3%) | 0.65 |
| TN duration (months) | 54 (29.5–108) | 24 (4.5–33) | 0.001 |
| Previous treatment | 9 (25.0%) | 3 (18.8%) | 0.62 |
| Vascular loop compressive to CNV* | 7 (31.8%) | 1 (7.7%) | 0.1 |
| Congenital absence of Meckel’s cave** | 0 (0.0%) | 1 (7.1%) | 0.19 |
| CNV thinning on ipsilateral TN side** | 3 (13.0%) | 1 (7.1%) | 0.57 |
| Meckel’s cave narrowing ipsilateral to TN side** | 2 (8.7%) | 1 (7.1%) | 0.87 |
| CNV enhancement on preop MRI | 0 (0.0%) | 3 (21.4%) | 0.01 |
| Foramen ovale morphology*** | |||
| Oval | 18 (58.1%) | 10 (58.8%) | 0.21 |
| Crescent | 6 (19.3%) | 0 (0.0%) | |
| Cordate | 1 (3.2%) | 0 (0.0%) | |
| Round | 2 (6.4%) | 3 (18.7%) | |
| Almond | 0 (0.0%) | 1 (6.2%) | |
| Elongated | 4 (12.9%) | 2 (12.5%) | |
| Foramen ovale maximum diameter*** | 9.35 (8.55–9.89) | 9.19 (7.87–11.08) | 0.74 |
| Facial numbness post-procedure | 27 (77.1%) | 12 (75.0%) | 0.87 |
| Resolution of TN pain in post-operative check | 16 (44.4%) | 6 (37.5%) | 0.64 |
| Annual Household income | 75.491 (62.068–92.826) | 72.300 (62.943–92.826) | 0.79 |
n (%), p-Value: Chi2 test
Median (IQR), p-value: Mann Whitney U test
Bold values: p-Value < 0.05
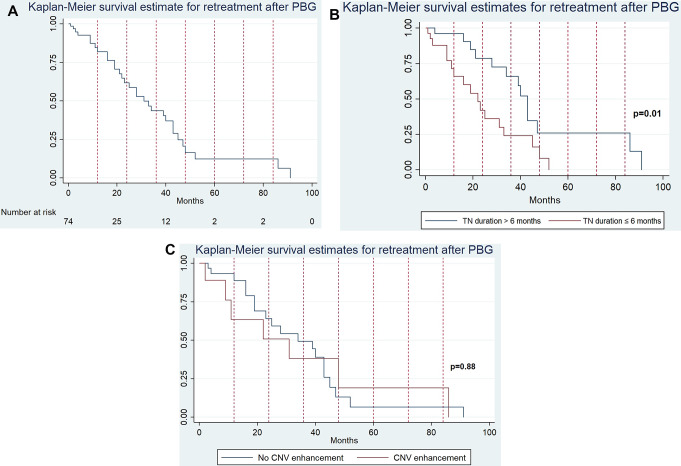
The two factors that were found to be significant on univariate analysis were CNV enhancement on pre-procedure MRI (p = 0.01) and TN disease duration ≤ 6 months prior to treatment with PBG (p < 0.001). However, after performing logistic regression, only TN duration ≤ 6 months remained significant OR 3.33 (95% CI 1.59–10.0; p = 0.003). We then performed a Kaplan-Meier survival analysis, which further confirmed that patients with a TN duration ≤ 6 months require retreatment earlier after PBG [22 months vs. 41 months; p = 0.01; Fig. 1]. An additional Kaplan-Meier survival analysis was performed comparing individuals with CN V enhancement to those without showing no significant difference between the two groups (p = 0.88).
Fig. 1.
KM curves showing survival estimate times to retreatment after PBG. (A) KM curve for the overall sample. (B) KM curve comparing time to retreatment according to the TN duration. There is an earlier retreatment occurrence for cases in which TN disease duration is ≤ 6 months (p = 0.01). (C) KM curve comparing time to retreatment according to the presence of CNV enhancement. There is no significant difference between the groups (p = 0.88)
Discussion
In this study, a retreatment rate of 30.8% was found for typical TN patients undergoing PBG. All of these patients had failed medical management despite adjustments to their pain control regimens. We found that the only factor that was a significant predictor for treatment failure and subsequent retreatment was a TN disease duration of ≤ 6 months (OR 3.33; 95% CI 1.59–10.0; p = 0.003), which has not been previously reported in the literature for PBG or other ablative procedures. Early trigeminal nerve neuronal plasticity changes that induce neuropathic pain could potentially explain this. These findings suggest that TN, present with or without an obvious vascular compression, may have a bimodal disease course. The acute phase (Six month or less of TN disease duration) seems to not respond adequately to percutaneous balloon ganglyolysis and perhaps to other ablative surgical techniques as well. Patients receiving PBG later during the chronic phase (after six months) show significantly better outcomes with lesser rates of pain recurrence. We hypothesize that the initial pathophysiological plasticity changes in the CNV nerve root that give rise to TN may not be completed in the early phase. Intervention before the completion of this process may be less effective and, thus, more prone to treatment failure. These findings support the initial medical management of patients with newly diagnosed TN, especially those who are not suitable candidates for MVD, hoping to get them past the acute phase so that intervention with PBG is more effective. Further studies should be performed to confirm these findings, which may impact treatment considerations in the future.
Our single-center retrospective analysis confirmed that PBG is an effective and safe treatment. Although we did see transient self-resolving bradycardia episodes during the procedure and no major cardiovascular complications, it is essential to note that contraindications to having a PBG performed include a history of arrhythmias or hypotension due to the higher risk of disproportionate vasovagal response [19]. On immediate post-operative check all patients reported improved or absent pain. After that, 82.6% reported a complete absence of pain on the first follow-up, while 68.8% reported adequate pain control or better on the last follow-up. Of those retreated, 75% were pain-free on the last follow-up. Post-procedure facial numbness was common (76.5%). Still, it was not found to be a reliable predictor of long-term successful treatment, contrary to what has been reported in the literature for other treatment modalities [1, 20].
The different treatment modalities for TN also have non-negligible rates of failure and need for eventual retreatment. Glycerol rhizotomy is 90% successful in relieving pain after initial intervention, but it has a pain relief duration of around three years in 50% of individuals. Additionally, the median time to recurrence of symptoms requiring some degree of medical management is only two years [20–22]. Additionally, studies have reported a 60% failure rate at 10-year follow-ups for stereotactic radiosurgery [23–25]. Lastly, MVD is known to have a failure rate range of 4–30% [13]. While the retreatment rates of the various surgical options are well documented, there is little consensus regarding the factors that predispose a patient to a favorable or unfavorable outcome.
MVD is a good option for young, healthy patients whose CNV shows neurovascular compression on imaging. This treatment’s efficacy, however, decreases when the compression is not present or with subsequent retreatments [9, 13, 26]. Additionally, it is the most invasive option, and the complication rate is substantially higher compared to other treatment modalities at 8% [15, 27]. It has a higher risk in those greater than 65 years old and is contraindicated for those who cannot tolerate a craniotomy or general anesthesia [19]. Glycerol rhizotomy’s effectiveness does not diminish with retreatments. Still, it has an increased risk of dysesthesia, corneal hypesthesia, and technical failures that make PBG a more appealing option in many circumstances [25, 28]. Finally, the efficacy of SRS is impacted by the presence of multiple sclerosis, the integral dose, and the mean dose of radiation given [23]. The literature agrees on a few unifying patient factors across modalities that predict a positive outcome, with one considered possibility being the presence of post-procedure numbness [1]. However, this has not been definitively proven.
Study limitations
Although the outcomes in this study are statistically robust, they represent outcomes from a small retrospective sample of patients at a single major academic center, and, therefore, it may contain all of the inherent biases that come with this research approach. Furthermore, all patients were treated following this surgeon’s specific protocol. Lack of achievement of the pear shape is associated with worse outcomes and thus this study’s findings may not be applicable to cases where it is not achieved or to surgeons cases whose protocol greatly differs [29]. we only included patients with typical trigeminal neuralgia; therefore, these findings cannot be extrapolated to atypical TN. Additionally, we acknowledge that 7 patients did not have a pre-operative MRI available for review. Of those 45 remaining that had MRI done, 7 did not have T2 DRIVE sequence, and 3 did not have contrast-enhanced sequences for review. Lastly, it is possible that factors outside the scope of this study could have contributed to the recurrence rate.
Conclusion
The main factor contributing to PBG intervention for TN failure and subsequent need for retreatment is a duration of symptoms of < 6 months or less. This points to the possibility that TN has both an acute and chronic phase that responds differently to PBG and possibly other ablative techniques.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Daniel Sconzo and Alejandro Enriquez-Marulanda collected and analyzed the data, wrote the manuscript, and were involved in the review process. Ekin Simwatachela collected and analyzed the radiographic data. Thai Vu assisted in data collection. All remaining authors as well as those mentioned above contributed to the study design, and reviewed the manuscript.
Funding
No funding was secured for this manuscript
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
This study was approved by the IRB and was given “exemption status” due to its retrospective nature. The offical IRB number is H-44098.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Córdoba JLD, Bach MG, Isach N, Piles S (2015) Percutaneous Balloon Compression for Trigeminal Neuralgia: imaging and technical aspects. Reg Anesth Pain Med 40(5):616–622. 10.1097/AAP.0000000000000292 [DOI] [PubMed] [Google Scholar]
- 2.Jones MR, Urits I, Ehrhardt KP et al (2019) A Comprehensive Review of Trigeminal Neuralgia. Curr Pain Headache Rep 23(10):74. 10.1007/s11916-019-0810-0 [DOI] [PubMed] [Google Scholar]
- 3.Valenzuela Cecchi B, Figueroa F, Contreras L, Bustos P, Maldonado F Percutaneous Balloon Compression for the treatment of trigeminal neuralgia: a review of 10 years of clinical experience. Cureus 15(8):e43645. 10.7759/cureus.43645 [DOI] [PMC free article] [PubMed]
- 4.Al-Quliti KW (2015) Update on neuropathic pain treatment for trigeminal neuralgia. Neurosciences (Riyadh) 20(2):107–114. 10.17712/nsj.2015.2.20140501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendtsen L, Zakrzewska JM, Heinskou TB et al (2020) Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol 19(9):784–796. 10.1016/S1474-4422(20)30233-7 [DOI] [PubMed] [Google Scholar]
- 6.Fisher R, Clarkson E (2024) Medication Management of Neuropathic Pain disorders. Dental Clin N Am 68(1):121–131. 10.1016/j.cden.2023.07.010 [DOI] [PubMed] [Google Scholar]
- 7.Rapisarda A, Battistelli M, Izzo A et al (2023) Outcome comparison of drug-resistant trigeminal Neuralgia Surgical Treatments—An Umbrella review of Meta-analyses and systematic reviews. Brain Sci 13(4):530. 10.3390/brainsci13040530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baabor MG, Perez-Limonte L (2011) Percutaneous balloon compression of the gasserian ganglion for the treatment of trigeminal neuralgia: personal experience of 206 patients. Acta Neurochir Suppl 108:251–254. 10.1007/978-3-211-99370-5_39 [DOI] [PubMed] [Google Scholar]
- 9.Grewal SS, Kerezoudis P, Garcia O, Quinones-Hinojosa A, Reimer R, Wharen RE (2018) Results of Percutaneous Balloon Compression in Trigeminal Pain syndromes. World Neurosurg 114:e892–e899. 10.1016/j.wneu.2018.03.111 [DOI] [PubMed] [Google Scholar]
- 10.Hu S, Huang Z, Wang H et al (2023) The value of a headless pear shape in Percutaneous Balloon Compression for Trigeminal Neuralgia. Operative Neurosurg 25(4):372. 10.1227/ons.0000000000000831 [DOI] [PubMed] [Google Scholar]
- 11.Huang P, Liu H, Liu Z et al (2023) Effectiveness of percutaneous balloon compression (PBC) in improving physical function and quality of life in trigeminal neuralgia: a retrospective study. Acta Neurochir (Wien) Published Online Oct 28. 10.1007/s00701-023-05823-z [DOI] [PubMed]
- 12.Skirving DJ, Dan NG (2001) A 20-year review of percutaneous balloon compression of the trigeminal ganglion. J Neurosurg 94(6):913–917. 10.3171/jns.2001.94.6.0913 [DOI] [PubMed] [Google Scholar]
- 13.Unal TC, Unal OF, Barlas O et al (2017) Factors determining the outcome in trigeminal neuralgia treated with Percutaneous Balloon Compression. World Neurosurg 107:69–74. 10.1016/j.wneu.2017.07.132 [DOI] [PubMed] [Google Scholar]
- 14.Montano N, Papacci F, Cioni B, Di Bonaventura R, Meglio M (2014) The role of percutaneous balloon compression in the treatment of trigeminal neuralgia recurring after other surgical procedures. Acta Neurol Belg 114(1):59–64. 10.1007/s13760-013-0263-x [DOI] [PubMed] [Google Scholar]
- 15.Bick SKB, Eskandar EN (2017) Surgical Treatment of Trigeminal Neuralgia. Neurosurg Clin North Am 28(3):429–438. 10.1016/j.nec.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 16.Gobel H (2024) 13.1.1 Trigeminal neuralgia. ICHD-3. Accessed September 21, https://ichd-3.org/13-painful-cranial-neuropathies-and-other-facial-pains/13-1-trigeminal-neuralgia/13-1-1-classical-trigeminal-neuralgia/
- 17.Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL (2000) Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of the Barrow Neurological Institute. Int J Radiat Oncol Biol Phys 47(4):1013–1019. 10.1016/S0360-3016(00)00513-7 [DOI] [PubMed] [Google Scholar]
- 18.Elnashar A, Patel SK, Kurbanov A, Zvereva K, Keller JT, Grande AW (2019) Comprehensive anatomy of the foramen ovale critical to percutaneous stereotactic radiofrequency rhizotomy: cadaveric study of dry skulls. J Neurosurg 132(5):1414–1422. 10.3171/2019.1.JNS18899 [DOI] [PubMed] [Google Scholar]
- 19.Parmar M, Sharma N, Modgill V, Naidu P (2013) Comparative evaluation of Surgical procedures for Trigeminal Neuralgia. J Maxillofac Oral Surg 12(4):400–409. 10.1007/s12663-012-0451-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slettebø H, Hirschberg H, Lindegaard KF (1993) Long-term results after percutaneous retrogasserian glycerol rhizotomy in patients with trigeminal neuralgia. Acta Neurochir (Wien) 122(3–4):231–235. 10.1007/BF01405534 [DOI] [PubMed] [Google Scholar]
- 21.North RB, Kidd DH, Piantadosi S, Carson BS (1990) Percutaneous retrogasserian glycerol rhizotomy. Predictors of success and failure in treatment of trigeminal neuralgia. J Neurosurg 72(6):851–856. 10.3171/jns.1990.72.6.0851 [DOI] [PubMed] [Google Scholar]
- 22.Chang KW, Jung HH, Chang JW (2022) Percutaneous procedures for trigeminal Neuralgia. J Korean Neurosurg Soc 65(5):622–632. 10.3340/jkns.2022.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti A, Acker G, Pontoriero A et al (2020) Factors affecting outcome in frameless non-isocentric stereotactic radiosurgery for trigeminal neuralgia: a multicentric cohort study. Radiat Oncol 15:115. 10.1186/s13014-020-01535-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conti A, Pontoriero A, Iatì G et al (2017) Frameless stereotactic radiosurgery for treatment of multiple sclerosis–related trigeminal Neuralgia. World Neurosurg 103:702–712. 10.1016/j.wneu.2017.04.102 [DOI] [PubMed] [Google Scholar]
- 25.Asplund P, Blomstedt P, Bergenheim AT (2016) Percutaneous Balloon Compression vs Percutaneous Retrogasserian glycerol rhizotomy for the primary treatment of trigeminal Neuralgia. Neurosurgery 78(3):421–428. 10.1227/NEU.0000000000001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leal PRL, Barbier C, Hermier M, Souza MA, Cristino-Filho G, Sindou M (2014) Atrophic changes in the trigeminal nerves of patients with trigeminal neuralgia due to neurovascular compression and their association with the severity of compression and clinical outcomes: clinical article. J Neurosurg 120(6):1484–1495. 10.3171/2014.2.JNS131288 [DOI] [PubMed] [Google Scholar]
- 27.Herta J, Schmied T, Loidl TB et al (2021) Microvascular decompression in trigeminal neuralgia: predictors of pain relief, complication avoidance, and lessons learned. Acta Neurochir (Wien) 163(12):3321–3336. 10.1007/s00701-021-05028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bender M, Pradilla G, Batra S et al (2012) Effectiveness of repeat glycerol rhizotomy in treating recurrent trigeminal neuralgia. Neurosurgery 70(5):1125–1133 discussion 1133–1134. 10.1227/NEU.0b013e31823f5eb6 [DOI] [PubMed] [Google Scholar]
- 29.Cao B, Li Y, Wang Y et al (2024) Analysis of recurrence factors after balloon compression for trigeminal neuralgia and the relationship between pear-shaped balloon and guidewire path. Clin Neurol Neurosurg 246:108548. 10.1016/j.clineuro.2024.108548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.