Abstract
Griscelli syndrome type 2 (GS2) is a rare, life-threatening immunodysregulatory disorder characterised by impaired cytotoxic activity leading to susceptibility to haemophagocytic lymphohistiocytosis (HLH) and hypopigmentation. We completed a literature review and analysis of clinical data of 149 patients with GS2 including 8 new patients.
We identified three founder mutations which show diverse phenotypic profiles (RAB27A c.244 C > T, p.R82C, c.514_518delCAAGC, p.Q172NfsX2, c.550 C > T, p.R184X). The most common presentation was HLH (119/149, 80%), with high proportion of central nervous system involvement (68/149, 46%). Features of partial albinism were present in 105 of 149 cases (70%). Hypopigmentation can be absent in GS2 and should not exclude the diagnosis. Patients with biallelic protein truncating variants (PTV) were more likely to have systemic HLH (44/56, 79%) and partial albinism (45/56, 80%), in comparison to hypomorphic variants (9/41, 22%; 20/41, 49%). Patients with hypomorphic variants presented later (5.4 years cf. 0.4 years, p = < 0.0001) and were more likely to have isolated CNS HLH (2% cf. 42%, p = 0.001).
Mortality was high in the cohort (50/149, 34%). Survival of cases post-HLH who underwent transplantation is superior to un-transplanted patients, suggesting adequate HLH control followed by early HSCT is highly beneficial. Mortality was reduced in HSCT recipients versus the un-transplanted group where follow-up data was available (14% compared to 58%).
Asymptomatic cases identified through family history/genetic screening may benefit from pre-emptive HSCT, but access and development of robust functional testing are required. High mortality related to HLH remains concerning and emphasises the need for improved molecular characterisation and clinical prognostic factors to guide management decisions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-024-01842-2.
Introduction
Griscelli syndrome is a rare autosomal recessive disorder characterized by partial albinism first described by the French paediatrician Claude Griscelli in 1978 [1]. Since then, three distinct types of the syndrome have been genetically defined, with biallelic RAB27A genetic mutations associated with Griscelli syndrome type 2 (GS2), (OMIM: 607624). This is a clinically heterogeneous syndrome primarily featuring hypopigmented skin, silvery-grey hair and cellular immunodeficiency with propensity to haemophagocytic lymphohistiocytosis (HLH) [2, 3]. It is distinct from the other hypopigmentary Griscelli syndromes in its association with HLH: type 1 (OMIM: 214450) caused by biallelic MYO5A mutations, and type 3 (OMIM: 609227) caused by biallelic MLPH pathogenic mutations.
The RAB27A gene is located on chromosome 15q and contains 7 exons, with the first 2 being non-coding. RAB27A encodes a small 95 kDa GTPase widely expressed in secretory cells and tissues, including melanocytes and granulocytes. Rab27a acts as a molecular switch by cycling between an active guanosine triphosphate (GTP) binding state, and the inactive hydrolysing GTP to guanosine diphosphate (GDP), translating it to its ‘inactive’ form. Two switch regions have been shown to change conformation upon GDP or GTP binding; switch I localised to 10 amino acids in exon 3, and switch II region, approximately 17 amino acids straddling exon 4 and 5 [4]. Biallelic loss of function mutations are thought to abolish RAB27A expression resulting in reduced vesicular formation and transport [5]. Rab27a protein is critical for multi-vesicular endosome trafficking, fusion and docking at the plasma membrane [6–8]. Specifically, it is required for the trafficking of melanosomes in melanocytes and facilitating the exocytosis of granules from cytotoxic T cells (CTL), neutrophils and natural killer (NK) cells [9, 10] which underlies the clinical manifestations of hypopigmentation and immune deficiency. Previous studies have sought to characterise critical protein binding sites of Munc13-4 and Slp2a, its effector protein, on Rab27a, particularly in the absence of specific functional domains of the protein. These have identified hypomorphic mutations of RAB27A which demonstrate potential melanophilin (Mlph) binding sites including R184 which disrupt granule formation in the absence of partial albinism [6, 11–13]. In RAB27A deficiency, intracellular cytotoxic granule release is disrupted resulting in failure to clear infected cells, with excessive activation of macrophages and failure to terminate the immune response [6, 12]. To confirm disruption of the cytotoxic granule pathway, a granule release assay can be used. This is a flow cytometric test which detects a cell surface protein CD107a, as a marker of functional cytotoxic granule formation in lymphocytes [14, 15]. The assay is specific in identifying lymphocyte defects critical for degranulation and exocytosis in lymphocytes such as Rab27a, Syntaxin 11, Munc 13 − 4, and Munc 18 − 2; the absence of these proteins are known to cause HLH [14, 15]. This functional assay can support the diagnosis in affected patients particularly where a genetic diagnosis is not yet made.
Mortality of patients affected by GS2 is high due to the predisposition to HLH [16]. The precise triggers for the development of HLH are varied but may not be identified. HLH is a life-threatening immunological disorder characterised by hyperinflammation and release of inflammatory cytokines, with fever, hepatosplenomegaly and cytopenia [17, 18]. Treatment protocols featuring immunosuppressive and cytotoxic medication have been developed to optimise outcomes in patients [19–21]. Haematopoietic stem cell transplantation (HSCT) is the only curative option for patients with GS2 [22–24].
Despite the many case reports of the clinical manifestations in GS2, several unresolved questions remain. Herein, we analyse the genetic, phenotypic features and outcomes of reported patients with GS2 in the literature, including 8 unpublished cases identified in our patient cohort [2, 3, 6, 11–13, 16, 22–68].
Methods
We aimed to collate all patients with GS2 at our centre and in the literature to determine collective clinical features and patient outcomes. A systematic search was carried out utilizing the PubMed/MEDLINE database up to November 2023. The key search terms employed were “Griscelli syndrome type 2, Griscelli, RAB27A, haemophagocytic lymphohistiocytosis, hemophagocytic lymphohistiocytosis, HLH” aimed at identifying pertinent articles published in the English language and indexed in this comprehensive database. A screening process was conducted through two independent co-authors (ARB, MS). Studies using animal models, in-vitro models, and articles in languages other than English were excluded. Articles that were duplicates or lacked clinical features relevant to GS2 were excluded. Data collection included the following parameters: epidemiological features, clinical symptoms, laboratory and molecular data of the patients, including treatment information where available. Patient ethnicity or race was reported as originally described in papers. We also included 8 patients with GS2 from our centre who were not previously reported (P1-8).
Statistical Analysis
Descriptive statistics were reported for quantitative data and performed using Graphpad Prism v10 (La Jolla, CA, USA). For variables with normal distribution, median and interquartile ranges (IQR) were reported. Associations between variables and clinical characteristics were compared with the use of the chi-square test (categorical variables) or unpaired t-test (continuous variables).
Haplotype Analysis
Multipoint linkage and LOD scores were performed using the Superlink-Online SNP 1.1 [69]. For the parametric linkage analysis, fully penetrant autosomal recessive inheritance was assumed with a disease allele frequency of < 0.001. Suggestive linkage peaks with LOD scores > 2 analysed with SNP markers were selected from the whole exome sequencing variants of probands, where whole genome sequencing data were available.
Granule Release Assay
Where available, patient-derived cells were interrogated for their ability to release cytotoxic granules by detecting the presence of CD107a, a cell surface protein on CTL and/or NK cells. Whole-blood samples (5–10 mL) were collected in EDTA-containing vials. A healthy control sample was processed in parallel to each patient sample. Peripheral blood mononuclear cells were separated from whole blood and stimulated overnight with IL-2. The cells were stimulated with PHA or anti-CD3 antibody in the presence of anti-CD107a antibody conjugated with FITC for 2 h, then stained with cell surface markers and analysed by flow cytometry. An abnormal result is reported when the increase in percentage of CD107a between stimulated and unstimulated samples was < 0.5% for cytotoxic T cells after anti-CD3 stimulation and/or < 5% for NK cells after PHA stimulation [70].
Results
Literature Review
In total, 114 articles were identified from the initial search, with 2 added after review. Following exclusion for duplicates or insufficient clinical details, 54 articles met the criteria and were deemed suitable for inclusion. The literature search identified 141 individual case reports of GS2 published in the literature until November 2023. In addition, 8 patients not previously described in the literature were identified from Great Ormond Street Hospital, London, United Kingdom for inclusion in the study following genetic diagnosis of RAB27A deficiency.
Demographic Characteristics
In total, 149 patients were included in the study. The median age at GS2 diagnosis was 1.5 years, with a wide range spanning from 10 days to 42 years. 74 were males (50%), 57 were females (38%), and data on sex were unavailable for 18 cases (12%). Patients of Qatari descent were most frequently found in our study cohort (n = 20, 14%), followed by Turkish descent (n = 15, 10%). Asymptomatic molecular diagnosis was made based on positive family history in 12 cases (8%). The demographic data of all patients is summarised in Table 1.
Table 1.
Demographic data of patients with RAB27A deficiency. Number of samples is denominator where data were available. Median with interquartile range (IQR) shown in age of onset/death)
| Parameters (number of patients) | Results (%, or IQR) |
|---|---|
| Study total: | 149 patients |
| Sex ratio, Male: Female | 74:57 (50:38) |
| Age of onset (years) | 1.5 (0.3-5) |
| Consanguinity | 97 (65.1%) |
| Age of HLH diagnosis (years) | 1.4 (0.4–4.1) |
| Age of death (years) | 4.75 (0.96-10) |
| Overall mortality | 50 (33.5%) |
| Late onset (≥ 10y) | 16 (10.7%) |
| Molecular diagnosis: | 139 patients |
| Protein-truncating variants (PTV) | 56 (37.6%) |
| Hypomorphic variants (Missense) | 41 (27.5%) |
| Other (compound PTV + missense) | 42 (28.1%) |
Molecular Analysis
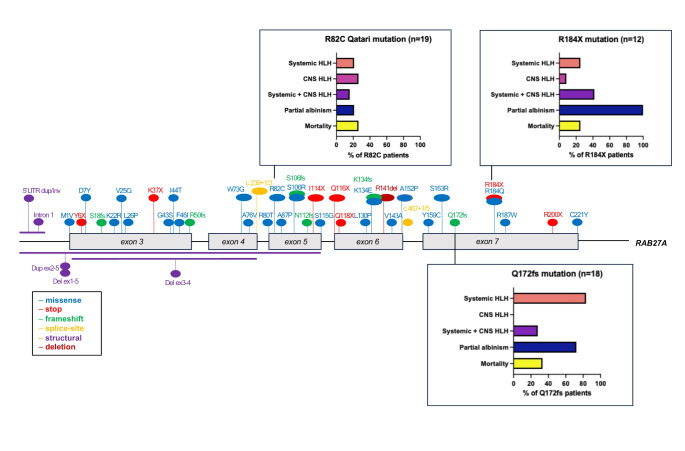
Genomic reports were available in 139 patients (93%). Overall, 56 distinct pathogenic sequence variants of RAB27A were identified, including intronic and structural variants. The mutations were distributed throughout the RAB27A gene, with several mutational hotspots identified (Fig. 1 and Supplemental Table 1). Analysis of haplotype blocks and literature review identified 3 potential founder mutations where genetic data were available: RAB27A c.244 C > T p.Arg82Cys, c.514_518del p.Gln172fs, c.550 C > T, p.Arg184X (NM_004580) [41, 71]. The RAB27A c.244 C > T p.Arg82Cys homozygous variant was only seen in Qatari patients. A shared haplotype block comprising 82 kilobases across the c.244 C > T p.Arg82Cys variant in 4 unrelated (Qatari) pedigrees. Shared haplotype blocks of 126 kilobases were found in 3 unrelated pedigrees across the c.514_518del p.Gln172fs variant and 5 unrelated patients with the c.550 C > T, p.Arg184X variant, suggestive of single recombination events from different common ancestors.
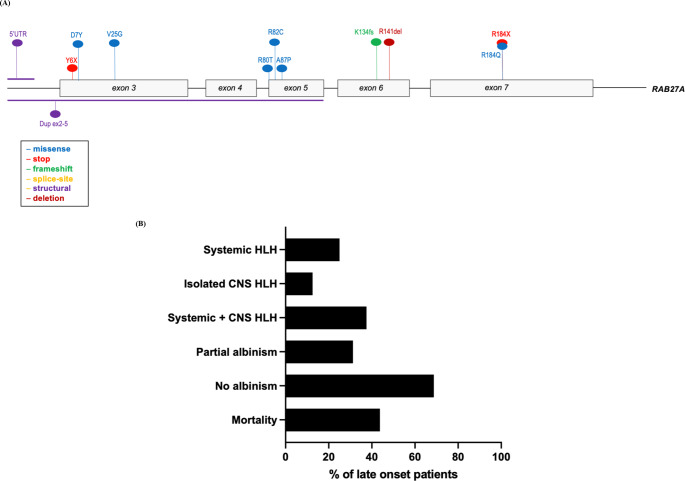
Fig. 1.
RAB27A biallelic pathogenic variants. Founder mutations with phenotypic features depicted in bar charts. The first 2 exons of RAB27A are non-coding
Figure 1 shows the three founder mutations identified with different phenotypic profiles and outcomes. Parental consanguinity was reported in nearly two-thirds of the cases reviewed (n = 97, 65%) with biallelic mutations mostly homozygous, indicative of consanguinity and founder effects.
Clinical Manifestations
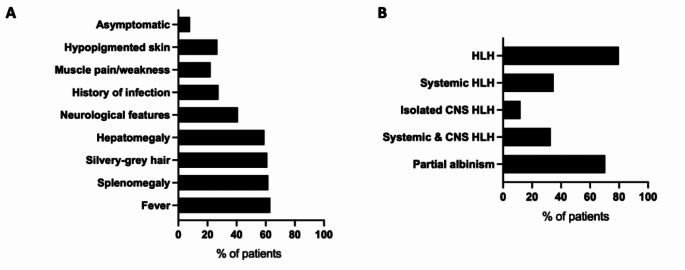
Clinical features were described in all 149 patients reported. Presenting clinical features included fever (n = 94, 63%), splenomegaly (n = 92, 62%), hepatomegaly (n = 88, 59%), neurological features (n = 61, 41%) and muscle weakness or myalgia (n = 33, 22%), (Fig. 2A). Neurological features include signs such as ataxia, strabismus (n = 55, 37%), seizures (n = 21, 14%), and developmental delay or cognitive dysfunction (n = 15, 10%), A large proportion of patients had silvery-grey hair (n = 75, 50%) with a smaller number having hypopigmented skin (n = 32, 21%) (Fig. 2A). A minority of patients were asymptomatic (n = 12, 8%), having received molecular diagnosis following positive family history. A history of infection was noted in a quarter of the cases evaluated (n = 40, 27%), with more than half reporting frequent viral infections (Fig. 2A).
Fig. 2.
A: Frequency of occurrence of clinical features. Values represent percentages of total cases (n = 149). B: Frequency of occurrence of diagnoses. Values represent percentages of total cases (n = 149)
Most patients developed HLH (n = 119, 80%). Information about HLH status was unavailable for 12 cases (8%) (Fig. 2B). Among the patients with HLH, nearly half presented with systemic HLH only (n = 52, 35%), combined systemic and central nervous system (CNS) HLH (n = 49, 33%), with the remaining cases presenting with isolated CNS HLH (n> = 18, 12%) HLH (Fig. 2B). HLH triggers were not adequately described in the literature to allow comment on this. Partial albinism was described in most patients as defined by the presence of one of the following: features of hypopigmented skin, silvery-grey hair, and uneven melanin pigmentation of the hair shaft detected by light microscopy (n = 105, 70%). 31 patients (21%) did not have any features of partial albinism. 5 patients in the cohort developed lymphoma which was successfully treated with chemotherapy (3%), with 2 specifically EBV-associated large B cell lymphoma [57, 58, 68].
Genotype-Phenotype Correlation
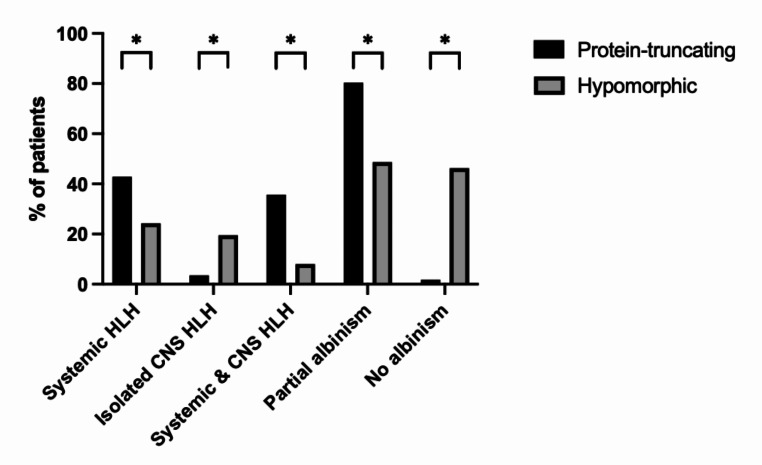
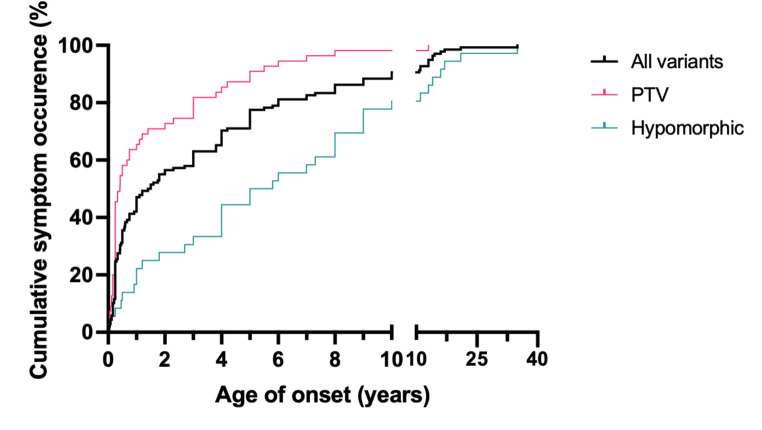
To analyse genotype-phenotype correlation, the patients were divided into those with: (1) protein-truncating variants (PTV) (n = 56; 38%), expected to abolish Rab27a protein expression and function, and (2) hypomorphic variants (n = 41; 28%) such as compound heterozygous or biallelic missense mutations that are predicted to have residual Rab27a expression. Our analysis found patients with biallelic PTV were more likely to have HLH, particularly systemic HLH (24/56, 43%) and partial albinism (45/56, 80%), in comparison to hypomorphic variants (9/41, 22%; 20/41, 49%) (Fig. 3). However, isolated CNS HLH occurred more frequently in patients with hypomorphic variants (8/41, 20% compared to 2/56, 4%, p = 0.002). Only 1 patient with biallelic PTV did not have any features of partial albinism, compared to 19 patients with hypomorphic variants (2% compared to 46%, p = 0.001). The median age of onset of symptoms was 1.5 years (IQR = 0.3-5 years), and patients with PTVs presented at an earlier age (median age: 0.4 years, IQR = 0.25–2.7 years) than patients with biallelic hypomorphic mutations (median age: 5.4 years, IQR = 1.65-9 years) (X2 = 24.54, p = < 0.0001) (Fig. 4).
Fig. 3.
Frequency of clinical features according to genotype groups. Compound variant types were excluded * denotes p-value < 0.05 from unpaired t-tests
Fig. 4.
Onset of any symptoms in patients with PTVs and hypomorphic variants in Griscelli syndrome type 2
The most common pathogenic variant was the Qatari homozygous variant c.244 C > T, p.R82C which was identified in 19 of the study cohort (13%). This variant did not have typical features of GS2 as only 4 patients presented with systemic HLH (21%) and 4 patients had features of partial albinism (21%), while 5 had CNS HLH (26%). This contrasts with the founder variant c.514–518delCAAGC, p.Q172NfsX2 (n = 18) which had 15 patients presenting with systemic HLH (83%) and 13 patients with partial albinism (72%). Isolated CNS HLH was not found in any patients with the Q172fs founder variant and co-occurred with systemic HLH in 5 cases (28%) (Fig. 1). The Qatari variant R82C also differed from the c.550 C > T, p.R184X homozygous variant which is found in 12 patients in the study cohort. All 12 patients presented features of partial albinism, 3 developed systemic HLH (25%) and 5 CNS HLH (41%) (Fig. 1).
Characteristics of Late-Onset Presentation
Late onset (at age ≥ 10 years) was noted in 16 cases (11%) (Fig. 5A) [13, 41]. 8 patients presented with homozygous missense hypomorphic variants (50%), while only 1 had a homozygous PTV (6%). The most common presenting feature in patients with late onset was systemic HLH (n = 10, 63%), with 8 patients also presenting with CNS HLH (50%) (Fig. 5B). Viral infections were found at HLH presentation in 8 patients (50%), with EBV implicated in 5 patients (31%). Late onset patients were more likely not to have partial albinism (n = 11, 69%) (Fig. 5B).
Fig. 5.
(A) RAB27A pathogenic variants in patients who presented at 10 years and over. The first 2 exons are non-coding (B) Phenotypic characteristics of patients with Griscelli syndrome type 2 presenting at age 10 or older (n = 16)
Granule Release Assay
Granule release assay results were available for 46 patients. 41 of 46 patients (88%) had reduced or absent granule release. In our local cohort (P1-8), we observed normal or equivocal granule release assay, and normal or equivocal NK cytotoxicity assay for P1-5 on repeated occasions, all patients being in an extended pedigree with Qatari R82C variant. Other R82C patients were found to have reduced NK cell cytotoxicity [11, 41].
Management Approach
For HLH-directed therapy, 22 patients received HLH-94 protocol (22/119, 18%) whilst 15 received the HLH-2004 protocol (15/119, 13%) as first-line treatments. Data specific for CNS HLH treatment were limited in patients with GS2, but individual reports describe successful regimens have included the use of high-dose corticosteroids and mycophenolate mofetil (MMF) [56, 58].
HSCT was undertaken in less than one-third of published cases (n = 44, 26%). The most common donors for HSCT were matched unrelated donors (n = 17), followed by matched related, sibling donors (n = 5). Haploidentical/mismatched related donors were used in 4 patients (15%). 5 patients were transplanted following isolated CNS HLH (5/44, 11%) with 4 patients surviving. 5 patients were transplanted pre-emptively without a prior diagnosis of HLH (n = 5, 11%) (Table 2) [39, 41, 43].
Table 2.
Characteristics of patients who had haematopoietic stem cell transplantation (HSCT). Subsections are given as percentage of number of patients where data is available
| HSCT Characteristics | Number of patients (percentage %) |
|---|---|
| Total number of patients | 44 |
| Age at transplant (years), median, IQR | 1.8 (0.5–6.6) |
| Patients who received 2 HSCT | 1 (2.2%) |
| Asymptomatic prior to HSCT | 5 (11.4%) |
| Mortality | 6 (13.6%) |
| HSC source | 44 |
| Bone marrow/stem cell | 38 (86.4%) |
| Peripheral blood | 1 (2.3%) |
| Cord blood | 5 (11.4%) |
| Donor | 26 |
| Matched unrelated | 17 (65.4%) |
| Matched related, sibling | 5 (19.2%) |
| Haploidentical/ Mismatched related | 4 (15.4%) |
| Complications | 34 |
| No complications | 18 (52.9%) |
| Neurological sequelae (HLH and non-HLH related) | 4 (11.8%) |
| Sepsis, infection | 6 (17.6%) |
| Fatal sepsis | 5 (14.7%) |
| Other | 6 (17.6%) |
Survival and Mortality Outcomes
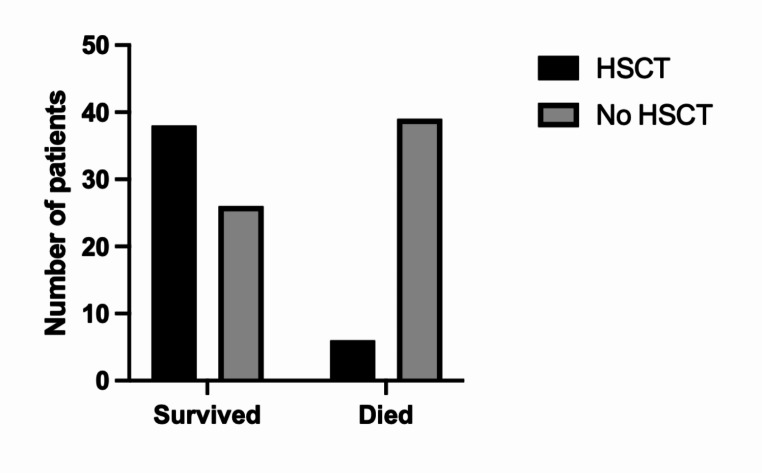
Overall, mortality was high (n = 50, 34%) in the study cohort. Data were not available to delineate specific causes of death or long-term outcomes in all patients. All patients who developed systemic HLH who did not receive HSCT shortly after, either died or were left with significant morbidity [43]. HSCT was undertaken in 44 patients with 18 having an uncomplicated post-HSCT course (41%) (Table 2). Donor information was only available in 25/44 patients (57%). Persistent neurological complications remained despite HSCT in 4 patients (9%), and fatal infections in the post-HSCT period occurred in 5 patients (11%). Among transplanted patients, 86% survived after HSCT (38/44). Of the 6 patients who died following HSCT, 5 had developed systemic and/or CNS HLH prior to HSCT (Fig. 6).
Fig. 6.
Comparison of outcomes in patients who received HSCT to those who did not
Discussion
GS2 caused by RAB27A pathogenic variants, is a rare syndromic inborn error of immunity. Our study collates the largest cohort of GS2 patients to date: a total of 149 cases. The main clinical features of immunodeficiency and partial albinism usually become apparent in the first few years of life with features of fever, splenomegaly and/or HLH. Despite the distinct characteristics of GS2, immunological and/or genetic evaluation was not initiated for all patients early in life. As the differential diagnosis of partial albinism is wide and includes secondary causes such as nutritional deficiencies, a clinical or genetic diagnosis in patients is often not sought or may not be readily available [12, 72]. Diagnosis of the syndrome often occurs in the context of HLH, which can consequently lead to challenges in management and high mortality. Detection of cell surface CD107a by granule release assay is a reliable test of granule exocytosis but was only carried out in a minority of patients. Of note, we found 5 patients with RAB27A c.244 C > T, p.R82C variant with no detectable deficit in granule release nor NK cell killing. Given this, specific genetic testing is still recommended to confirm the diagnosis. Awareness of the condition may expedite immunological and genetic testing in children with suspected GS2 and promote monitoring for complications such as HLH.
We found biallelic PTV, missense and structural RAB27A pathogenic variants were distributed throughout the gene without any apparent clustering regions. We also found survival rates of patients with PTV mutations were lower compared to those with hypomorphic mutations. This suggests residual RAB27A protein expression may be protective, or that some genetic variants may cause milder disease via their specific interactions at a cellular level. Further studies are required to ascertain the molecular basis of these findings, to explore potential genotype-phenotype correlations and to gain a deeper understanding of the underlying mechanisms driving this complex and rare disorder.
In addition, we identified 3 founder mutations with distinct genotype: phenotype characteristics. Consanguinity was present in more than 65% of cases with 3 founder variants identified in predominantly European and Middle Eastern populations. Our genetic studies confirm a high prevalence of patients with a founder mutation due to co-existing consanguinity.
Heterogenous and atypical presentations can cause diagnostic delays. Consistent with previous studies, our study shows that partial albinism is not a pre-requisite feature of GS2 [6, 12]. We observed 31 patients (21%) did not have features of partial albinism and data are suggestive of favourable outcome in this group. The homozygous missense variant RAB27A c.244 C > T, p.R82C was most frequently encountered, but presented with atypical features such as isolated CNS HLH and normal pigmentation, the combination of which may not prompt suspicion of GS2 and lead to diagnostic delay. Patients with this founder variant are therefore subject to diagnostic delay and may present with symptoms later in life. While it may be expected to under-diagnose features of partial albinism in patients with European descent, we found that the opposite with partial albinism more likely to be present in these populations. Genetic counselling and screening should be considered for these patients with a family history, particularly in the founder regions. In addition, our findings show a larger than expected proportion of GS2 patients presenting with neurological manifestations or muscle weakness, which precede the development of either isolated CNS, or combined systemic and CNS HLH.
Sixteen patients in the cohort developed initial symptoms at age 10 years or over (11%). The most common presenting feature is systemic HLH, with only 31% having features of partial albinism. This latter feature may explain in part the late diagnosis. We also note that hypomorphic mutations tended to present later, with a slower onset of symptoms. Further functional tests are warranted to determine if residual RAB27A activity explains the late onset of symptoms.
HSCT is a potential curative therapeutic option in patients with GS2. Our study confirms the reports of RAB27A deficiency causing propensity to HLH and resulting in high mortality. Survival rates of patients were higher in those offered HSCT, suggesting previous assertions that pre-emptive HSCT may be a more favourable treatment option [56, 73]. The R82C variant poses a clinical dilemma regarding the timing and need of HSCT intervention in asymptomatic patients. HLH, malignancy risk, family history and a personalised approach should be considered in guiding families to consider pre-emptive HSCT. HSCT is recommended once HLH has developed for all patients due to high mortality without HSCT. HSCT-related complications were not consistently recorded, and further studies are needed to assess optimal transplant characteristics and HSCT-related outcomes. We propose the collation of follow-up data and outcomes, particularly for those undergoing HSCT in the form of a curated registry. Open access to the data will also aid the design of prospective studies, with an aim to establish optimal treatment protocols for HSCT.
As a retrospective, literature-based review, this study is limited by the phenotypic data presented by other authors, with the largest study comprising 16 patients. The study is prone to publication bias of those with unusual characteristics that may differ from a typical GS2 presentation.
In summary, this evaluation of genetic and phenotypic features of GS2 highlights a condition with high fatality secondary to HLH. The gathering of data from the literature allows us to define new significant genotype: phenotype correlations. Our data also highlight the need for an improved molecular understanding of the precise mechanisms of initiation of HLH activation, and the need for specific diagnostic testing to be available in clinical decision making. These data will help in making early diagnoses of RAB27A deficiency and enable timely HSCT, or in the future, specific precision therapies ultimately improving outcomes for patients with GS2.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge the patients and their families in this study who consented to data analysis. We also acknowledge the authors of the collated cases in the UK and internationally, and by extension, the patients and families in our study cohort. We would also like to acknowledge the staff of Great Ormond Street and King’s College London hospitals who cared for the patients.
Abbreviations
- ATG
Anti-thymocyte globulin
- CTL
Cytotoxic T lymphocyte
- EBV
Epstein Barr Virus
- FITC
Fluorescein isothiocyanate
- GRA
Granule release assay
- GS2
Griscelli syndrome type 2
- GTPase
Guanosine triphosphate hydrolase enzyme
- HLH
Haemophagocytic lymphohistiocytosis
- HSCT
Haematopoietic stem cell transplantation
- IL-2
Interleukin-2
- IQR
Interquartile range
- MLPH
Melanophilin
- MMF
Mycophenolate mofetil
- MYO5A
Myosin V-A
- NK
Natural killer
- OMIM
Online Mendelian in Man
- PHA
Phytohaemagglutinin
- PTV
Protein-truncating variant
- RAB27A
Ras-related protein Rab-27 A
- SNP
Single nucleotide polymorphism
Author Contributions
JM, RE, MK: Conceptualization, Visualization, Writing - review & editing. JM, ARB, MS: Data curation, Methods, Investigation. JM: Writing - original draft, Writing - review & editing. JM, KG: Methods, software. CB, CC, LC, TBO, YH, ML, GG, KR, RE, MK: Supervision, Clinical care, Writing - review & editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institute for Health Research Integrated Academic Training scheme (JM).
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Competing Interests
Dr Lim reported receiving personal fees from Octapharma, Roche, Novartis, and Amgen. All other authors have no conflict of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Griscelli C, Prunieras M. Pigment dilution and immunodeficiency: a new syndrome. Int J Dermatol. 1978;17(10):788–91. [DOI] [PubMed] [Google Scholar]
- 2.Russ A, et al. Griscelli Type 2 syndrome and Hemophagocytic Lymphohistiocytosis: sisters with the same mutation but different presentations. J Pediatr Hematol Oncol. 2019;41(6):473–7. [DOI] [PubMed] [Google Scholar]
- 3.Ménasché G, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25(2):173–6. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc2. Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J Biol Chem. 2003;278(17):15373–80. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65(18):2801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohishi Y, et al. Griscelli Syndrome type 2 sine albinism: unraveling Differential RAB27A Effector Engagement. Front Immunol. 2020;11:612977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrowski M, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. sup pp 1–13. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y et al. The regulation of exosome generation and function in physiological and pathological processes. Int J Mol Sci, 2023. 25(1). [DOI] [PMC free article] [PubMed]
- 9.Stinchcombe JC, et al. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152(4):825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ménasché G, et al. A newly identified isoform of Slp2a associates with Rab27a in cytotoxic T cells and participates to cytotoxic granule secretion. Blood. 2008;112(13):5052–62. [DOI] [PubMed] [Google Scholar]
- 11.Netter P, et al. A novel Rab27a mutation binds melanophilin, but not Munc13-4, causing immunodeficiency without albinism. J Allergy Clin Immunol. 2016;138(2):599–e6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cetica V, et al. Patients with Griscelli syndrome and normal pigmentation identify RAB27A mutations that selectively disrupt MUNC13-4 binding. J Allergy Clin Immunol. 2015;135(5):1310–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zondag TCE, et al. Novel RAB27A variant Associated with late-onset Hemophagocytic Lymphohistiocytosis alters effector protein binding. J Clin Immunol. 2022;42(8):1685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruikshank M, et al. Screening assays for primary haemophagocytic lymphohistiocytosis in children presenting with suspected macrophage activation syndrome. Pediatr Rheumatol. 2015;13(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryceson YT, et al. A prospective evaluation of degranulation assays in the rapid diagnosis of familial hemophagocytic syndromes. Blood. 2012;119(12):2754–63. [DOI] [PubMed] [Google Scholar]
- 16.Sefsafi Z, et al. Macrophage activation syndrome associated with griscelli syndrome type 2: case report and review of literature. Pan Afr Med J. 2018;29:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34(4):101515. [DOI] [PubMed] [Google Scholar]
- 18.Brisse E, Wouters CH, Matthys P. Advances in the pathogenesis of primary and secondary haemophagocytic lymphohistiocytosis: differences and similarities. Br J Haematol. 2016;174(2):203–17. [DOI] [PubMed] [Google Scholar]
- 19.La Rosée P, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–77. [DOI] [PubMed] [Google Scholar]
- 20.Trottestam H, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(17):4577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergsten E, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta D, et al. Hematopoietic stem cell transplantation in children with Griscelli syndrome type 2: experience and outcomes. Indian J Pathol Microbiol. 2019;62(2):279–82. [DOI] [PubMed] [Google Scholar]
- 23.Aricò M, et al. Successful treatment of Griscelli syndrome with unrelated donor allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 2002;29(12):995–8. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, et al. Successful rescue of a lethal Griscelli syndrome type 2 presenting with neurological involvement and hemophagocytic lymphohistiocytosis: a case report. BMC Pediatr. 2021;21(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamishi S, et al. Analysis of RAB27A gene in griscelli syndrome type 2: novel mutations including a deletion hotspot. J Clin Immunol. 2008;28(4):384–9. [DOI] [PubMed] [Google Scholar]
- 26.Onay H, et al. A further Turkish case of Griscelli syndrome with new RAB27A mutation. J Am Acad Dermatol. 2008;58(5 Suppl 1):S115–6. [DOI] [PubMed] [Google Scholar]
- 27.Aslan D, et al. Griscelli syndrome: description of a case with Rab27A mutation. Pediatr Hematol Oncol. 2006;23(3):255–61. [DOI] [PubMed] [Google Scholar]
- 28.Meschede IP, et al. Griscelli syndrome-type 2 in twin siblings: case report and update on RAB27A human mutations and gene structure. Braz J Med Biol Res. 2008;41(10):839–48. [DOI] [PubMed] [Google Scholar]
- 29.Schuster F, et al. Griscelli syndrome: report of the first peripheral blood stem cell transplant and the role of mutations in the RAB27A gene as an indication for BMT. Bone Marrow Transpl. 2001;28(4):409–12. [DOI] [PubMed] [Google Scholar]
- 30.Anikster Y, et al. Evidence that Griscelli syndrome with neurological involvement is caused by mutations in RAB27A, not MYO5A. Am J Hum Genet. 2002;71(2):407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bizario JC, et al. Griscelli syndrome: characterization of a new mutation and rescue of T-cytotoxic activity by retroviral transfer of RAB27A gene. J Clin Immunol. 2004;24(4):397–410. [DOI] [PubMed] [Google Scholar]
- 32.Masri A, et al. Griscelli syndrome type 2: a rare and lethal disorder. J Child Neurol. 2008;23(8):964–7. [DOI] [PubMed] [Google Scholar]
- 33.Aksu G, et al. Griscelli syndrome without hemophagocytosis in an eleven-year-old girl: expanding the phenotypic spectrum of Rab27A mutations in humans. Am J Med Genet A. 2003;116a(4):329–33. [DOI] [PubMed] [Google Scholar]
- 34.Sarper N, et al. A rare syndrome in the differential diagnosis of hepatosplenomegaly and pancytopenia: report of identical twins with Griscelli disease. Ann Trop Paediatr. 2003;23(1):69–73. [DOI] [PubMed] [Google Scholar]
- 35.Sanal O, et al. Griscelli disease: genotype-phenotype correlation in an array of clinical heterogeneity. J Clin Immunol. 2002;22(4):237–43. [DOI] [PubMed] [Google Scholar]
- 36.Gazit R, et al. NK cytotoxicity mediated by CD16 but not by NKp30 is functional in Griscelli syndrome. Blood. 2007;109(10):4306–12. [DOI] [PubMed] [Google Scholar]
- 37.Rajadhyax M, et al. Neurological presentation of Griscelli syndrome: obstructive hydrocephalus without haematological abnormalities or organomegaly. Brain Dev. 2007;29(4):247–50. [DOI] [PubMed] [Google Scholar]
- 38.Sheela SR, Latha M, Injody SJ. Griscelli syndrome: Rab 27a mutation. Indian Pediatr. 2004;41(9):944–7. [PubMed] [Google Scholar]
- 39.Westbroek W, et al. A novel missense mutation (G43S) in the switch I region of Rab27A causing Griscelli syndrome. Mol Genet Metab. 2008;94(2):248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westbroek W, et al. Rab27b is up-regulated in human Griscelli syndrome type II melanocytes and linked to the actin cytoskeleton via exon F-Myosin va transcripts. Pigment Cell Res. 2004;17(5):498–505. [DOI] [PubMed] [Google Scholar]
- 41.Al-Sulaiman R, et al. A founder RAB27A variant causes Griscelli syndrome type 2 with phenotypic heterogeneity in Qatari families. Am J Med Genet A. 2020;182(11):2570–80. [DOI] [PubMed] [Google Scholar]
- 42.Ariffin H, et al. Griscelli syndrome. Med J Malaysia. 2014;69(4):193–4. [PubMed] [Google Scholar]
- 43.Durmaz A, et al. Molecular analysis and clinical findings of Griscelli syndrome patients. J Pediatr Hematol Oncol. 2012;34(7):541–4. [DOI] [PubMed] [Google Scholar]
- 44.Shamsian BS, et al. A novel RAB27A mutation in a patient with Griscelli syndrome type 2. J Investig Allergol Clin Immunol. 2010;20(7):612–5. [PubMed] [Google Scholar]
- 45.Yeetong P, Suphapeetiporn K, Shotelersuk V. Mutation analysis and prenatal diagnosis of a family with Griscelli syndrome type 2: two novel mutations in the RAB27A gene. World J Pediatr. 2017;13(4):392–4. [DOI] [PubMed] [Google Scholar]
- 46.Zur Stadt U, et al. Mutation spectrum in children with primary hemophagocytic lymphohistiocytosis: molecular and functional analyses of PRF1, UNC13D, STX11, and RAB27A. Hum Mutat. 2006;27(1):62–8. [DOI] [PubMed] [Google Scholar]
- 47.Grandin V, et al. A RAB27A duplication in several cases of Griscelli syndrome type 2: an explanation for cases lacking a genetic diagnosis. Hum Mutat. 2017;38(10):1355–9. [DOI] [PubMed] [Google Scholar]
- 48.Mishra K, et al. Griscelli syndrome type 2: a novel mutation in RAB27A gene with different clinical features in 2 siblings: a diagnostic conundrum. Korean J Pediatr. 2014;57(2):91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent LM, et al. Novel 47.5-kb deletion in RAB27A results in severe Griscelli syndrome type 2. Mol Genet Metab. 2010;101(1):62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajyalakshmi R, Chakrapani RN. Griscelli syndrome type 2: a rare and fatal syndrome in a south Indian boy. Indian J Pathol Microbiol. 2016;59(1):113–6. [DOI] [PubMed] [Google Scholar]
- 51.Minocha P, et al. Griscelli syndrome subtype 2 with hemophagocytic lympho-histiocytosis: a case report and review of literature. Intractable Rare Dis Res. 2017;6(1):76–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tewari N, et al. Oral features of Griscelli syndrome type II: a rare case report. Spec Care Dentist. 2018;38(6):421–5. [DOI] [PubMed] [Google Scholar]
- 53.Gotesman R, et al. Cutaneous granulomas as the presenting manifestation of Griscelli syndrome type 2. Pediatr Dermatol. 2021;38(1):194–7. [DOI] [PubMed] [Google Scholar]
- 54.Nodehi H, et al. Neonatal onset of Hemophagocytic Lymphohistiocytosis due to prenatal varicella-zoster infection in a neonate with Griscelli Syndrome Type 2. Iran J Allergy Asthma Immunol. 2022;21(4):488–93. [DOI] [PubMed] [Google Scholar]
- 55.Gironi LC et al. Congenital Hypopigmentary disorders with Multiorgan Impairment: a Case Report and an overview on Gray Hair syndromes. Med (Kaunas), 2019. 55(3). [DOI] [PMC free article] [PubMed]
- 56.Castaño-Jaramillo LM, et al. Diagnostic and therapeutic caveats in Griscelli syndrome. Scand J Immunol. 2021;93(6):e13034. [DOI] [PubMed] [Google Scholar]
- 57.Woodward KE, et al. Considering immunologic and genetic evaluation for HLH in neuroinflammation: a case of Griscelli syndrome type 2 with neurological symptoms and a lack of albinism. Pediatr Blood Cancer. 2020;67(8):e28312. [DOI] [PubMed] [Google Scholar]
- 58.Tesi B, et al. A RAB27A 5’ untranslated region structural variant associated with late-onset hemophagocytic lymphohistiocytosis and normal pigmentation. J Allergy Clin Immunol. 2018;142(1):317–e3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohbayashi N, et al. Functional characterization of two RAB27A missense mutations found in Griscelli syndrome type 2. Pigment Cell Melanoma Res. 2010;23(3):365–74. [DOI] [PubMed] [Google Scholar]
- 60.Emanuel PO, Sternberg LJ, Phelps RG. Griscelli Syndrome Skinmed. 2007;6(3):147–9. [DOI] [PubMed] [Google Scholar]
- 61.Messinger YH, et al. Delayed diagnosis of Griscelli syndrome type 2 with compound heterozygote RAB27A variants presenting with pulmonary failure. Pediatr Hematol Oncol. 2021;38(6):593–601. [DOI] [PubMed] [Google Scholar]
- 62.Rossi A, et al. Griscelli syndrome type 2: long-term follow-up after unrelated donor bone marrow transplantation. Dermatology. 2009;218(4):376–9. [DOI] [PubMed] [Google Scholar]
- 63.Panigrahi I, et al. Seizure as the presenting manifestation in Griscelli syndrome type 2. Pediatr Neurol. 2015;52(5):535–8. [DOI] [PubMed] [Google Scholar]
- 64.Lee JYW, et al. Further evidence for genotype-phenotype disparity in Griscelli syndrome. Br J Dermatol. 2017;176(4):1086–9. [DOI] [PubMed] [Google Scholar]
- 65.Hinson A, et al. Congenital hemophagocytic lymphohistiocytosis presenting as thrombocytopenia in a newborn. J Pediatr Hematol Oncol. 2015;37(4):300–3. [DOI] [PubMed] [Google Scholar]
- 66.Szczawinska-Poplonyk A, et al. Pulmonary lymphomatoid granulomatosis in Griscelli syndrome type 2. Viral Immunol. 2011;24(6):471–3. [DOI] [PubMed] [Google Scholar]
- 67.Meeths M, et al. Clinical presentation of Griscelli syndrome type 2 and spectrum of RAB27A mutations. Pediatr Blood Cancer. 2010;54(4):563–72. [DOI] [PubMed] [Google Scholar]
- 68.Brauer N, et al. Immunodeficiency with susceptibility to lymphoma with complex genotype affecting energy metabolism (FBP1, ACAD9) and vesicle trafficking (RAB27A). Front Immunol. 2023;14:1151166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silberstein M, et al. A system for exact and approximate genetic linkage analysis of SNP data in large pedigrees. Bioinformatics. 2013;29(2):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wheeler RD, et al. A novel assay for investigation of suspected familial haemophagocytic lymphohistiocytosis. Br J Haematol. 2010;150(6):727–30. [DOI] [PubMed] [Google Scholar]
- 71.Elgaali E, et al. Genetic background of primary and familial HLH in Qatar: registry data and population study. Front Pediatr. 2024;12:1326489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheinfeld NS. Syndromic albinism: a review of genetics and phenotypes. Dermatol Online J. 2003;9(5):5. [PubMed] [Google Scholar]
- 73.Kuskonmaz B, et al. Hematopoietic stem cell transplantation in children with Griscelli syndrome: a single-center experience. Pediatr Transplant. 2017;21(7):e13040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.