Abstract
Purpose
Healthy diets are believed to be associated with a reduced risk of experiencing common mental disorders (CMDs) and related symptomatology (such as ruminative thinking), and with healthier brain chemistry and structure, especially in the frontal regions implicated in CMDs, cognitive control, and food choice. Nevertheless, there is very limited research on the relationship between diet health/quality and brain function. In this study we assessed the associations between adherence to the Mediterranean diet and resting state functional connectivity (rs-FC) of the prefrontal cortex (PFC) with the whole brain and whether this connectivity would be associated with ruminative thinking as a transdiagnostic factor for CMDs.
Methods
Thirty-seven adults (Mean Age = 25.57, SD = 7.18) completed the Mediterranean Diet Adherence Screener (MEDAS) and were classified into high- and low-quality diet groups and completed the Ruminative Response Scale. All participants underwent resting-state functional MRI (fMRI) to determine whole-brain rs-FC of the medial prefrontal cortex (mPFC).
Results
Participants in the high MEDAS group (vs. low MEDAS group) exhibited significantly greater rs-FC of the mPFC seed with the thalamus, caudate and putamen. Additionally, the strength of rs-FC of the mPFC seed with these regions was positively associated with the MEDAS scores across groups in both crude and adjusted models. There were no significant associations between the strength of rs-FC of the mPFC seed with the cluster of voxels with the thalamus, caudate, and putamen and ruminative thinking.
Discussion
This work shows that healthy dietary patterns are associated with rs-FC in the frontal-subcortical circuitry in healthy volunteers. Considering the implications of the dysregulation of this circuity, adhering to healthy dietary patterns may offer a promising alternative/complementary method to improve CMDs, cognitive control, and food choices.
Keywords: Mediterranean diet, Diet quality, Prefrontal cortex, Thalamus, Caudate, Putamen
Introduction
A growing body of research suggests that several healthy dietary patterns such as the Mediterranean diet (MED), as well as individual food types (e.g., fruits and vegetables) and nutrients (e.g., dietary fibre) that comprise the MED, have been shown to be associated with a lower likelihood of experiencing common mental disorders (CMDs) such as depression and anxiety, as well as CMD-related symptomatology [1–5]. Additionally, data from multiple randomised clinical trials have also demonstrated a causal effect of the healthy dietary patterns improving these outcomes [6–9], possibly via modulation of various mechanisms including, but not limited to, oxidative stress, plasticity, microbiota–gut–brain axis, and inflammatory responses [10, 11].
Nonetheless, there is limited research on the relationship between diet and brain imaging biomarkers. Studies focusing on dietary patterns showed that the MED is associated with structural alterations in the brain, such as greater total brain volume [12, 13], greater grey matter volume in the frontal lobes [13, 14] and preserved integrity of white matter bundles linking limbic regions with the frontal regions [15].
Recently, work from our group has shown that adherence to the MED was associated with increased gamma-aminobutyric acid (GABA) and reduced glutamate (GLU) concentrations, as well as increased grey matter volume in the areas in the prefrontal cortex (PFC; an area that is involved in the aetiology and maintenance of CMD [14, 16–19] and regulation of food intake (type, quantity, and quality) and appetite [20]), and these neural changes were associated with rumination (a transdiagnostic factor for CMDs) [14]. Importantly, the PFC is structurally and functionally connected to the thalamus and striatal regions [21] which are involved in food choice, reward, and motivation [22]. Because there may be a bidirectional relationship between making healthy dietary choices and/or adhering to MED style diets and better CMD outcomes, it may be that stronger resting state functional connectivity (rs-FC) of the PFC with the thalamus and/or striatum is associated with a greater adherence to the MED diet.
Converging evidence from a health neuroscience framework of obesity/overeating highlighted that reduced PFC activity could be viewed as a predictor, cause, or outcome of poor dietary choices/adhering to unhealthy dietary patterns, through downregulation of the PFC (i.e., poor cognitive control, hence dietary self-regulation and food choice) and upregulation of the striatum (i.e., increased reward sensitivity) [23]. Similar to this model, diet-related neurochemical and neurostructural changes in the PFC might, therefore, alter the functioning of the PFC, thalamus, and striatum. This may offer an additional mechanistic explanation as to why there may be a bidirectional relationship between making healthy dietary choices and/or following the MED style diets and better CMD outcomes. However, apart from a small number of studies that identified (i) localised changes in functional brain activity in response to intake of specific nutrients [24] (which fails to account for the synergistic and cumulative effects of multiple nutrients within a comprehensive dietary pattern [25]), and (ii) increased within-network and between-network resting-state functional connectivity (rs-FC) in individuals who adhere to the MED [26], and reduced rs-FC in the structures that are involved in pathophysiology of obesity/overeating [27], no studies have investigated the associations between MED and rs-FC of the prefrontal cortex. To this end, we performed a seed-based analysis to assess the associations between adherence to healthy eating patterns (i.e., the MED), resting-state functional connectivity of the PFC, and the CMD transdiagnostic factor, rumination. We hypothesised that connectivity of the PFC with those regions identified as important for CMDs, food choice, reward, and motivation would be weaker in individuals with low adherence to the Mediterranean diet, and that this connectivity would be associated with rumination.
Methods
Participants
One hundred and sixty-four students (from the University of Roehampton and University of Royal Holloway) and members of the public were screened online (Qualtrics; https://www.qualtrics.com) using the Mediterranean Diet Adherence Screener (MEDAS) [28]. Thirty-eight participants were selected based on the upper and lower quartiles to establish high MEDAS score (High MEDAS; >8, n = 19) and low MEDAS score (Low MEDAS; <6, n = 19) groups.
Exclusion criteria included presence of contraindications for MRI scanning (i.e., presence of metal, etc.), current use of prescribed medication for neuropsychiatric disorders or illicit substances misuse, or history of or presence of psychiatric and neurological disorders, body mass index < 18.5 kg/m2 and > 29.9 kg/m2, and having diabetes mellitus, hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg) or cardiovascular diseases (clinical history). All participants provided informed consent and received £50 for participation. The research protocol was approved by the Ethical committee at the University of Roehampton (Reference: PSYC 22/ 444) on 30/01/2023. The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Demographic, dietary, and clinical assessment
In order to ensure that High vs. Low MEDAS groups were matched for demographic and environmental/lifestyle factors, all participants were asked to complete a demographics form assessing age, sex, level of education, handedness (assessed via Annett Hand Preference Questionnaire [29]), income, alcohol consumption (units per day), and tobacco consumption (cigarettes per day)…etc. Additionally, EPIC Norfolk Food Frequency Questionnaire (FFQ) was used [30] to estimate habitual food intake. Participants reported (i) how frequently they consume 130 food items, with options ranging from ‘never or less than once a month’ to ‘6 + per day’ and (ii) other foods they consume. FETA software was used to convert food frequency questionnaire data into nutrient and food group values [31].
A-14-item MEDAS [28] was used to estimate adherence to the Mediterranean style diets. This measure involves 12 questions on food consumption frequency (e.g., “How many servings of whole fruit do you eat per day?”) and 2 questions on food intake habit (e.g., “Do you use olive oil as the main source of fat for cooking?”). Each question is scored 0 or 1, and total scores range from 0 to 14 where higher scores represent higher adherence to the Mediterranean diet, hence higher diet quality. The MEDAS demonstrates good test-retest reliability (r = 0.69) [32] and validity (coefficients ranging from 0.52 to 0.79) [28, 32, 33] in healthy and unhealthy adult populations in the UK and in other European countries.
Ruminative Response Scale (RRS) [34], a 22-item questionnaire, was used to assesses reflection, brooding, and depression-related rumination. Each item (e.g., “How often do you Think about how sad you feel?”) is rated on a 5-point Likert scale from 1 (almost never) to 4 (almost always) and higher scores reflect increased levels of ruminative thinking. The RRS demonstrates good test-retest reliability (r = 0.67) and validity (0.90) [34].
High and Low MEDAS groups were compared on demographic and clinical measures by using chi-square or independent sample t-tests (two-tailed) on IBM® SPSS Statistics Version 26. A threshold of p < 0.05 was applied throughout.
fMRI data acquisition
Resting-state fMRI images were acquired over 10 min using a 3-T Siemens AG Trio MRI system with a 32-channel head coil while participants were instructed to keep their eyes closed (300 T2*-weighted echoplanar images; repetition time = 2 s; echo time = 30 milliseconds; slice thickness = 4 mm; flip angle = 90°; matrix: 64 × 64; field of view = 192 mm). A T1-weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scan was acquired for registration purposes, including spatial normalization to standard space (Montreal Neurological Institute; MNI).
fMRI data preprocessing
Image analysis was performed using FMRIB Software Library (FSL) version 6.0.6 (www.fmrib.ox.ac.uk/fsl). The time course of the fMRI data was first realigned to compensate for head movements as in Jenkinson, et al. [35]Jenkinson, et al. [35], and all non-brain matter was removed using FSL’s brain extraction tool (BET). Time-series statistical analysis was carried out using FSL’s Improved Linear Model, with local autocorrelation correction as in [36], after high-pass temporal filtering (Gaussian-weighted least square fit (LSF) straight line fitting with sigma = 50 s).
None of the fMRI data were deemed to have had excessive head motion (> 2.5 mm translation). Motion cleaning and noise reduction were performed using a 32-parameter linear regression model [37] used in our previous publications [38–40]. Specifically, this included 6 motion parameters (3 translational dimensions along the X, Y and Z axes as well as the 3 rotational dimensions of ‘pitch’, ‘roll’ and ‘yaw’) combined with the timeseries from the CSF and white matter (all of which provides 8 parameters), as well as their temporal derivatives to provide 16 parameters, and the quadratic of these to provide 32 parameters in total. Furthermore, frame-wise displacement (FD) was determined with root-mean squared matrix calculation (using the tool ‘fsl_motion_outliers’) to obtain the average rotation and translation parameter differences across EPIs. Time points where motion exceeded acceptable FD thresholds as expressed in [41, 42] were censored using separate regressors for each of these time points in the model. A fixed FD threshold for all participants was determined via calculation of the SD of FD across all data points and computing the following equation: 0.25 mm + 2 * SD as in Satterthwaite, Elliott, Gerraty, Ruparel, Loughead, Calkins, Eickhoff, Hakonarson, Gur, Gur and Wolf [37]Satterthwaite, Elliott, Gerraty, Ruparel, Loughead, Calkins, Eickhoff, Hakonarson, Gur, Gur and Wolf [37]. The timeseries of the resultant residuals from the regression model was then scaled and normalised at each voxel: ([residuals – mean]/SD) + 100.
A seed-based approach was implemented by creating a 10 mm sphere in the mPFC with a centre-of-mass (in MNI space) of X = 45, Y = 85 and Z = 48 (Fig. 1). This placement of the seed was chosen to mirror the placement of the voxel for the 1H-Magnetic Resonance Spectroscopy scans performed on the same participants as shown in our previous paper [14]. This seed mask was transformed to each participant’s native space, and the timeseries data from within this mask were then extracted from the scaled and normalised residuals of the 32-parameter regression model and then included as a single explanatory variable in a linear model at the first (i.e., single-subject) level. Contrast images representing voxel-wise effects resulting from this model were then registered to the MPRAGE structural image and then into standard MNI space using a 12-paramater affine transformation [43]. Registration from MPRAGE structural images into standard space was further refined using nonlinear registration [i.e. FNIRT; 44]. Finally, images were smoothed using a 5 mm full-width at half-maximum Gaussian kernel.
Fig. 1.
Placement of seed in the mPFC in standard (MNI) space
fMRI data analysis
Group level analyses were performed using FSL’s Local Analysis of Mixed Effects (FLAME1) with outlier de-weighting applied.
To assess the influence of group (High vs. Low MEDAS) on whole-brain connectivity of the mPFC seed, a model with five explanatory variables (EVs) that controlled for BMI, age and sex was constructed. The first EV denoted the High MEDAS group, the second EV denoted the Low MEDAS group, the third EV denoted BMI (demeaned), the fourth EV denoted age (demeaned), and the fifth EV denoted sex (demeaned). Two contrasts were computed in order to determine which in regions (if any) (i) the high MEDAS group exhibited greater connectivity with the mPFC seed and (ii) the low MEDAS group exhibited greater connectivity with the same mPFC seed.
To assess the relationship between scores on the MEDAS and whole-brain connectivity of the mPFC seed, a model with four explanatory variables (EVs) that controlled for BMI, age and sex was constructed. In this model, the first EV denoted MEDAS scores, the second EV denoted BMI, the third EV denoted age, and the fourth EV denoted sex (all demeaned). Two contrasts were computed to determine which regions (if any) exhibited resting-state functional connectivity that (i) positively correlated and (ii) negative correlated with MEDAS scores in the whole group.
For all models, statistical maps were cluster-corrected for multiple comparisons (voxel height threshold: Z > 3.09, cluster significance p < 0.05).
To examine the relationships of rumination with group differences in connectivity, connectivity values (z scores) were extracted from significant clusters and entered into the IBM® SPSS Statistics Version 26 for correlation with the RRS.
Results
Participant characteristics
Due to data loss, we report results from 37 participants (19 High MEDAS and 18 Low MEDAS). Table 1 provides a full summary of participant characteristics across the sample and in High MEDAS and Low MEDAS groups. The groups did not differ for sex, age, education, income, physical activity, BMI, energy and macronutrient intakes, and handedness, but by design, differed significantly on the MEDAS scores.
Table 1.
Participant characteristic across the sample and in the high MEDAS and low MEDAS groups
| Total Sample (n = 37) | High MEDAS (n = 19) | Low MEDAS (n = 18) | t/χ2 | p | |
|---|---|---|---|---|---|
| Sex (N: M/F) | 13/24 | 7/12 | 6/12 | 0.05 | 0.82 |
| Age (M ± SD) | 25.57 ± 7.18 | 26.21 ± 8.71 | 24.89 ± 5.28 | − 0.55 | 0.58 |
| MEDAS Score (M ± SD) | 6.92 ± 2.30 | 8.84 ± 0.90 | 4.89 ± 1.37 | -10.45 | < 0.001 |
| RRS Score (M ± SD) | 46.46 ± 14.66 | 46.58 ± 15.25 | 46.33 ± 14.45 | 0.05 | 0.96 |
| Education (N) | 3.88 | 0.42 | |||
| GCSE/O levels | 1 | 0 | 1 | ||
| A levels/secondary | 8 | 5 | 3 | ||
| Degree commenced | 6 | 2 | 4 | ||
| Degree completed | 12 | 8 | 4 | ||
| Postgraduate | 10 | 4 | 6 | ||
| Income/annum (N) | 1.82 | 0.87 | |||
| Less than £18,000 | 7 | 4 | 3 | ||
| £18, 000 to £30, 999 | 8 | 4 | 4 | ||
| £31, 000 to £51, 999 | 8 | 5 | 3 | ||
| £52, 000 to £100, 000 | 3 | 2 | 1 | ||
| More than £100, 000 | 5 | 2 | 3 | ||
| Do not know | 6 | 2 | 4 | ||
| Physical Activity/week (N) | 2.67 | 0.62 | |||
| Less than 30 min | 3 | 1 | 2 | ||
| 30–90 min | 6 | 3 | 3 | ||
| 90–150 min | 13 | 8 | 5 | ||
| 150–300 min | 9 | 3 | 6 | ||
| More than 300 min | 6 | 4 | 2 | ||
| BMI (kg/m²) (M ± SD) | 24.37 ± 4.69 | 23.53 ± 4.35 | 25.26 ± 5.00 | -1.12 | 0.27 |
| Energy/day (kcal) (M ± SD) | 1738.09 ± 637.26 | 1764.12 ± 688.43 | 1710.61 ± 597.17 | 0.25 | 0.80 |
| Carbohydrate/day (g) (M ± SD) | 201.26 ± 82.25 | 199.09 ± 88.63 | 204.79 ± 77.39 | − 0.21 | 0.84 |
| Protein/day (g) (M ± SD) | 85.65 ± 32.81 | 85.26 ± 36.66 | 86.14 ± 29.27 | − 0.08 | 0.94 |
| Fat/day (g) (M ± SD) | 67.00 ± 28.44 | 71.34 ± 30.36 | 64.46 ± 26.68 | 0.73 | 0.47 |
| Handedness (N: R/L) | 34/3 | 18/1 | 16/2 | 0.42 | 0.52 |
N: number; M: male; F: female; MEDAS: Mediterranean Diet Adherence Screener; RRS: Ruminative Response Scale; kg: kilograms; m: metre; kcal: kilocalories; g: grams; R: right; L: left
Resting state functional connectivity
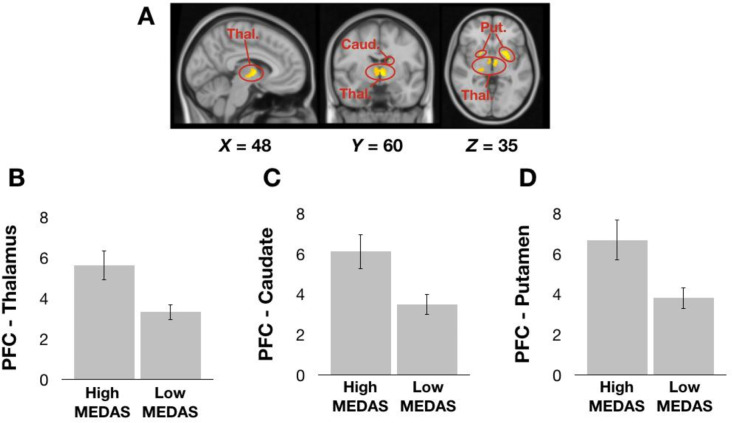
Whole-brain, voxel-wise GLM analyses revealed that compared to individuals in the Low MEDAS group, those in the High MEDAS group exhibited greater resting-state functional connectivity of the mPFC seed with the thalamus, caudate and putamen (Fig. 2). We did not observe any significant clusters for Low MEDAS > High MEDAS contrast.
Fig. 2.
A: Cluster of voxels in which resting-state functional connectivity with the mPFC seed depicted in Fig. 1 is greater in the High MEDAS group than in the Low MEDAS group. B-D: Mean z values from the cluster of voxels in the Thalamus (B), Caudate (C) and Putamen (D) in High MEDAS individuals and Low MEDAS individuals separately. Thal. = Thalamus; Caud. = Caudate; Put. = Putamen. Error bars denote 1 standard error of the mean
The GLM that examined the relationship between whole-brain connectivity of the mPFC seed and continuous MEDAS scores in the whole group revealed no significant clusters. As such, we extracted the values from each of the three separate clusters depicted in Fig. 2 and correlated these with MEDAS scores in all participants in an exploratory fashion. Bivariate correlations revealed that, in the whole group, MEDAS scores positively correlated with resting-state functional connectivity of the mPFC seed with the cluster of voxels with the thalamus (r = 0.362, p = 0.028), caudate (r = 0.375, p = 0.022) and putamen (r = 0.337, p = 0.041) (Fig. 3). Partial correlations revealed that these correlations remained significant when controlling for the effects of BMI, age, and sex revealed (thalamus: r = 0.398, p = 0.020, caudate: r = 0.377, p = 0.028; putamen: r = 0.346, p = 0.045).
Fig. 3.
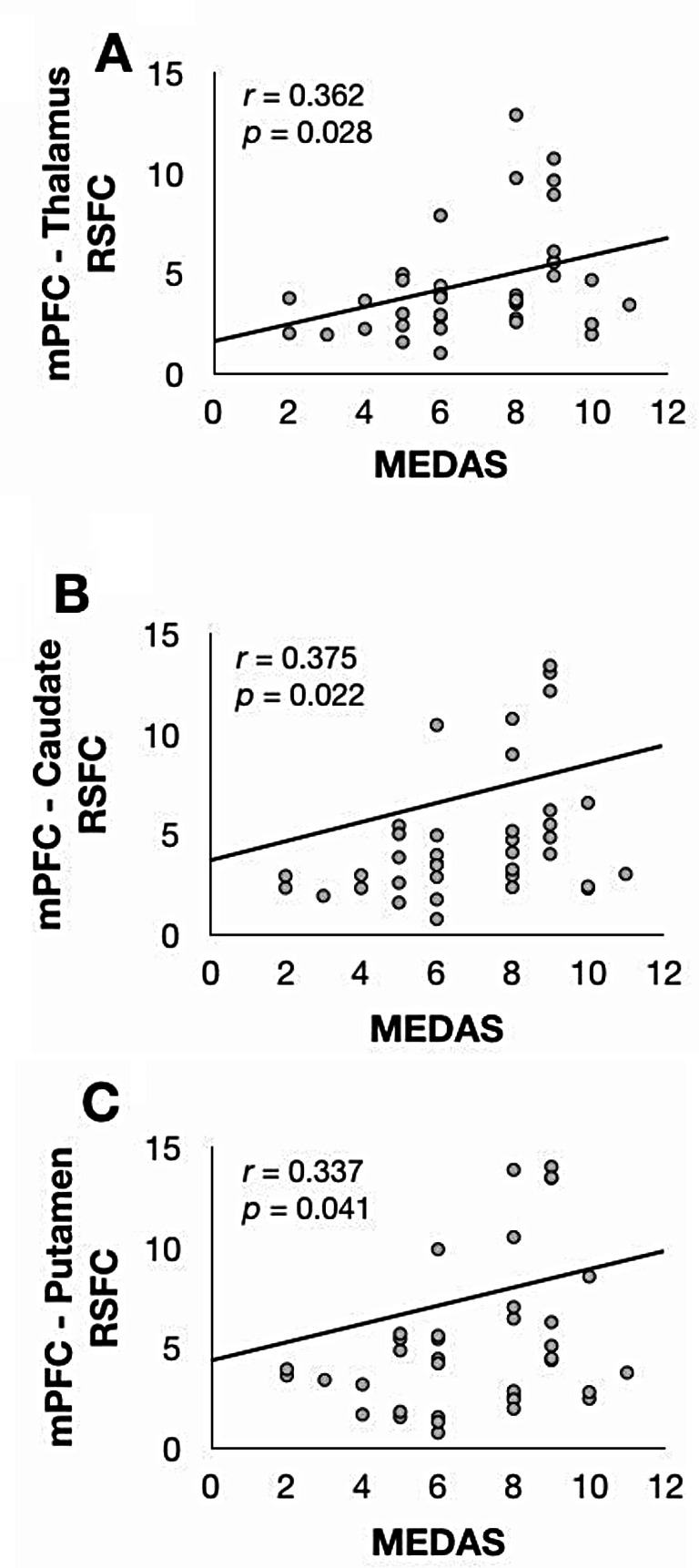
Scatterplots depicting positive bivariate correlations between MEDAS scores and z scores extracted from within the A: Thalamus; B: Caudate; and C: Putamen clusters depicted in Fig. 2 above
Bivariate and partial correlations did not reveal significant associations between RRS (i.e. rumination scores) and resting-state functional connectivity of the mPFC seed with the cluster of voxels with the thalamus, caudate, and putamen.
Discussion
The aim of the current study was to perform a seed-based analysis to assess the associations between adherence to healthy eating patterns (i.e., the MED) and rs-FC of the PFC with those regions identified as important for CMDs, food choice, reward, and motivation. Results revealed greater rs-FC of the mPFC seed with the thalamus, caudate and putamen in individuals in the high (vs. low) MEDAS groups. Additionally, rs-FC of the mPFC seed with the cluster of voxels with the thalamus, caudate, and putamen were positively associated with the MEDAS scores across groups in both crude and adjusted models. However, rs-FC of the PFC with thalamus, caudate, and putamen were not significantly associated with the RRS scores.
Our study provides the first direct evidence showing greater rs-FC of the mPFC seed with the striatal regions (including caudate and putamen) and thalamus in individuals in the high (vs. low) MEDAS groups. Additionally, rs-FC of the mPFC was positively correlated with MEDAS scores across both groups. Previous research showed that weaker PFC activity is implicated in impairments in cognitive control [45, 46] (which is linked to the aetiology of CMDS [47]), possibly through modulation of GABAergic, glutamatergic, and dopaminergic neurotransmission/metabolism in this area [18, 48–50]. Increased striatal/thalamic activity, on the other hand, is known to be involved in impairments in impulsivity/compulsivity [51, 52], reward [53], as well as motivation [22], and intake of palatable calorie-dense foods increases striatal cortical activity [54], possibly through decreased dopaminergic signalling/activity, as previously observed in the forms of (i) decreased binding potential for dopamine D2 receptors in obese humans [55] (as a consequence of insulin resistance [56]) and decreased dopamine D2 receptors in the striatum in rats who were fed high fat (vs. normal chow) diets [57, 58] and/or (ii) increased microglia proliferation, hence, metabolic inflammation following high-fat diet consumption [52]. As depicted in models of obesity/over-eating, downregulation of PFC and upregulation of striatum is implicated in impairments in food choice-related self-control and cognitive control, which may increase tendency to consume unhealthy foods/follow unhealthy dietary patterns [59–61]. Additionally, PFC-thalamus connectivity is an integral part of decision-making [62] and external eating behaviours (i.e., eating in response to food cues) [63]. Taken together, adherence to unhealthy dietary patterns may impact the functional calibration of this frontal–subcortical circuitry, by evoking functional dysregulation of the PFC and striatal and thalamic regions (potentially through diet-induced alterations in neurotransmission/metabolism of GABA/GLU/Dopamine). In turn, this may result in potential impairments in cognitive control and thus in experiencing symptoms of CMDs, and/or adherence to unhealthy dietary patterns/making poor food choices through altered impulsivity, motivation, and reward processing. Therefore, dietary approaches that alter frontal and striatal GABA, GLU, and dopamine neurotransmission/metabolism, may offer a promising approach to recalibrate this frontal–subcortical circuitry, and potentially improve CMDs and CMD-related symptomatology and/or making healthy food choices. This may be true in part due to the fact that adherence to low GLU diets and consumption of high dietary GABA have already been shown to affect brain and behaviour (improved subjective stress and sleep-related outcomes and altered cognitive performance, blood oxygen level dependent (BOLD) response and functional connectivity) [3, 64, 65], possibly through GABA-modulating bacteria of the human gut microbiota [66].
The MED emphasises food groups intrinsically related to brain health (hence potentially to better affective and cognitive processing and outcomes) and is characterised by high intakes of vegetables, fruits and nuts, legumes, and unprocessed cereals, low intakes of meat and meat products [67] that are associated with various metabolic processes [10, 11]. Hence, the MED may also potentially alter neuro-chemistry,-structure, and/or -function, and related affective and cognitive mechanisms through inhibition of oxidisation and inflammatory pathways [68, 69] and through modulating peripheral and central glucose and insulin metabolism [68, 70] and the gut microbiota [71–73].
Although in our previous study we observed a significant negative correlation between rumination and rPCG-GMV and a marginally significant positive association between rumination and mPFC-GLU concentrations [14], in the current study rs-FC of the mPFC with the striatal and thalamic regions were not correlated with RRS scores. Given that our measure of rumination in the current study was based on self-report, the lack of correlations may reflect diminished insight into ruminative-thinking processes driven by lower regional connectivity in the frontal and higher regional connectivity the striatal areas that were reported to be associated with more self-reported unwanted thoughts [74].
There are several notable caveats to be aware of in this study. Firstly, due to the cross-sectional nature of our study, we could not determine cause and effect relationships, therefore, further longitudinal studies are warranted to allow stronger causal inferences to examine the impact of diet quality on rs-FC. Secondly, the small sample may have led to Type II errors, especially when examining associations of connectivity with RRS scores. Lastly, due to small sample size, we could not examine potential sex-dependent effects, however, future studies are encouraged to consider stratification of results/analysis by sex in order to fully elucidate sexual dimorphism in the context of nutrition and health.
In conclusion, our findings suggest that adhering to healthy dietary patterns may be associated with stronger rs-FC between specific brain structures involved in CMD physiopathology and food choice, reward, and motivation, and may therefore, offer a promising alternative and/or complementary method to improve CMDs and CMD-related symptomatology and/or making further healthy food choices. Considering the potential implications of the PFC dysregulation discussed above, and that (i) the PFC follows a protracted development [75], and (ii) overall brain health may encompass a wide range of adverse outcomes including dementia and functional impairment, the timing of preventative interventions may be especially pertinent.
Acknowledgements
We thank Mr Ari Lingeswaran at the Combined Universities Brain Imaging Centre, Royal Holloway, University of London for his technical assistance and all our volunteers who participated in this work.
Data availability
The data presented in this study are available on request from the corresponding author.
Declarations
Ethics approval
The research protocol was approved by the Ethical committee at the University of Roehampton (Reference: PSYC 22/ 444) on 30/01/2023. The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants provided informed consent.
Conflict of interest
PH, AC, and PA have received research funding, consultancy, travel support, and speaking honoraria from various industrial companies. MHS is employed by FrieslandCampina, Amersfoort, the Netherlands. Other authors declare that they have no conflicts of interest. Funding for this study was provided by FrieslandCampina whom had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication (Ref: 3129AQ00).
References
- 1.Sarris J (2019) Nutritional Psychiatry: from Concept to the clinic. Drugs 79:929–934. 10.1007/s40265-019-01134-9 [DOI] [PubMed] [Google Scholar]
- 2.Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanzá-Martínez V, Freeman MP, Hibbeln J, Matsuoka Y, Mischoulon D, Mizoue T et al (2015) Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2:271–274. 10.1016/s2215-0366(14)00051-0 [DOI] [PubMed] [Google Scholar]
- 3.Hepsomali P, Groeger JA, Nishihira J, Scholey A (2020) Effects of oral Gamma-Aminobutyric Acid (GABA) administration on stress and sleep in humans: a systematic review. Front Neurosci 14:923. 10.3389/fnins.2020.00923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hepsomali P, Groeger JA (2021) Diet, sleep, and mental health: insights from the UK Biobank study. Nutrients 13:2573. 10.3390/nu13082573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraal AZ, Arvanitis NR, Jaeger AP, Ellingrod VL (2020) Could Dietary Glutamate play a role in Psychiatric Distress? Neuropsychobiology 79:13–19. 10.1159/000496294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacka FN, O’Neil A, Opie RS, Itsiopoulos C, Cotton S, Mohebbi M, Castle D, Dash S, Mihalopoulos C, Chatterton ML et al (2017) A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med 15. 10.1186/s12916-017-0791-y [DOI] [PMC free article] [PubMed]
- 7.Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani A, Itsiopoulos C, Niyonsenga T, Blunden S, Meyer BJ et al (2019) A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: a randomized controlled trial (HELFIMED). Nutr Neurosci 22:474–487. 10.1080/1028415x.2017.1411320 [DOI] [PubMed] [Google Scholar]
- 8.Bayes J, Schloss J, Sibbritt D (2022) The effect of a Mediterranean diet on the symptoms of depression in young males (the AMMEND: a Mediterranean Diet in MEN with Depression study): a randomized controlled trial. Am J Clin Nutr 116:572–580. 10.1093/ajcn/nqac106 [DOI] [PubMed] [Google Scholar]
- 9.Bizzozero-Peroni B, Martínez-Vizcaíno V, Fernández-Rodríguez R, Jiménez-López E, de Núñez S, Saz-Lara A, Díaz-Goñi V, Mesas AE (2024) The impact of the Mediterranean diet on alleviating depressive symptoms in adults: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev nuad176. 10.1093/nutrit/nuad176 [DOI] [PubMed]
- 10.Marx W, Moseley G, Berk M, Jacka F (2017) Nutritional psychiatry: the present state of the evidence. Proc Nutr Soc 76:427–436. 10.1017/s0029665117002026 [DOI] [PubMed] [Google Scholar]
- 11.Marx W, Lane M, Hockey M, Aslam H, Berk M, Walder K, Borsini A, Firth J, Pariante CM, Berding K et al (2020) Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry. 10.1038/s41380-020-00925-x [DOI] [PubMed] [Google Scholar]
- 12.Titova OE, Ax E, Brooks SJ, Sjögren P, Cederholm T, Kilander L, Kullberg J, Larsson E-M, Johansson L, Åhlström H et al (2013) Mediterranean diet habits in older individuals: associations with cognitive functioning and brain volumes. Exp Gerontol 48:1443–1448. 10.1016/j.exger.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Brickman AM, Stern Y, Habeck CG, Razlighi QR, Luchsinger JA, Manly JJ, Schupf N, Mayeux R, Scarmeas N (2015) Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 85:1744–1751. 10.1212/wnl.0000000000002121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hepsomali P, Costabile A, Schoemaker M, Imakulata F, Allen P (2024) Adherence to unhealthy diets is associated with altered frontal gamma-aminobutyric acid and glutamate concentrations and grey matter volume: preliminary findings. Nutr Neurosci 1–13. 10.1080/1028415X.2024.2355603 [DOI] [PubMed]
- 15.Rodrigues B, Coelho A, Portugal-Nunes C, Magalhães R, Moreira PS, Castanho TC, Amorim L, Marques P, Soares JM, Sousa N et al (2020) Higher adherence to the Mediterranean Diet is Associated with preserved White Matter Integrity and altered structural connectivity. Front NeuroSci 14. 10.3389/fnins.2020.00786 [DOI] [PMC free article] [PubMed]
- 16.Nemeroff CB (2003) The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacol Bull 37:133–146 [PubMed] [Google Scholar]
- 17.Sanacora G, Treccani G, Popoli M (2012) Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77. 10.1016/j.neuropharm.2011.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page CE, Coutellier L (2019) Prefrontal excitatory/inhibitory balance in stress and emotional disorders: evidence for over-inhibition. Neurosci Biobehavioral Reviews 105:39–51. 10.1016/j.neubiorev.2019.07.024 [DOI] [PubMed] [Google Scholar]
- 19.Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- 20.Delgado TC (2013) Glutamate and GABA in Appetite Regulation. Front Endocrinol (Lausanne) 4. 10.3389/fendo.2013.00103 [DOI] [PMC free article] [PubMed]
- 21.Haber SN, Liu H, Seidlitz J, Bullmore E (2022) Prefrontal connectomics: from anatomy to human imaging. Neuropsychopharmacology 47:20–40. 10.1038/s41386-021-01156-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley AE, Baldo BA, Pratt WE, Will MJ (2005) Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86:773–795. 10.1016/j.physbeh.2005.08.066 [DOI] [PubMed] [Google Scholar]
- 23.Lowe CJ, Reichelt AC, Hall PA (2019) The Prefrontal Cortex and Obesity: a Health Neuroscience Perspective. Trends Cogn Sci 23:349–361. 10.1016/j.tics.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 24.Gaynor AM, Varangis E, Song S, Gazes Y, Noofoory D, Babukutty RS, Habeck C, Stern Y, Gu Y (2022) Diet moderates the effect of resting state functional connectivity on cognitive function. Sci Rep 12:16080. 10.1038/s41598-022-20047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA (2009) Mediterranean diet and mild cognitive impairment. Arch Neurol 66:216–225 10.1001/archneurol.2008.536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu Y, Burns E, Habeck C, Mensing A, Noofoory D, Babukutty RS, Stern Y (2021) Diet and resting state brain functional connectivity across the adult lifespan. Alzheimer’s Dement 17:e054768. 10.1002/alz.054768 [Google Scholar]
- 27.García-Casares N, Bernal-López MR, Roé-Vellvé N, Gutiérrez-Bedmar M, Fernández-García JC, García-Arnés JA, Ramos-Rodriguez JR, Alfaro F, Santamaria-Fernández S, Steward T et al (2017) Brain functional connectivity is modified by a Hypocaloric Mediterranean Diet and physical activity in obese women. Nutrients 9. 10.3390/nu9070685 [DOI] [PMC free article] [PubMed]
- 28.Schröder H, Fitó M, Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Lamuela-Raventós R, Ros E, Salaverría I, Fiol M et al (2011) A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr 141:1140–1145. 10.3945/jn.110.135566 [DOI] [PubMed] [Google Scholar]
- 29.Annett M (1970) A classification of hand preference by association analyis. Br J Psychol 61:303–321. 10.1111/j.2044-8295.1970.tb01248.x [DOI] [PubMed] [Google Scholar]
- 30.Bingham SA, Gill C, Welch A, Cassidy A, Runswick SA, Oakes S, Lubin R, Thurnham DI, Key TJ, Roe L et al (1997) Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol 26:S137–S137. 10.1093/ije/26.suppl_1.S137 [DOI] [PubMed] [Google Scholar]
- 31.Mulligan AA, Luben RN, Bhaniani A, Parry-Smith DJ, Connor L, Khawaja AP, Forouhi NG, Khaw K (2014) -T. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open 4:e004503. 10.1136/bmjopen-2013-004503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papadaki A, Johnson L, Toumpakari Z, England C, Rai M, Toms S, Penfold C, Zazpe I, Martínez-González MA, Feder G (2018) Validation of the English version of the 14-Item Mediterranean Diet Adherence Screener of the PREDIMED Study, in people at High Cardiovascular Risk in the UK. Nutrients 10. 10.3390/nu10020138 [DOI] [PMC free article] [PubMed]
- 33.García-Conesa MT, Philippou E, Pafilas C, Massaro M, Quarta S, Andrade V, Jorge R, Chervenkov M, Ivanova T, Dimitrova D et al (2020) Exploring the validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): a cross-national study in seven European countries around the Mediterranean Region. Nutrients 12. 10.3390/nu12102960 [DOI] [PMC free article] [PubMed]
- 34.Treynor W, Gonzalez R, Nolen-Hoeksema S (2003) Rumination reconsidered: a psychometric analysis. Cogn Therapy Res 27:247–259. 10.1023/A:1023910315561 [Google Scholar]
- 35.Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841. 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- 36.Woolrich MW, Ripley BD, Brady M, Smith SM (2001) Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage 14:1370–1386. 10.1006/nimg.2001.0931 [DOI] [PubMed] [Google Scholar]
- 37.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE et al (2013) An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage 64:240–256. 10.1016/j.neuroimage.2012.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faulkner P, Ghahremani DG, Tyndale RF, Hellemann G, London ED (2018) Functional connectivity of the Raphe nuclei: link to Tobacco Withdrawal in smokers. Int J Neuropsychopharmacol 21:800–808. 10.1093/ijnp/pyy054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faulkner P, Ghahremani DG, Tyndale RF, Paterson NE, Cox C, Ginder N, Hellemann G, London ED (2019) Neural basis of smoking-induced relief of craving and negative affect: contribution of nicotine. Addict Biol 24:1087–1095. 10.1111/adb.12679 [DOI] [PubMed] [Google Scholar]
- 40.Faulkner P, Dean AC, Ghahremani DG, London ED (2020) Neural basis of smoking-related difficulties in emotion regulation. Int J Neuropsychopharmacol 23:409–416. 10.1093/ijnp/pyaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2013) Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. NeuroImage 76:439–441. 10.1016/j.neuroimage.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014) Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 84, 320–341, 10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed]
- 43.Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. 10.1016/s1361-8415(01)00036-6 [DOI] [PubMed] [Google Scholar]
- 44.Andersson J, Jenkinson M, Smith S (2007) FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford
- 45.Menon V, D’Esposito M (2022) The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 47:90–103. 10.1038/s41386-021-01152-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman NP, Robbins TW (2022) The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 47:72–89. 10.1038/s41386-021-01132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snyder HR, Miyake A, Hankin BL (2015) Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front Psychol 6. 10.3389/fpsyg.2015.00328 [DOI] [PMC free article] [PubMed]
- 48.Morgenroth E, Orlov N, Lythgoe DJ, Stone JM, Barker H, Munro J, Eysenck M, Allen P (2019) Altered relationship between prefrontal glutamate and activation during cognitive control in people with high trait anxiety. Cortex 117:53–63. 10.1016/j.cortex.2019.02.021 [DOI] [PubMed] [Google Scholar]
- 49.Bari A, Robbins TW (2013) Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108:44–79. 10.1016/j.pneurobio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 50.Dalley JW, Roiser JP (2012) Dopamine, serotonin and impulsivity. Neuroscience 215:42–58. 10.1016/j.neuroscience.2012.03.065 [DOI] [PubMed] [Google Scholar]
- 51.Guzulaitis R, Palmer LM (2023) A thalamocortical pathway controlling impulsive behavior. Trends Neurosci 46:1018–1024. 10.1016/j.tins.2023.09.001 [DOI] [PubMed] [Google Scholar]
- 52.Cheng J, Ma X, Li C, Ullah R, Wang X, Long J, Yuan Z, Liu S, Fu J, Chen Z et al (2022) Diet-induced inflammation in the anterior paraventricular thalamus induces compulsive sucrose-seeking. Nat Neurosci 25:1009–1013. 10.1038/s41593-022-01129-y [DOI] [PubMed] [Google Scholar]
- 53.Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002) Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehavioral Reviews 26:321–352. 10.1016/S0149-7634(02)00007-6 [DOI] [PubMed] [Google Scholar]
- 54.Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE, Alonso-Alonso M, Audette M, Malbert CH, Stice E (2015) Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage: Clin 8:1–31. 10.1016/j.nicl.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang G-J, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusll N, Fowler JS (2001) Brain dopamine and obesity. Lancet 357:354–357. 10.1016/S0140-6736(00)03643-6 [DOI] [PubMed] [Google Scholar]
- 56.Kahleova H, Tintera J, Thieme L, Veleba J, Klementova M, Kudlackova M, Malinska H, Oliyarnyk O, Markova I, Haluzik M et al (2021) A plant-based meal affects thalamus perfusion differently than an energy- and macronutrient-matched conventional meal in men with type 2 diabetes, overweight/obese, and healthy men: a three-group randomized crossover study. Clin Nutr 40:1822–1833. 10.1016/j.clnu.2020.10.005 [DOI] [PubMed] [Google Scholar]
- 57.Johnson PM, Kenny PJ (2010) Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13:635–641. 10.1038/nn.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones KT, Woods C, Zhen J, Antonio T, Carr KD, Reith MEA (2017) Effects of diet and insulin on dopamine transporter activity and expression in rat caudate-putamen, nucleus accumbens, and midbrain. J Neurochem 140:728–740. 10.1111/jnc.13930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allan JL, Johnston M, Campbell N (2010) Unintentional eating. What determines goal-incongruent chocolate consumption? Appetite. 54:422–425. 10.1016/j.appet.2010.01.009 [DOI] [PubMed]
- 60.Allom V, Mullan B (2014) Individual differences in executive function predict distinct eating behaviours. Appetite 80:123–130. 10.1016/j.appet.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 61.Hare TA, Camerer CF, Rangel A (2009) Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324:646–648. 10.1126/science.1168450 [DOI] [PubMed] [Google Scholar]
- 62.Sieveritz B, García-Muñoz M, Arbuthnott GW (2019) Thalamic afferents to prefrontal cortices from ventral motor nuclei in decision-making. Eur J Neurosci 49:646–657. 10.1111/ejn.14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J, Wu G, Wang M, Li W, Wang Y, Ren X, Wei X, Yang Z, Li Z, Wang Z et al (2023) Exploring the thalamus: a crucial hub for brain function and communication in patients with bulimia nervosa. J Eat Disorders 11:207. 10.1186/s40337-023-00933-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holton KF, Kirkland AE, Baron M, Ramachandra SS, Langan MT, Brandley ET, Baraniuk JN (2020) The low glutamate Diet effectively improves Pain and other symptoms of Gulf War Illness. Nutrients 12. 10.3390/nu12092593 [DOI] [PMC free article] [PubMed]
- 65.Langan MT, Kirkland AE, Rice LC, Mucciarone VC, Baraniuk J, VanMeter A, Holton KF (2022) Low glutamate diet improves working memory and contributes to altering BOLD response and functional connectivity within working memory networks in Gulf War Illness. Sci Rep 12:18004. 10.1038/s41598-022-21837-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, McDonald D, Dietrich D, Ramadhar TR, Lekbua A et al (2019) GABA-modulating bacteria of the human gut microbiota. Nat Microbiol 4:396–403. 10.1038/s41564-018-0307-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trichopoulou A, Martínez-González MA, Tong TYN, Forouhi NG, Khandelwal S, Prabhakaran D, Mozaffarian D, de Lorgeril M (2014) Definitions and potential health benefits of the Mediterranean diet: views from experts around the world. BMC Med 12. 10.1186/1741-7015-12-112 [DOI] [PMC free article] [PubMed]
- 68.Muth A-K, Park SQ (2021) The impact of dietary macronutrient intake on cognitive function and the brain. Clin Nutr 40:3999–4010. 10.1016/j.clnu.2021.04.043 [DOI] [PubMed] [Google Scholar]
- 69.Sandoval-Salazar C, Oviedo-Solís CI, Lozoya-Gloria E, Aguilar-Zavala H, Solís-Ortiz MS, Pérez-Vázquez V, Balcón-Pacheco CD, Ramírez-Emiliano J (2019) Strawberry Intake ameliorates oxidative stress and decreases GABA levels Induced by High-Fat Diet in Frontal cortex of rats. Antioxidants 8. 10.3390/antiox8030070 [DOI] [PMC free article] [PubMed]
- 70.Merat S, Casanada F, Sutphin M, Palinski W, Reaven PD (1999) Western-type diets induce insulin resistance and hyperinsulinemia in LDL receptor-deficient mice but do not increase aortic atherosclerosis compared with normoinsulinemic mice in which similar plasma cholesterol levels are achieved by a fructose-rich diet. Arterioscler Thromb Vasc Biol 19:1223–1230. 10.1161/01.atv.19.5.1223 [DOI] [PubMed] [Google Scholar]
- 71.Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV et al (2019) The Microbiota-Gut-Brain Axis. Physiol Rev 99:1877–2013. 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- 72.Khavandegar A, Heidarzadeh A, Angoorani P, Hasani-Ranjbar S, Ejtahed H-S, Larijani B, Qorbani M (2024) Adherence to the Mediterranean diet can beneficially affect the gut microbiota composition: a systematic review. BMC Med Genom 17:91. 10.1186/s12920-024-01861-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merra G, Noce A, Marrone G, Cintoni M, Tarsitano MG, Capacci A, De Lorenzo A (2021) Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 13. 10.3390/nu13010007 [DOI] [PMC free article] [PubMed]
- 74.Kühn S, Vanderhasselt M-A, De Raedt R, Gallinat J (2014) The neural basis of unwanted thoughts during resting state. Soc Cogn Affect Neurosci 9:1320–1324. 10.1093/scan/nst117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caballero A, Granberg R, Tseng KY (2016) Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci Biobehav Rev 70:4–12. 10.1016/j.neubiorev.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.