Abstract
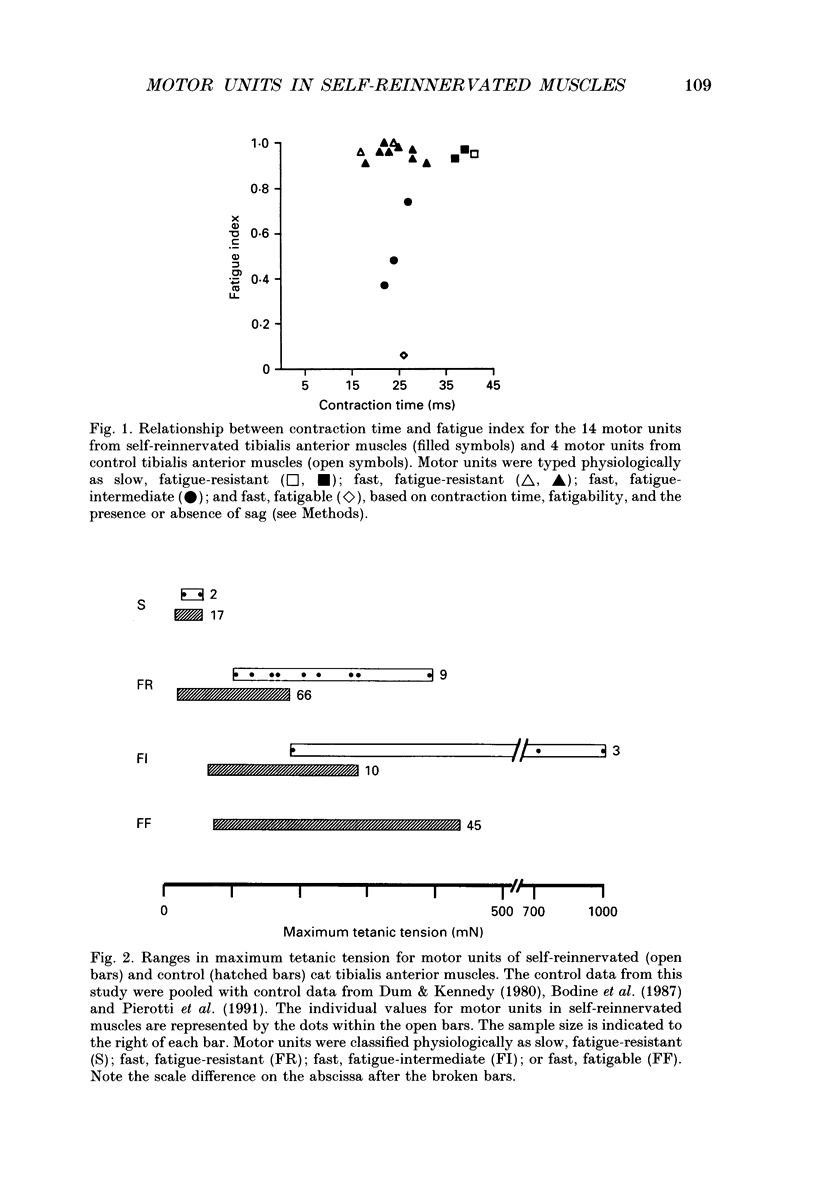
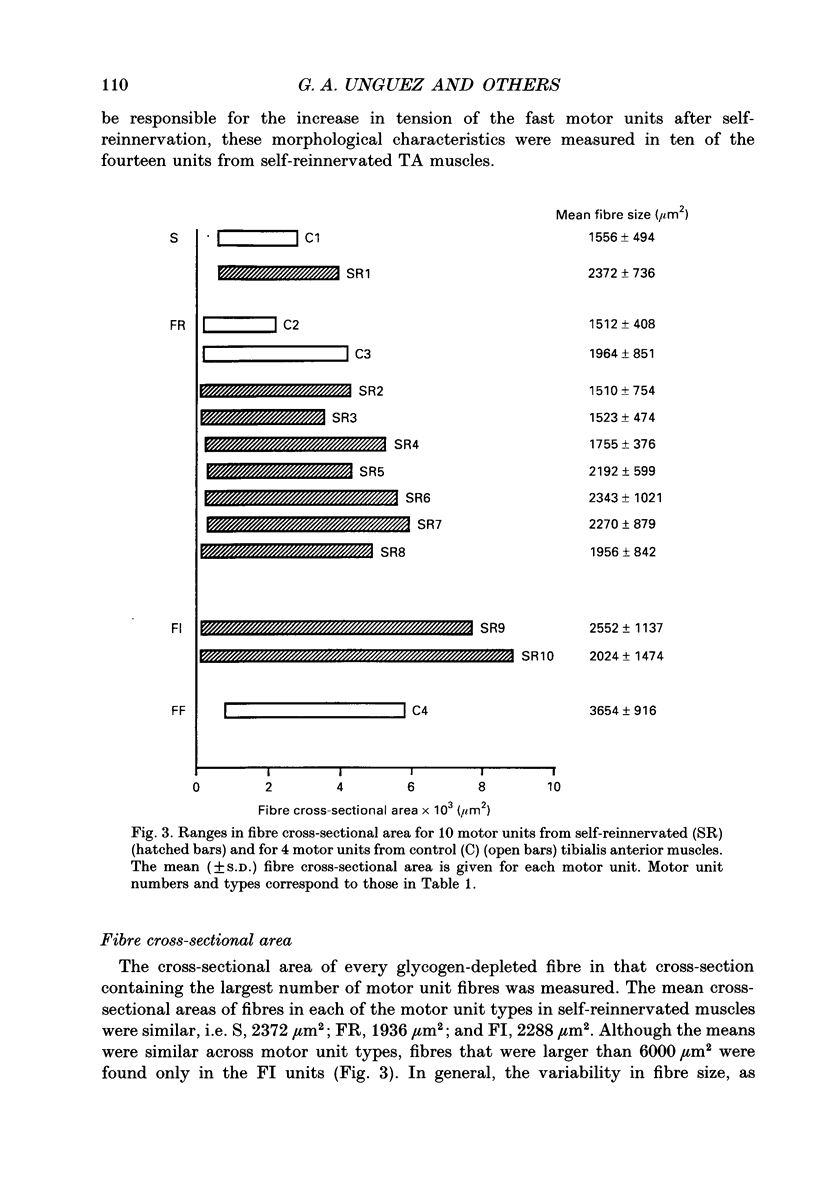
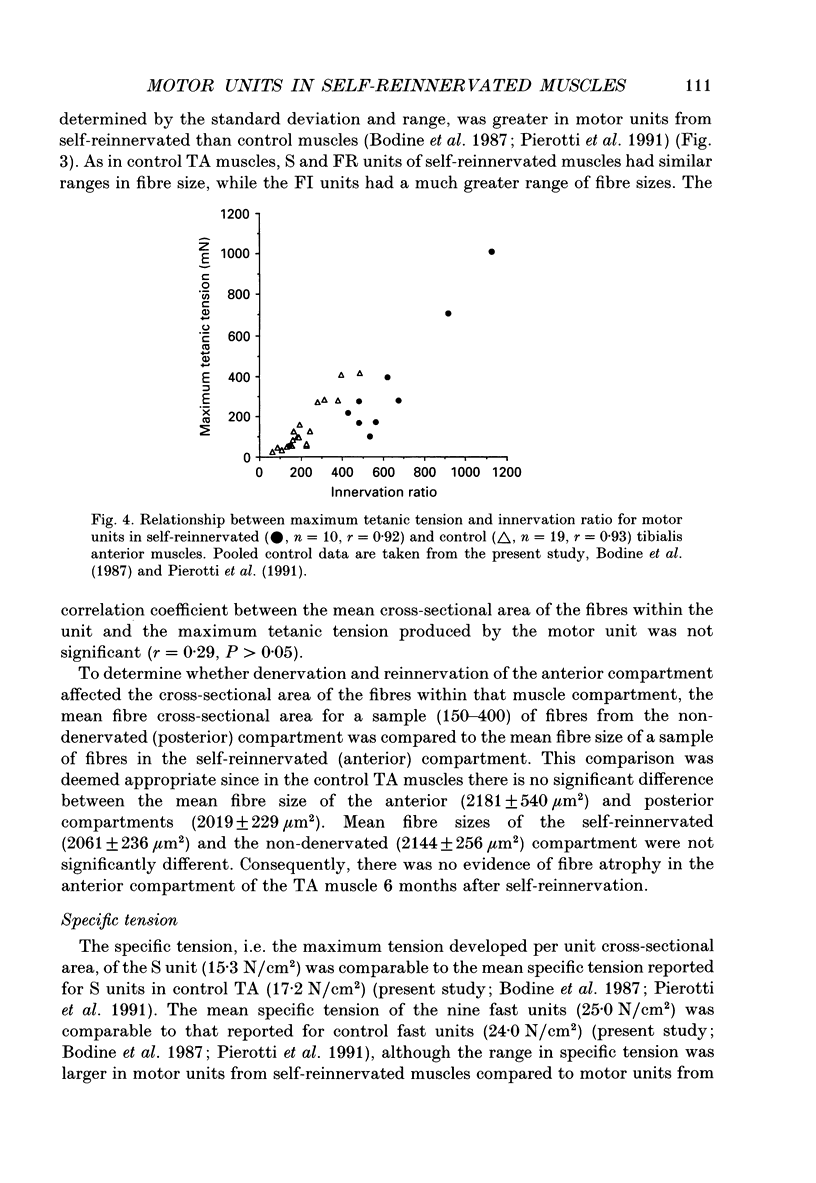
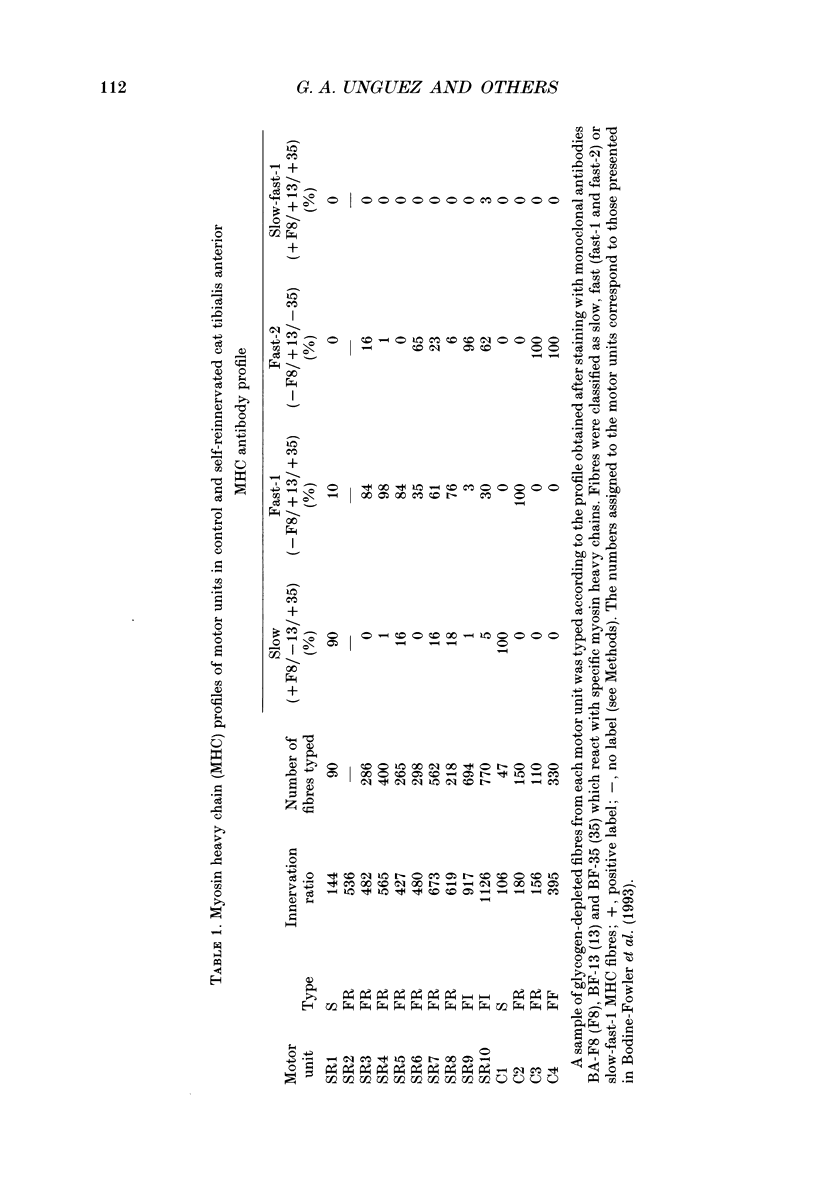
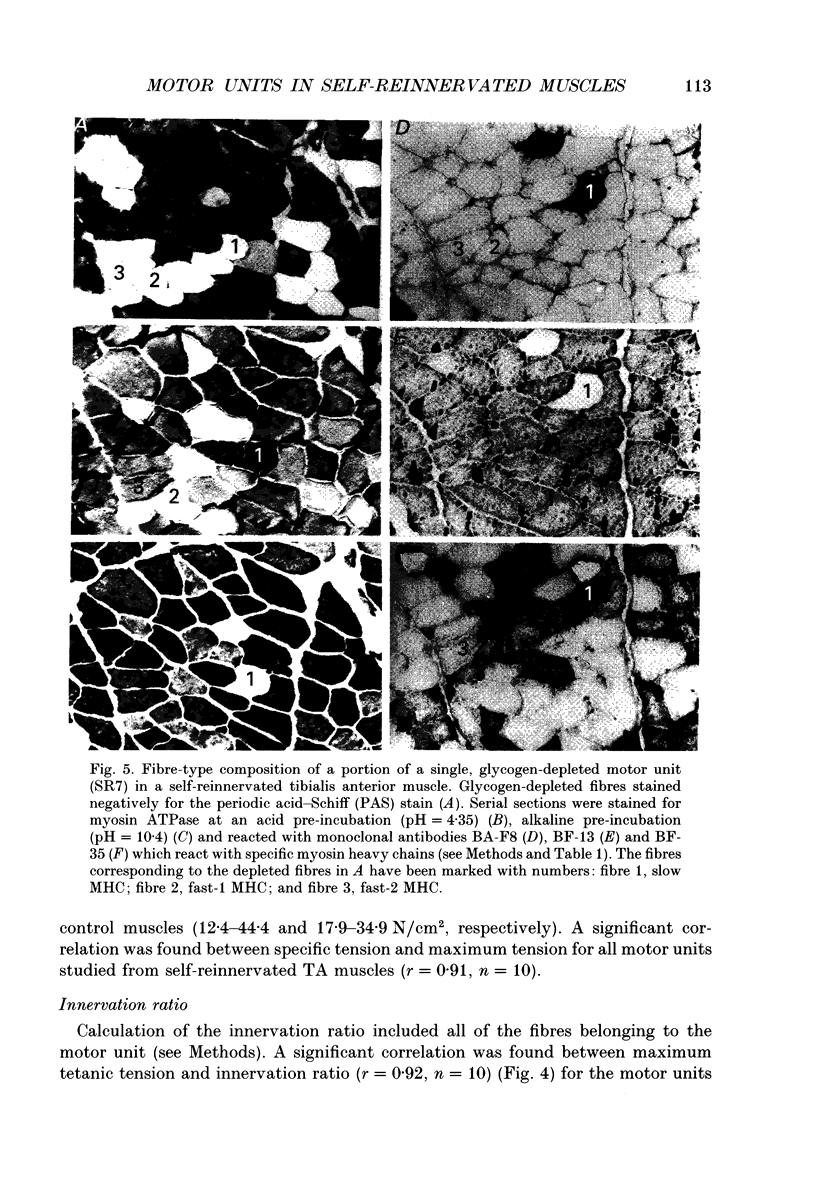
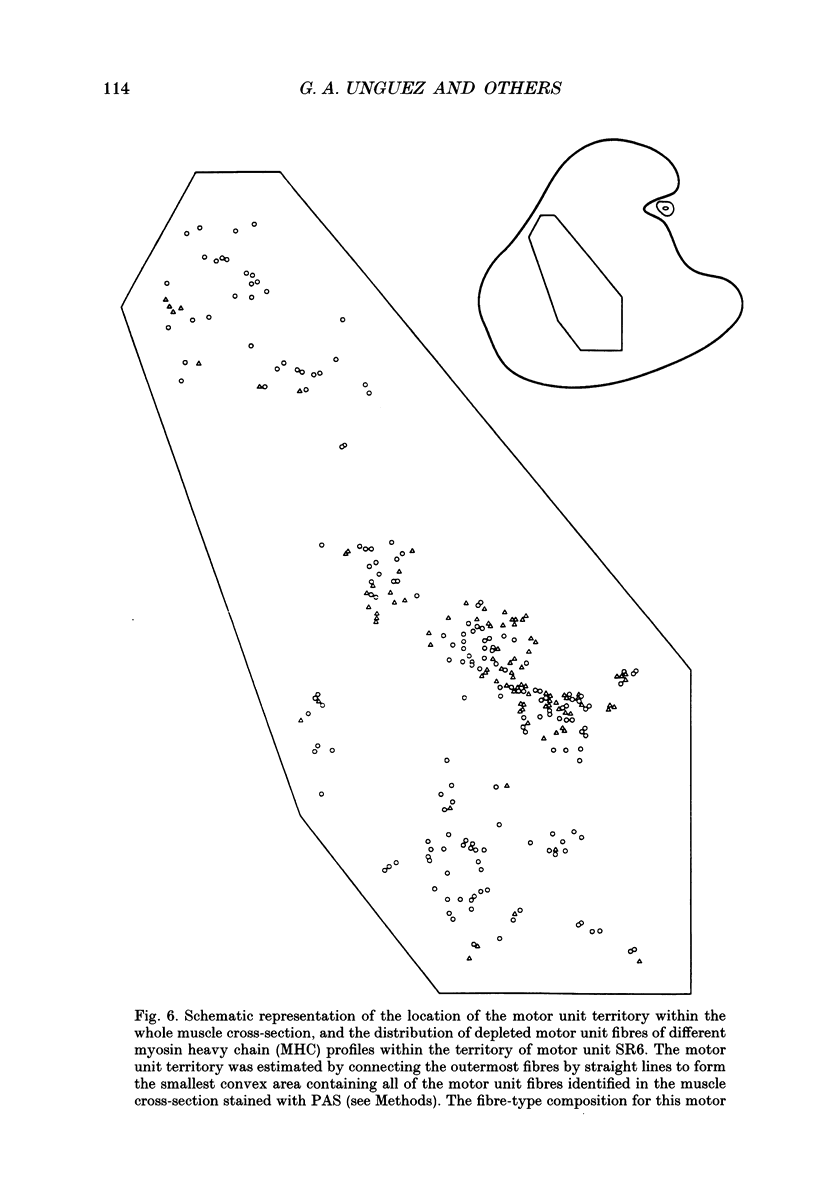
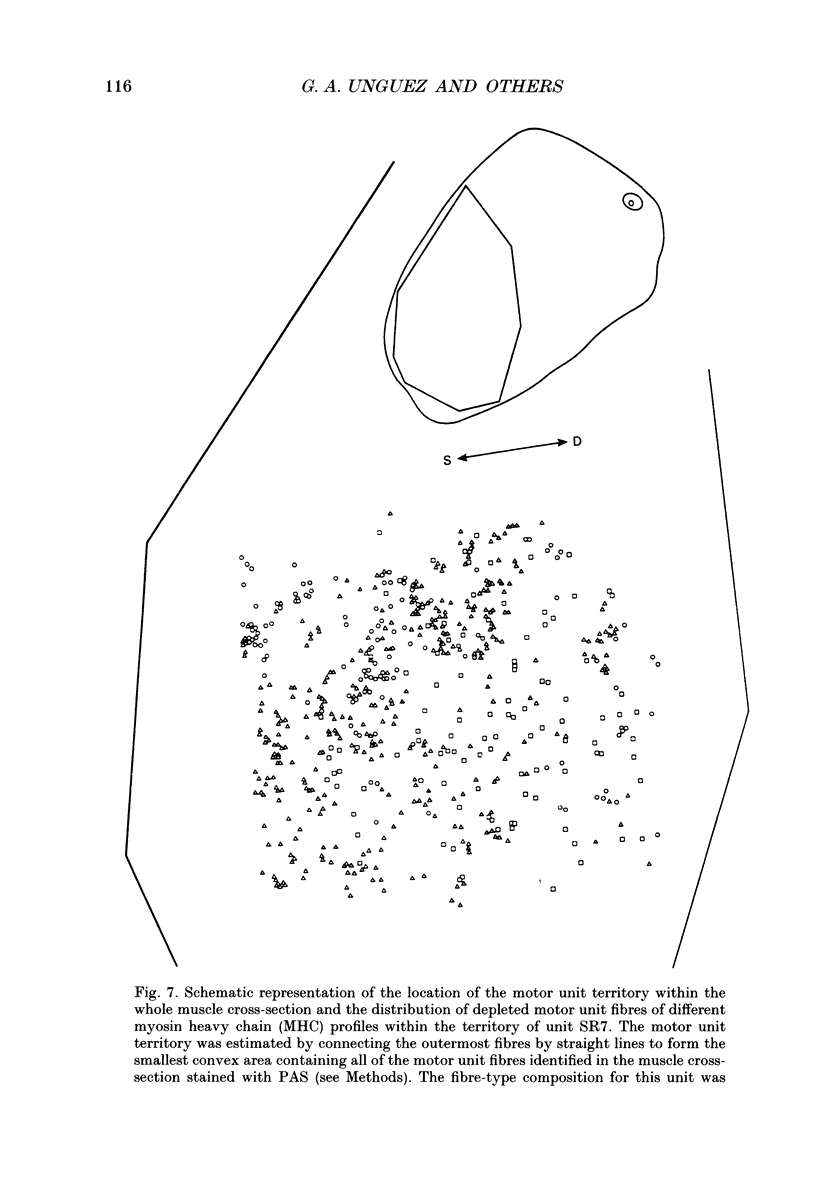
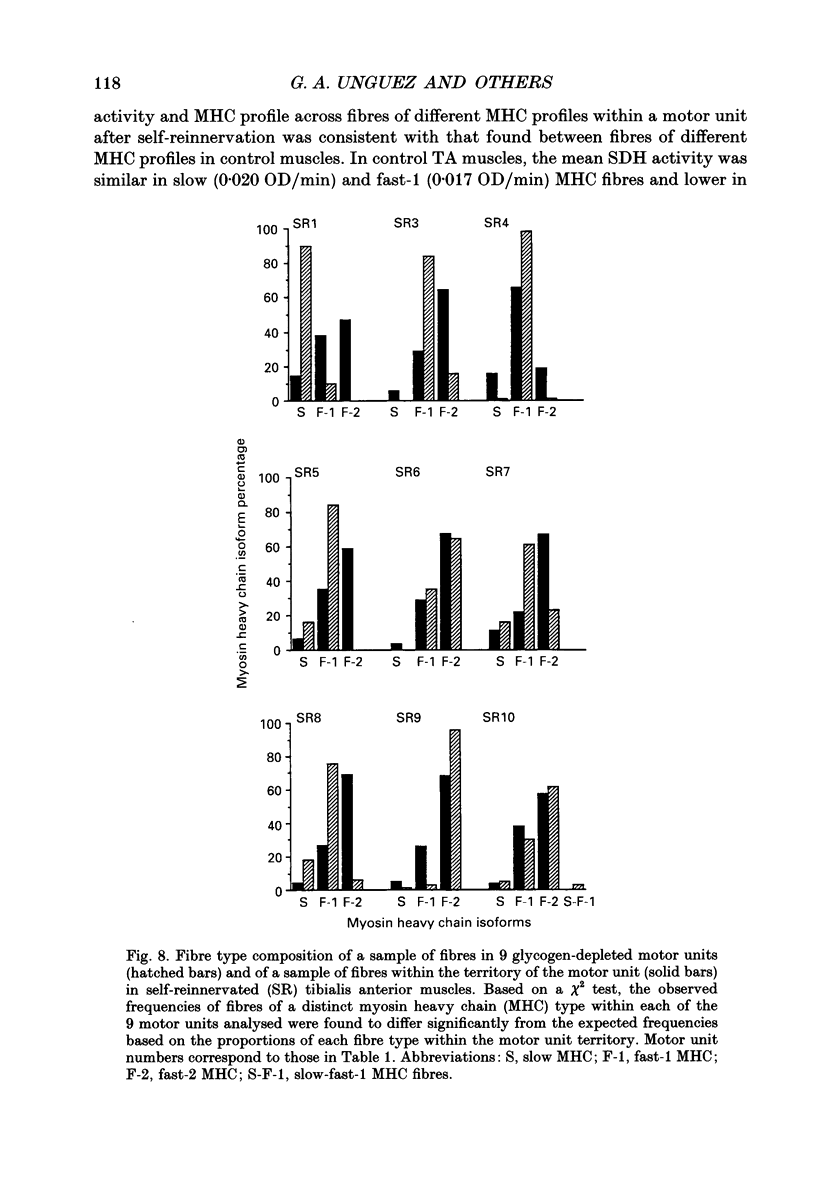
1. The mechanical, morphological and biochemical properties of single motor units from the anterior compartment of the tibialis anterior muscle in adult cats were studied six months after the nerve branches to that compartment were cut and resutured in close proximity to the muscle. 2. In these self-reinnervated muscles, the maximum tetanic tensions were lower in slow than fast units, a relationship similar to that observed among motor units from control adult muscles. The maximum tetanic tensions produced by the fast units were larger than those produced by the same motor unit types in control muscles. Direct counts of muscle fibres belonging to a motor unit showed that factors controlling the number of muscle fibres innervated by a motoneurone type persist during the reinnervation process in that fast motoneurones reinnervated more muscle fibres than slow motoneurones. Thus, the number of muscle fibres reinnervated by a motoneurone principally accounted for the difference in the maximum tension outputs among motor unit types, a relationship similar to that observed in control tibialis anterior muscles. 3. Monoclonal antibodies for specific myosin heavy chains were used to differentiate fibre types. By this criterion, motor units from control muscles were found to contain a homogeneous fibre type composition. In contrast, a heterogeneous, yet markedly biased, fibre type composition was observed in each unit analysed from self-reinnervated muscles. 4. Although not all of the muscle fibres of a motor unit developed the same type-associated parameters after reinnervation, the relationships among myosin heavy chain profile, succinate dehydrogenase activity and the fibre size were similar in fibres of control and self-reinnervated muscles. 5. The processes which dictate both motor unit size and the matching between motoneurone and muscle fibre type during the reinnervation process must be interdependent and result from a hierarchy of decisions which reflects their relative importance. The mechanisms responsible for these two processes may be a combination of: (1) selective innervation which may or may not incorporate a pruning process if multiple synaptic connections are initially formed and/or (2) conversion of enough fibres of a motor unit to form a predominant type.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagust J., Lewis D. M. Isometric contractions of motor units in self-reinnervated fast and slow twitch muscles of the cat. J Physiol. 1974 Feb;237(1):91–102. doi: 10.1113/jphysiol.1974.sp010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon R. J., Thompson W. J. Synaptic rearrangements and alterations in motor unit properties in neonatal rat extensor digitorum longus muscle. J Physiol. 1988 Apr;398:191–210. doi: 10.1113/jphysiol.1988.sp017038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of blocking the nerve with a local anaesthetic on the evolution of multiinnervation at the regenerating neuromuscular junction of the rat. Brain Res. 1978 Jun 23;149(1):89–96. doi: 10.1016/0006-8993(78)90589-9. [DOI] [PubMed] [Google Scholar]
- Bodine-Fowler S. C., Unguez G. A., Roy R. R., Armstrong A. N., Edgerton V. R. Innervation patterns in the cat tibialis anterior six months after self-reinnervation. Muscle Nerve. 1993 Apr;16(4):379–391. doi: 10.1002/mus.880160407. [DOI] [PubMed] [Google Scholar]
- Bodine-Fowler S., Garfinkel A., Roy R. R., Edgerton V. R. Spatial distribution of muscle fibers within the territory of a motor unit. Muscle Nerve. 1990 Dec;13(12):1133–1145. doi: 10.1002/mus.880131208. [DOI] [PubMed] [Google Scholar]
- Bodine S. C., Roy R. R., Eldred E., Edgerton V. R. Maximal force as a function of anatomical features of motor units in the cat tibialis anterior. J Neurophysiol. 1987 Jun;57(6):1730–1745. doi: 10.1152/jn.1987.57.6.1730. [DOI] [PubMed] [Google Scholar]
- Bottinelli R., Schiaffino S., Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991 Jun;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol. 1978 May;278:325–348. doi: 10.1113/jphysiol.1978.sp012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain S., Lewis D. M. Contractile characteristics and innervation ratio of rat soleus motor units. J Physiol. 1989 May;412:1–21. doi: 10.1113/jphysiol.1989.sp017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. K., Edgerton V. R., Goslow G. E., Jr, Kurata H., Rasmussen S. A., Spector S. A. Histochemical and physiological properties of cat motor units after self-and cross-reinnervation. J Physiol. 1982 Nov;332:343–361. doi: 10.1113/jphysiol.1982.sp014417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum R. P., Kennedy T. T. Physiological and histochemical characteristics of motor units in cat tibialis anterior and extensor digitorum longus muscles. J Neurophysiol. 1980 Jun;43(6):1615–1630. doi: 10.1152/jn.1980.43.6.1615. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R., Martin T. P., Bodine S. C., Roy R. R. How flexible is the neural control of muscle properties? J Exp Biol. 1985 Mar;115:393–402. doi: 10.1242/jeb.115.1.393. [DOI] [PubMed] [Google Scholar]
- Foehring R. C., Munson J. B. Motoneuron and muscle-unit properties after long-term direct innervation of soleus muscle by medial gastrocnemius nerve in cat. J Neurophysiol. 1990 Sep;64(3):847–861. doi: 10.1152/jn.1990.64.3.847. [DOI] [PubMed] [Google Scholar]
- Foehring R. C., Sypert G. W., Munson J. B. Properties of self-reinnervated motor units of medial gastrocnemius of cat. I. Long-term reinnervation. J Neurophysiol. 1986 May;55(5):931–946. doi: 10.1152/jn.1986.55.5.931. [DOI] [PubMed] [Google Scholar]
- Gates H. J., Ridge R. M. The importance of competition between motoneurones in developing rat muscle; effects of partial denervation at birth. J Physiol. 1992 Jan;445:457–472. doi: 10.1113/jphysiol.1992.sp018933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Burke R. E., Lowey S., Hobbs A. W. Myosin isozymes in normal and cross-reinnervated cat skeletal muscle fibers. J Cell Biol. 1983 Sep;97(3):756–771. doi: 10.1083/jcb.97.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T., Stein R. B. Reorganization of motor-unit properties in reinnervated muscles of the cat. J Neurophysiol. 1982 Nov;48(5):1175–1190. doi: 10.1152/jn.1982.48.5.1175. [DOI] [PubMed] [Google Scholar]
- Gorio A., Carmignoto G., Finesso M., Polato P., Nunzi M. G. Muscle reinnervation--II. Sprouting, synapse formation and repression. Neuroscience. 1983 Mar;8(3):403–416. doi: 10.1016/0306-4522(83)90188-4. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., OLSON C. B. RELATIONS BETWEEN STRUCTURE AND FUNCTION IN THE DESIGN OF SKELETAL MUSCLES. J Neurophysiol. 1965 May;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- Iliya A. R., Dum R. P. Somatotopic relations between the motor nucleus and its innervated muscle fibers in the cat tibialis anterior. Exp Neurol. 1984 Nov;86(2):272–292. doi: 10.1016/0014-4886(84)90186-9. [DOI] [PubMed] [Google Scholar]
- Karpati G., Engel W. K. "Type grouping" in skeletal muscles after experimental reinnervation. Neurology. 1968 May;18(5):447–455. doi: 10.1212/wnl.18.5.447. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Edström L., Abbruzzese M. Mapping of motor units in experimentally reinnervated rat muscle. Interpretation of histochemical and atrophic fibre patterns in neurogenic lesions. J Neurol Neurosurg Psychiatry. 1970 Jun;33(3):319–329. doi: 10.1136/jnnp.33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff A. R., Hatcher D. D., Torkko K. Enlarged motor units resulting from partial denervation of cat hindlimb muscles. J Neurophysiol. 1988 May;59(5):1377–1394. doi: 10.1152/jn.1988.59.5.1377. [DOI] [PubMed] [Google Scholar]
- Martin T. P., Bodine-Fowler S., Roy R. R., Eldred E., Edgerton V. R. Metabolic and fiber size properties of cat tibialis anterior motor units. Am J Physiol. 1988 Jul;255(1 Pt 1):C43–C50. doi: 10.1152/ajpcell.1988.255.1.C43. [DOI] [PubMed] [Google Scholar]
- Nemeth P. A., Cope T. C., Kushner S., Nemeth P. M. Spatial arrangement and metabolic capacity of fiber types in self-reinnervated cat muscle. Am J Physiol. 1993 Feb;264(2 Pt 1):C411–C418. doi: 10.1152/ajpcell.1993.264.2.C411. [DOI] [PubMed] [Google Scholar]
- Nemeth P. M., Turk W. R. Biochemistry of rat single muscle fibres in newly assembled motor units following nerve crush. J Physiol. 1984 Oct;355:547–555. doi: 10.1113/jphysiol.1984.sp015437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry D. J., Wilkinson R. S. The effect of reinnervation on the distribution of muscle fibre types in the tibialis anterior muscle of the mouse. Can J Physiol Pharmacol. 1990 May;68(5):596–602. doi: 10.1139/y90-086. [DOI] [PubMed] [Google Scholar]
- Pierotti D. J., Roy R. R., Bodine-Fowler S. C., Hodgson J. A., Edgerton V. R. Mechanical and morphological properties of chronically inactive cat tibialis anterior motor units. J Physiol. 1991 Dec;444:175–192. doi: 10.1113/jphysiol.1991.sp018872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich M. M., Lichtman J. W. In vivo visualization of pre- and postsynaptic changes during synapse elimination in reinnervated mouse muscle. J Neurosci. 1989 May;9(5):1781–1805. doi: 10.1523/JNEUROSCI.09-05-01781.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soileau L. C., Silberstein L., Blau H. M., Thompson W. J. Reinnervation of muscle fiber types in the newborn rat soleus. J Neurosci. 1987 Dec;7(12):4176–4194. doi: 10.1523/JNEUROSCI.07-12-04176.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS P. K. CHANGES IN THE ENDONEURIAL SHEATHS OF PERIPHERAL MYELINATED NERVE FIBRES DURING WALLERIAN DEGENERATION. J Anat. 1964 Apr;98:175–182. [PMC free article] [PubMed] [Google Scholar]
- Taxt T. Local and systemic effects of tetrodotoxin on the formation and elimination of synapses in reinnervated adult rat muscle. J Physiol. 1983 Jul;340:175–194. doi: 10.1113/jphysiol.1983.sp014757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tötösy de Zepetnek J. E., Zung H. V., Erdebil S., Gordon T. Innervation ratio is an important determinant of force in normal and reinnervated rat tibialis anterior muscles. J Neurophysiol. 1992 May;67(5):1385–1403. doi: 10.1152/jn.1992.67.5.1385. [DOI] [PubMed] [Google Scholar]
- West S. P., Roy R. R., Edgerton V. R. Fiber type and fiber size of cat ankle, knee, and hip extensors and flexors following low thoracic spinal cord transection at an early age. Exp Neurol. 1986 Jan;91(1):174–182. doi: 10.1016/0014-4886(86)90035-x. [DOI] [PubMed] [Google Scholar]



