Abstract
Aims/hypothesis
Metabolic abnormalities such as central obesity, insulin resistance, dyslipidaemia and hypertension, often referred to as ‘the metabolic syndrome’ (or ‘combined metabolic abnormalities’), are increasingly being identified in people living with type 1 diabetes, accelerating the risk for CVD. As a result, in recent years, treatment in people living with type 1 diabetes has shifted to improving overall metabolic health rather than glucose control alone. In Belgium, diabetes care for people living with type 1 diabetes is centrally organised. The Initiative for Quality Improvement and Epidemiology in Diabetes, imposed by the Belgian health insurance system, has systematically collected data from patients on intensive insulin therapy treated in all 101 diabetes clinics in Belgium since 2001. The aim of this real-world study is to describe the evolution of treatment and metabolic health, including the prevalence of obesity and combined metabolic abnormalities, in people living with type 1 diabetes over the past 20 years, and to compare the treatment and prevalence of complications between those with and without combined metabolic abnormalities.
Methods
We analysed data on adults (≥16 years old) living with type 1 diabetes, who were diagnosed at age ≤45 years and who had a diabetes duration ≥1 year, collected between 2001 and 2022. The evolution of HbA1c, BMI, LDL-cholesterol, systolic BP, lipid-lowering therapy and antihypertensive therapy over time was analysed. The prevalence of individual and multiple metabolic abnormalities according to various definitions of the metabolic syndrome/combined metabolic abnormalities was analysed, and the association between combined metabolic abnormalities and metabolic health indicators, complications and treatment was investigated in the 2022 data.
Results
The final dataset consisted of 26,791 registrations of adults living with type 1 diabetes collected between 2001 and 2022. Although glycaemic and lipid control generally improved over time, the prevalence of obesity strongly increased (12.1% in 2001 vs 21.7% in 2022, p<0.0001), as did the presence of combined metabolic abnormalities (WHO criteria: 26.9% in 2001 vs 42.9% in 2022 in women, p<0.0001; 30.4% in 2001 vs 52.1% in 2022 in men, p<0.0001; WHO criteria without albuminuria: 22.3% in 2001 vs 40.6% in 2022 in women, p<0.0001; 25.1% in 2001 vs 49.2% in 2022 in men, p<0.0001; NCEP-ATPIII criteria: 39.9% in 2005 vs 57.2% in 2022 in women, p<0.0001; 40.8% in 2005 vs 60.9% in 2022 in men, p<0.0001; IDF criteria: 43.9% in 2005 vs 59.3% in 2022 in women, p<0.001; 33.7% in 2005 vs 50.0% in 2022 in men, p<0.0001). People with combined metabolic abnormalities had higher glucose levels compared to those without combined metabolic abnormalities (HbA1c >58 mmol in men: 48.9% vs 36.9%; HbA1c >58 mmol in women: 53.3% vs 41.1%, p<0.0001). People with combined metabolic abnormalities were more often treated with adjunct therapies such as metformin, sodium–glucose transport protein 2 inhibitors and glucagon-like peptide-1 receptor agonists. In both men and women, the presence of combined metabolic abnormalities was strongly related to the presence of eye complications, peripheral neuropathy, chronic kidney disease and CVD, corrected for age, diabetes duration and HbA1c.
Conclusions/interpretation
Overweight, obesity and combined metabolic abnormalities are increasingly being identified in people living with type 1 diabetes, further accelerating the risk of microvascular and macrovascular complications. Early identification of the presence of combined metabolic abnormalities should enable therapeutic interventions to be modified towards multifactorial approaches, with attention to education on avoidance of overweight (e.g. dietary counselling) in addition to strict glycaemic control and intensification of use of antihypertensive agents and statins. Use of adjunct therapies in this population as a tool should be explored more thoroughly to reduce risk of complications.
Graphical Abstract
Keywords: Combined metabolic abnormalities, Diabetes complications, Diabetes treatment, Metabolic health, Metabolic syndrome, Obesity, Real-world data, Type 1 diabetes
Introduction
Since the DCCT/Epidemiology of Diabetes Interventions and Complications trial demonstrated a reduction in chronic complications of diabetes in people on intensive insulin therapy [1–3], treatment in type 1 diabetes has mainly focused on reducing blood glucose levels. However, the profile of people living with type 1 diabetes is evolving, with overweight and obesity coming to the fore, and overall metabolic health, rather than glucose control alone, contributing to outcomes [4, 5]. People living with type 1 diabetes still have an almost threefold higher mortality rate compared with the general population, with CVD being a major cause of this increased mortality risk [6, 7]. In addition, achieving and maintaining strict glucose control, which is the primary therapeutic goal in most patients living with type 1 diabetes, requires a high level of self-management and follow-up, but also increases the risk for insulin-induced weight gain, and may thus aggravate cardiovascular risk [8].
The presence of a combination of metabolic abnormalities, including central obesity, insulin resistance, dyslipidaemia and hypertension, in addition to hyperglycaemia, often referred to by clinicians as ‘the metabolic syndrome’ or ‘combined metabolic abnormalities’, is a hallmark of type 2 diabetes and increases the risk of CVD in this population as well as in the general population [9, 10]. This combination of metabolic abnormalities is also increasingly found in people living with type 1 diabetes, partly related to lifestyle choices and rising obesity rates in the general population, but also secondary to insulin therapy, as insulin-induced weight gain may induce peripheral insulin resistance [11]. This cluster of metabolic abnormalities may be a target for intervention, identifying people living with type 1 diabetes at high risk of developing CVD who may benefit from adjunct treatments that address insulin resistance and cardiometabolic risk [10, 12].
The aim of this real-world observational study is to describe the evolution of treatment and metabolic health, including the prevalence of obesity and combined metabolic abnormalities, over the past 20 years in people in Belgium living with type 1 diabetes, and to compare the treatment and prevalence of complications between those with and without combined metabolic abnormalities in the setting of centrally organised diabetes care with free-of-charge access to diabetes education, regular follow-up by a multidisciplinary team, and reimbursement of medication, sensors and pumps to manage diabetes.
Methods
Study population
We used data from the Initiative for Quality Improvement and Epidemiology in Diabetes (IQED), a national project allowing monitoring and improvement of the quality of care for people living with diabetes in Belgium, and study of their epidemiology [13, 14].
For this study, data on adults (aged ≥18 years until 2015; aged ≥16 years from 2016) living with type 1 diabetes were cross-sectionally collected between 2001 and 2022. Patients with a history of pancreas or islet cell transplantation, dementia or pregnant women were excluded from the IQED study. Data from people treated with continuous subcutaneous insulin infusion (CSII) were not collected between 2006 and 2014. Each centre was asked to review their medical records and complete a standardised electronic questionnaire using the patient’s most recent data from the previous year (the audit period) for 10% of the total number of people living with diabetes and treated at their centre. The 10% sample was defined by the first letter of the family name, chosen randomly at the start of each data collection period. Data were pseudonymised. More information about the data collected and the data collection process is available online [13].
Final dataset
The people living with type 1 diabetes were defined based on the clinical diagnosis encoded in the electronic patient file. The IQED database contained 40,449 registrations of adults living with type 1 diabetes. We excluded registrations for people for whom information was missing: sex (n=1), age (n=1), diabetes duration (n=627), HbA1c (n=752), LDL-cholesterol (n=3761), HDL-cholesterol (n=3275), triglycerides (TG) (n=3018), lipid-lowering therapy (n=1277), antihypertensive therapy (n=1183), systolic BP (n=927), diastolic BP (n=951) or BMI (n= 3220). To eliminate mis-classified type 2 diabetes as much as possible, people with an age at diagnosis ≥45 years (n=4596) and a diabetes duration of less than 1 year (n=617) were also excluded. Thus the final dataset consisted of 26,791 registrations of adults living with type 1 diabetes cross-sectionally collected between 2001 and 2022.
Parameters
We used various definitions of the metabolic syndrome/combined metabolic abnormalities: (1) the WHO definition [10]; (2) the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATPIII) definition [15], which is equivalent to the Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention, National Heart, Lung and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society and the International Association for the Study of Obesity [16] using their waist circumference (WC) thresholds; and (3) the International Diabetes Federation (IDF) definition [17]. In addition to diabetes, the WHO definition of the metabolic syndrome/combined metabolic abnormalities requires the presence of two or more of the following conditions: obesity (BMI >30 kg/m2), hypertension (BP ≥140/90 mmHg and/or treatment with antihypertensive drugs), triglycerides ≥1.7 mmol/l (≥150 mg/dl), HDL-cholesterol <0.9 mmol/l (<35 mg/dl) in men or <1 mmol/l (<40 mg/dl) in women and/or treatment with lipid-lowering drugs, or albuminuria (albumin 0.3 g/l [>30 mg/dl]). Applying the NCEP-ATPIII definition in people living with diabetes, the metabolic syndrome/combined metabolic abnormalities requires the presence of two or more of the following conditions: WC ≥102 cm in men (≥88 cm in women), hypertension (BP ≥130/85 mmHg and/or treatment with antihypertensive drugs), TG ≥1.7 mmol/l (≥150 mg/dl), or HDL-cholesterol <1 mmol/l (<40 mg/dl) in men/<1.3 mmol/l (<50 mg/dl) in women and/or treatment with lipid-lowering drugs. In addition to the presence of central obesity (WC >94 cm in men, >80 cm in women), the IDF definition of the metabolic syndrome/combined metabolic abnormalities in people living with diabetes, requires the presence of one or more of the following conditions: hypertension (BP ≥130/85 mmHg and/or treatment with antihypertensive drugs), TG ≥1.7 mmol/l (≥150 mg/dl), or HDL-cholesterol <1 mmol/l (<40 mg/dl) in men/<1.3 mmol/l (<50 mg/dl) in women and/or treatment with lipid-lowering drugs.
The estimated glucose disposal rate (eGDR) was used as a measure of insulin resistance, and was calculated using the formula: eGDRWC = 21.158 + (−0.09 × WC, in cm) + (−3.407 × presence or absence of hypertension, where presence = 1/absence = 0) + (−0.551 × HbA1c, %), whereby the presence of hypertension was defined as BP ≥140/90 mmHg or current use of any antihypertensive drugs [18]. As WC was only reported from audit 4 onwards and for the minority of the patients, the following formula was used as an alternative: eGDRBMI = 19.02 − (0.22 × BMI, in kg/m2) − (3.26 × hypertension, presence = 1/absence = 0) − (0.61 × HbA1c, %) [11].
LDL-cholesterol was calculated by the Friedewald formula for patients with TG <4.52 mmol/l (<400 mg/dl), regardless of whether the blood sample was obtained under fasting or non-fasting conditions [19, 20].
Statistical analysis
Results are expressed as proportions for categorical variables, mean ± SD for normally distributed variables, or median (IQR) for non-normally distributed variables.
The statistical significance of the trend over time for study population characteristics and the individual metabolic abnormalities was tested using generalised estimating equations (GEE), using logistic regression for dichotomous outcome variables and the normal probability distribution for continuous outcome variables, with exchangeable correlation structure (diabetes centre and patient) and audit year (defined as the midpoint of the audit year) as continuous explanatory variables.
Statistical comparisons of mean HbA1c, BMI, LDL-cholesterol, systolic BP or the proportion of people using lipid-lowering and antihypertensive drugs between 2001 and 2022 were tested using GEE as described above, with audit year as the categorical explanatory variable. Comparisons were adjusted using the Tukey method.
Pairwise differences in the prevalence of combined metabolic abnormalities by sex between 2001 and 2022 were analysed using GEE as described above, with audit year as the categorical explanatory variable and comparisons adjusted using the Tukey method. Statistical analyses were also adjusted for age (continuous) and diabetes duration (continuous) using GEE. The GEE model predictions are presented with the corresponding 95% CI.
Statistical comparisons of metabolic health indicators and treatment rates between people with and without combined metabolic abnormalities in 2022 was tested using GEE as described above, with the presence of combined metabolic abnormalities as the categorical explanatory variable and comparisons adjusted using the Tukey method. Analysis of the prevalence of complications by sex was adjusted for age (continuous) and diabetes duration (continuous). The GEE model predictions are presented with the corresponding 95% CI. Analyses were repeated with additional adjustment for HbA1c (continuous) or smoking status (categorical).
All p values were two-sided. p values <0.05 were considered statistically significant. Data analyses were performed using SAS software version 9.4 (SAS Institute, USA).
Results
Population characteristics
Table 1 shows the general characteristics of the study population for each audit. The number of people included in each audit increased over time. Since 2001, the general characteristics of the study population have significantly changed: people living with type 1 diabetes are older, have a longer diabetes duration and a younger age at diagnosis. The proportion of smokers significantly decreased, whereas BMI and WC significantly increased. Insulin resistance, as measured by a decrease in eGDRWC and eGDRBMI, significantly increased. The proportion of CSII users also increased tenfold from 2% in 2001 to 20% in 2022.
Table 1.
General characteristics of people living with type 1 diabetes
| Audit number | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Audit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Trend since 2001, p value | Trend since 2016, p value |
| Audit period | 11/2000–11/2001 | 10/2001–09/2002 | 03/2003–02/2004 | 02/2005–01/2006 | 10/2006–09/2007 | 03/2008–03/2009 | 09/2010–08/2011 | 12/2013–12/2014 | 10/2015–09/2016 | 10/2017–09/2018 | 03/2020–02/2021 | 10/2021–09/2022 | ||
| Audit year (midpoint) | 2001 | 2002 | 2003 | 2005 | 2007 | 2008 | 2011 | 2014 | 2016 | 2018 | 2020 | 2022 | ||
| Number of patients | 1256 | 1399 | 1459 | 1652 | 2412 | 2373 | 2502 | 2570 | 2650 | 2714 | 2815 | 2989 | ||
| Men | 718 (57.2) | 799 (57.1) | 840 (57.6) | 934 (56.5) | 1384 (57.4) | 1423 (60.0) | 1507 (60.2) | 1509 (58.7) | 1527 (57.6) | 1578 (58.1) | 1612 (57.3) | 1697 (56.8) | ns | ns |
| Age, years | 42.3 (33.3–52.3) | 42.2 (33.2–52.2) | 41.7 (32.7–52.7) | 43.6 (33.6–52.6) | 43.2 (34.2–53.2) | 44.7 (34.7–53.7) | 45.2 (34.2–55.2) | 45.5 (35.5–57.5) | 46.2 (34.2–57.2) | 46.2 (34.2–57.2) | 46.7 (34.7–58.7) | 46.2 (35.2–58.2) | <0.0001 | <0.0001 |
| Diabetes duration, years | 17.3 (9.3–27.3) | 17.2 (9.2–27.2) | 17.7 (9.7–28.7) | 17.7 (9.6–29.6) | 18.2 (10.2–29.2) | 17.7 (9.7–28.7) | 19.2 (10.2–31.2) | 20.5 (11.5–32.5) | 21.0 (11.6–32.2) | 21.2 (11.8–32.2) | 21.7 (12.7–33.7) | 22.2 (12.8–33.3) | <0.0001 | <0.0001 |
| Age at diagnosis, years | 24.0 (15.0–33.0) | 24.0 (15.0–33.0) | 23.0 (14.0–32.0) | 24.0 (14.0–32.0) | 24.0 (14.0–32.0) | 24.0 (15.0–33.0) | 24.0 (15.0–32.0) | 24.0 (14.0–33.0) | 23.0 (14.0–32.1) | 23.0 (13.6–32.0) | 23.0 (13.0–31.5) | 22.9 (13.0–31.5) | <0.0001 | ns |
| Smoker |
304 (25.7) (N=1185) |
337 (24.7) (N=1365) |
320 (22.6) (N=1418) |
349 (22.6) (N=1543) |
542 (23.4) (N=2315) |
526 (23.3) (N=2257) |
546 (23.0) (N=2374) |
495 (20.7) (N=2397) |
513 (20.5) (N=2501) |
552 (21.4) (N=2580) |
520 (19.3) (N=2690) |
548 (19.2) (N=2848) |
<0.0001 | ns |
| BMI, kg/m2 | 25.4 ± 4.2 | 25.2 ± 4.0 | 25.4 ± 4.3 | 25.6 ± 4.2 | 25.5 ± 4.3 | 25.8 ± 4.4 | 26.0 ± 4.7 | 26.0 ± 4.5 | 26.1 ± 4.6 | 26.2 ± 4.7 | 26.8 ± 4.8 | 26.7 ± 4.8 | <0.0001 | <0.0001 |
| WC, cm |
90.6 ± 16.3 (N= 317) |
89.5 ± 13.4 (N= 985) |
90.7 ± 13.4 (N=1066) |
91.9 ± 13.4 (N=1107) |
91.0 ± 13.1 (N=1086) |
92.0 ± 14.3 (N=1153) |
91.5 ± 14.0 (N=1081) |
94.1 ± 14.4 (N= 899) |
94.0 ± 14.9 (N=1028) |
<0.0001 | <0.0001 | |||
| eGDRWC, mg/kg/min |
6.9 ± 2.6 (N= 317) |
7.1 ± 2.4 (N= 985) |
6.9 ± 2.5 (N=1066) |
6.8 ± 2.4 (N=1107) |
7.0 ± 2.4 (N=1086) |
6.8 ± 2.5 (N=1153) |
6.9 ± 2.5 (N=1081) |
6.4 ± 2.5 (N= 899) |
6.6 ± 2.6 (N=1028) |
<0.0001 | <0.01 | |||
| eGDRBMI, mg/kg/min | 7.2 ± 2.3 | 7.3 ± 2.3 | 7.1 ± 2.3 | 6.9 ± 2.2 | 7.0 ± 2.2 | 6.9 ± 2.3 | 6.9 ± 2.3 | 6.9 ± 2.2 | 6.9 ± 2.2 | 6.9 ± 2.3 | 6.6 ± 2.3 | 6.9 ± 2.3 | <0.0001 | ≤0.05 |
| CSII |
23 (1.8) (N=1246) |
53 (3.8) (N=1389) |
18 (1.2) (N=1446) |
/ | / | / | / | / |
280 (10.7) (N=2615) |
344 (12.9) (N=2658) |
416 (15.0) (N=2765) |
577 (19.7) (N=2933) |
<0.0001 | <0.0001 |
Values are n (%) for categorical variables and median (IQR) or mean ± SD for continuous variables
Change over time of the general characteristics of people living with type 1 diabetes was analysed from 2001 until 2022 (trend since 2001) and from 2016 until 2022 (trend since 2016). CSII users were not eligible for inclusion in the IQED study between audit 4 and audit 9. Data on smoking habits, WC and CSII use were only available for the indicated populations (N). For WC, the trend since 2001 is actually measured from 2005, as WC data are only available from audit 4 onwards
N, population size; n, number of observations; ns, not significant
Evolution of HbA1c, BMI, lipids and BP
The mean BMI increased from 25.4 ± 4.2 kg/m2 in 2001 to 26.7 ± 4.8 kg/m2 in 2022 (p<0.0001), whereas the mean HbA1c decreased from 64 ± 18 mmol/mol (8.0 ± 1.6%) in 2001 to 59 ± 13 mmol/mol (7.6 ± 1.2%) in 2022 (p<0.0001) (Fig. 1a). The mean LDL-cholesterol value decreased from 2.9 ± 0.9 mmol/l in 2001 to 2.3 ± 0.8 mmol/l in 2022 (p<0.0001), which is mainly explained by an increase in the rate of lipid-lowering therapy from 12.7% in 2001 to 39.5% in 2011 (p<0.0001) and 48.1% in 2022 (p<0.0001 vs 2011) (Fig. 1b). The mean systolic BP value did not change over time (129 ± 18 mmHg in 2001 vs 129 ± 17 mmHg in 2022). The rate of antihypertensive therapy increased from 24.3% in 2001 to 36.3% in 2007 (p<0.0001) and remained stable afterwards (Fig. 1c).
Fig. 1.
(a) Evolution of the mean HbA1c value (blue circles) and mean BMI value (red circles) over the audit years. (b) Evolution of the mean LDL-cholesterol value (blue circles) and the proportion of people living with type 1 diabetes and treated with lipid-lowering therapy (blue bars) over the audit years. Lipid-lowering therapy was defined as use of statins, fibrates (included in the data collection from 2006) or ezetimibe (included in the data collection from 2011). (c) Evolution of the mean systolic BP value (blue circles) and the proportion of people living with type 1 diabetes and treated with antihypertensive therapy (blue bars) over the audit years. Antihypertensive therapy was defined as use of ACE inhibitors (including sartans) or other antihypertensive drugs (included in the data collection from 2006). The solid lines are the fitted LOESS curves; the shaded bands represent the 95% CI of the fitted LOESS curve. The size of the study population in each audit year is indicated below the graphs. SBP, systolic BP
Evolution of metabolic abnormalities in people living with type 1 diabetes
Table 2 shows the evolution of the presence of the individual metabolic abnormalities used by the various definitions of the metabolic syndrome/combined metabolic abnormalities. The proportion of people with below-target HDL-cholesterol or receiving lipid-lowering therapy strongly increased, but the prevalence of hypertriglyceridaemia remained stable. The prevalence of hypertension slightly increased. The prevalence of obesity doubled, and the proportion of people with above-target WC increased. The prevalence of albuminuria did not change significantly from 2001.
Table 2.
Evolution of metabolic abnormalities in people living with type 1 diabetes
| Audit number | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Audit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Audit period | 11/2000–11/2001 | 10/2001–09/2002 | 03/2003–02/2004 | 02/2005–01/2006 | 10/2006–09/2007 | 03/2008–03/2009 | 09/2010–08/2011 | 12/2013–12/2014 | 10/2015–09/2016 | 10/2017–09/2018 | 03/2020–02/2021 | 10/2021–09/2022 | Trend since 2001, p value | Trend since 2016, p value |
| Audit year, midpoint | 2001 | 2002 | 2003 | 2005 | 2007 | 2008 | 2011 | 2014 | 2016 | 2018 | 2020 | 2022 | ||
| Number of patients | 1256 | 1399 | 1459 | 1652 | 2412 | 2373 | 2502 | 2570 | 2650 | 2714 | 2815 | 2989 | ||
| Reduced HDL-cholesterola | ||||||||||||||
| <0.9 mmol/l in men (<1 mmol/l in women) | 207 (16.5) | 209 (14.9) | 224 (15.4) | 433 (26.2) | 770 (31.9) | 872 (36.7) | 1043 (41.7) | 1097 (42.7) | 1178 (44.5) | 1219 (44.9) | 1333 (47.4) | 1502 (50.3) | <0.0001 | <0.0001 |
| <1 mmol/l in men (<1.3 mmol/l in women) | 292 (23.2) | 281 (20.1) | 293 (20.1) | 504 (30.5) | 863 (35.8) | 984 (41.5) | 1146 (45.8) | 1193 (46.4) | 1263 (47.7) | 1311 (48.3) | 1449 (51.5) | 1634 (54.7) | <0.0001 | <0.0001 |
| TG ≥1.7 mmol/l | 217 (17.3) | 213 (15.2) | 253 (17.3) | 237 (14.3) | 346 (14.3) | 358 (15.1) | 362 (14.5) | 362 (14.1) | 369 (13.9) | 404 (14.9) | 433 (15.4) | 453 (15.2) | ns | ns |
| Hypertensionb | ||||||||||||||
| BP ≥140/90 mmHg | 539 (42.9) | 590 (42.2) | 623 (42.7) | 753 (45.6) | 1172 (48.6) | 1151 (48.5) | 1230 (49.2) | 1256 (48.9) | 1325 (50.0) | 1357 (50.0) | 1512 (53.7) | 1499 (50.2) | <0.0001 | <0.01 |
| BP ≥130/85 mmHg | 760 (60.5) | 827 (59.1) | 871 (59.7) | 992 (60.0) | 1536 (63.7) | 1482 (62.5) | 1513 (60.5) | 1640 (63.8) | 1692 (63.8) | 1721 (63.4) | 1949 (69.2) | 1927 (64.5) | <0.0001 | ns |
| BMI >30 kg/m2 | 152 (12.1) | 159 (11.4) | 178 (12.2) | 240 (14.5) | 319 (13.2) | 374 (15.8) | 436 (17.4) | 449 (17.5) | 456 (17.2) | 501 (18.5) | 643 (22.8) | 648 (21.7) | <0.0001 | <0.0001 |
| Central obesity | ||||||||||||||
| WC >102 cm in men (>88 cm in women) |
94 (29.7) (N=317) |
232 (23.6) (N=985) |
311 (29.2) (N=1066) |
352 (31.8) (N=1107) |
320 (29.5) (N=1086) |
397 (34.4) (N=1153) |
349 (32.3) (N=1081) |
328 (36.5) (N=899) |
394 (38.3) (N=1028) |
<0.0001 | ns | |||
| WC >94 cm in men (>80 cm in women) |
154 (48.6) (N=317) |
452 (45.9) (N=985) |
517 (48.5) (N=1066) |
581 (52.5) (N=1107) |
561 (51.7) (N=1086) |
618 (53.6) (N=1153) |
571 (52.8) (N=1081) |
545 (60.6) (N=899) |
620 (60.3) (N=1028) |
<0.0001 | <0.0001 | |||
| Albuminuria |
184 (17.4) (N=1060) |
170 (14.6) (N=1166) |
187 (15.2) (N=1232) |
249 (17.3) (N=1436) |
321 (15.7) (N=2042) |
337 (16.4) (N=2057) |
306 (14.3) (N=2145) |
300 (13.9) (N=2163) |
314 (14.5) (N=2170) |
323 (14.8) (N=2180) |
312 (14.2) (N=2190) |
319 (13.5) (N=2367) |
ns | ns |
Values are n (%) for categorical variables
Data on WC and albuminuria were only available for the indicated populations (N). For WC, the trend since 2001 is actually measured from 2005, as WC data are only available from audit 4 onwards
aThe presence of reduced HDL-cholesterol is defined by the thresholds given in the rows below and/or use of lipid-lowering therapy
bThe presence of hypertension is defined by the thresholds given in the rows below and/or use of antihypertensive therapy
N, population size; n, number of observations; ns, not significant
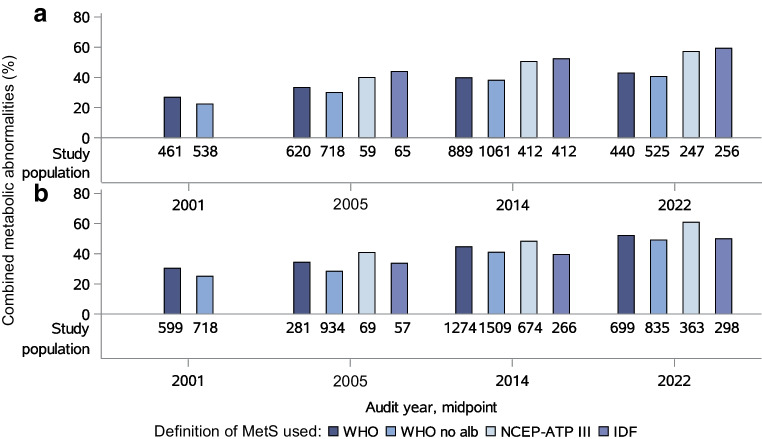
Between 2001 and 2022, the prevalence of combined metabolic abnormalities based on the various definitions of the metabolic syndrome/combined metabolic abnormalities increased (Fig. 2). Based on the WHO criteria, the prevalence of combined metabolic abnormalities increased from 26.9% in 2001 to 42.9% in 2022 in women (p<0.0001), and from 30.4% in 2001 to 52.1% in 2022 in men (p<0.0001). This increase remained when albuminuria was removed from the definition (from 22.3% to 40.6% in women [p<0.0001] and from 25.1% to 49.2% in men [p<0.0001]). Based on the NCEP-ATPIII criteria, the prevalence of combined metabolic abnormalities increased from 39.9% in 2005 to 57.2% in 2022 in women (p<0.0001) and from 40.8% in 2005 to 60.9% in 2022 in men (p<0.0001). Use of the IDF criteria (which require the presence of central obesity) showed an increase in the prevalence of combined metabolic abnormalities from 43.9% in 2005 to 59.3% in 2022 in women (p<0.001) and from 33.7% in 2005 to 50.0% in 2022 in men (p<0.0001).
Fig. 2.
Prevalence of combined metabolic abnormalities in 2001, 2005, 2014 and 2022 for women (a) and men (b), according to the various definitions of the metabolic syndrome/combined metabolic abnormalities. The sizes of the study populations are indicated below the bars. MetS, combined metabolic abnormalities (metabolic syndrome); no alb, no albuminuria
Similar increases were seen between 2005 and 2022 after adjustment for age and diabetes duration: from 27.5% (95% CI 23.2, 32.3) to 40.2% (37.2, 43.3) in women (p<0.01) and from 31.7% (27.7, 36.1) to 49.7% (46.9, 52.5) in men (p<0.0001) using the WHO criteria; from 21.8% (18.2, 25.8) to 38.2% (35.5, 40.9) in women (p<0.0001) and from 24.7% (21.4, 28.4) to 46.4% (43.9, 48.9) in men (p<0.0001) using the WHO criteria without albuminuria; from 43.3% (35.6, 51.4) to 56.9% (51.8, 61.9) in women (not significant) and from 42.3% (35.1, 49.9) to 58.7% (54.5,62.8) in men (p<0.01) using the NCEP-ATPIII criteria; and from 42.5% (36.0,49.3) to 60.6% (56.2,64.9) in women (p<0.001) and from 28.2% (22.1, 35.1) to 52.7% (49.1, 56.2) in men (p<0.0001) using the IDF criteria.
Metabolic health indicators, diabetes complications and treatment in people with and without combined metabolic abnormalities
People living with type 1 diabetes with combined metabolic abnormalities (defined using the WHO criteria without albuminuria) were less often treated with CSII, but use of metformin was threefold higher in those with combined metabolic abnormalities compared to those without combined metabolic abnormalities. In addition to antihypertensive and lipid-lowering drugs, those with combined metabolic abnormalities received sodium–glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP1) receptor agonists more frequently (Table 3).
Table 3.
Comparison of the prevalence of metabolic health indicators, complications and treatments in people living with type 1 diabetes with or without combined metabolic abnormalities (defined by the WHO definition of the metabolic syndrome/combined metabolic abnormalities, without albuminuria), stratified by sex (2022 data)
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Presence of combined metabolic abnormalities | No | Yes | p value | No | Yes | p value |
| Number of patients | 862 | 835 | 767 | 525 | ||
| Metabolic health indicators | ||||||
| Smokera | 187 (22.7) (N=822) | 168 (20.9) (N=804) | ns | 109 (15.2) (N=715) | 84 (16.6) (N=507) | ns |
| HbA1c >58 mmol/mol (>7.5%) | 318 (36.9) | 408 (48.9) | <0.0001 | 315 (41.1) | 280 (53.3) | <0.0001 |
| BMI >30 kg/m2b | 35 (4.1) | 320 (38.3) | <0.0001 | 61 (8.0) | 232 (44.2) | <0.0001 |
| Hypertensionb | 185 (21.5) | 734 (87.9) | <0.0001 | 132 (17.2) | 448 (85.3) | <0.0001 |
| Dyslipidaemiab | 197 (22.9) | 730 (87.4) | <0.0001 | 138 (18.0) | 437 (83.2) | <0.0001 |
| TG ≥1.7 mmol/lb | 57 (6.6) | 230 (27.5) | <0.0001 | 43 (5.6) | 123 (23.4) | <0.0001 |
| eGDRWCa | 8.1 ± 1.8 (N=291) | 4.5 ± 1.8 (N=305) | <0.0001 | 8.6 ± 1.9 (N= 257) | 5.2 ± 1.9 (N=175) | <0.0001 |
| eGDRBMI | 8.3 ± 1.7 | 5.2 ± 1.5 | <0.0001 | 8.4 ± 1.6 | 5.2 ± 1.7 | <0.0001 |
| Complicationsc | ||||||
| Eye complications | 208 (29.0 [25.4, 32.8]) (N=838) | 444 (44.8 [40.7, 48.9] (N=819) | <0.0001 | 184 (27.6 [24.0, 31.6] (N=743) | 268 (41.7 [36.6, 47.0]) (N=493) | <0.0001 |
| Peripheral neuropathy |
26 (4.6 [3.1, 6.7]) (N=602) |
103 (10.6 [8.2, 13.6]) (N=602) |
<0.001 |
32 (6.3 [4.5, 8.8]) (N=548) |
68 (12.6 [9.2, 17.0]) (N=374) |
<0.01 |
| Chronic kidney disease | 39 (8.6 [6.3, 11.6]) (N=502) | 131 (14.4 [11.6, 17.7]) (N=631) | <0.01 | 40 (7.8 [5.7, 10.5]) (N=536) | 116 (16.8 [13.1, 21.3]) (N=435) | <0.0001 |
| CVD |
29 (4.8 [3.2, 7.1]) (N=544) |
111 (8.6 [6.3, 11.7]) (N=561) |
<0.01 |
24 (4.1 [2.7, 6.3]) (N=516) |
67 (9.1 [6.4, 12.8]) (N=342) |
<0.01 |
| Treatment | ||||||
| Glucose-lowering drugs | ||||||
| CSIIa | 133 (15.8) (N=842) | 104 (12.7) (N=816) | ns | 225 (29.8) (N=755) | 115 (22.1) (N=520) | <0.01 |
| Metformina | 43 (5.0) (N=859) | 145 (17.5) (N=830) | <0.0001 | 45 (5.9) (N=762) | 91 (17.5) (N=519) | <0.0001 |
| Cardiovascular drugs | ||||||
| Lipid-lowering drugsb | 188 (21.8) | 700 (83.8) | <0.0001 | 130 (16.9) | 420 (80.0) | <0.0001 |
| Antihypertensive drugsb | 84 (9.7) | 573 (68.6) | <0.0001 | 82 (10.7) | 354 (67.4) | <0.0001 |
| SGLT2 inhibitorsa | 11 (1.3) (N=855) | 38 (4.6) (N=824) | <0.001 | 14 (1.8) (N=764) | 14 (2.7) (N=518) | ns |
| GLP1 receptor agonistsa | 3 (0.3) (N=858) | 25 (3.0) (N=824) | <0.001 | 19 (2.5) (N=766) | 31 (6.0) (N=518) | <0.01 |
Values are n (%) for categorical variables and mean ± SD for continuous variables
Hypertension is defined as BP ≥140/90 mmHg and/or treatment with antihypertensive drugs, dyslipidaemia is defined as HDL-cholesterol <0.9 mmol/l (<35 mg/dl) in men (<1 mmol/l (<40 mg/dl) in women) and/or treatment with lipid-lowering drugs. Eye complications is defined as ever been treated (laser photocoagulation and/or intravitreal injection) for diabetic retinopathy or diabetic maculopathy, or the presence of diabetic retinopathy (proliferative or non-proliferative) or blindness. Peripheral neuropathy is defined as an abnormal sensitivity test or treatment for peripheral neuropathy. Chronic kidney disease is defined as eGFR <60 ml/min per 1.73 m2 (Modification of Diet in Renal Disease study equation [40]). CVD is defined as the presence of myocardial infarction, heart attack, coronary revascularisation (percutaneous coronary intervention or coronary artery bypass grafting), carotid revascularisation, heart failure, absence of foot pulses or peripheral bypass surgery. Lipid-lowering therapy is defined as use of statins, fibrates or ezetimibe. Antihypertensive therapy is defined as the use of either ACE inhibitors (including sartans) or other antihypertensive drugs
aOnly known for the indicated population (N)
bParameters included in the definition of the metabolic syndrome/combined metabolic abnormalities
cThe prevalence of complications was analysed using the GEE model, adjusted for age and diabetes duration, presented as n (% [95% CI])
N, population size; n, number of observations; ns, not significant
Except for smoking, both men and women with combined metabolic abnormalities had worse metabolic health compared with people living with type 1 diabetes without combined metabolic abnormalities (Table 3). As expected, people with combined metabolic abnormalities had a higher prevalence of all individual metabolic abnormalities included in the cluster, but also had a significantly higher prevalence of elevated HbA1c (>58 mmol/mol or >7.5%) and lower eGDRWC and eGDRBMI.
Furthermore, the presence of combined metabolic abnormalities was associated with a higher prevalence of micro- and macrovascular complications, corrected for age and diabetes duration (Table 3). The prevalence of eye complications was about 50% higher in both men and women with combined metabolic abnormalities compared to those without combined metabolic abnormalities. The prevalence of peripheral neuropathy was twice as high in both men and women with combined metabolic abnormalities compared to those without combined metabolic abnormalities. Chronic kidney disease and CVD were 65% and 80%, respectively, more prevalent in men with combined metabolic abnormalities compared to those without combined metabolic abnormalities, and twice as prevalent in women with combined metabolic abnormalities compared to those without combined metabolic abnormalities. The results did not change upon additional adjustment for HbA1c or smoking (data not shown).
Discussion
Data collected by the quality control system of the Belgian healthcare system enabled the study of a large, real-world population of well-characterised people living with type 1 diabetes. Over the last 20 years, we have observed an improvement in glycaemic control and LDL-cholesterol levels, but an increase in the prevalence of overweight, obesity and combined metabolic abnormalities in people living with type 1 diabetes.
In recent decades, the prevalence of obesity in the general population has increased, mainly due to the adoption of a progressively more sedentary lifestyle and the consumption of less healthy diets [21, 22]. In Belgium, age-adjusted obesity has increased from 11.5% in men and 11.1% in women in 1997 to 16.4% in men and 14.5% in women in 2018 [23]. In the current study, we show that the proportion of obesity in adults with type 1 diabetes in Belgium doubled from 12% in 2001 to 22% in 2022, confirming the finding that obesity is a growing emergency in people living with type 1 diabetes [4, 5, 22, 24–26].
The presence of a combination of metabolic abnormalities, such as central obesity, insulin resistance, dyslipidaemia and hypertension, often referred to as the metabolic syndrome or combined metabolic abnormalities, increases the risk of CVD in the general population as well as in people living with type 2 diabetes [9, 16, 27]. The prevalence of combined metabolic abnormalities ranges from 20–50% in the general adult population [6, 10, 28], but reaches almost 80% in people living with type 2 diabetes [6, 28]. An international review by Belete et al reported a pooled prevalence of combined metabolic abnormalities of 25.9% (95% CI 20.5, 31.6) in women and 22.5% (95% CI 16.7, 28.9) in men living with type 1 diabetes (studies performed between 2005 and 2020), with rates varying widely depending on patient characteristics and definition used [28]. Time-based subgroup analyses revealed a higher prevalence of combined metabolic abnormalities in the studies performed between 2015 and 2020 (26.6%) compared with those performed between 2005 and 2014 (21.8%) [28].
In the present study, we confirmed an increasing prevalence of combined metabolic abnormalities in adults living with type 1 diabetes, corrected for age and diabetes duration, irrespectively of the definition used. In 2001, combined metabolic abnormalities were identified in 27.5% of women and 31.7% of men, according to the WHO definition. In 2022, these proportions increased to 40.2% and 49.7% in women (p<0.01) and men (p<0.0001), respectively. The WHO definition of the metabolic syndrome/combined metabolic abnormalities includes microalbuminuria as a criterion, reflecting the pathophysiology of albuminuria seen in patients with type 2 diabetes, which may lead to a higher prevalence of combined metabolic abnormalities compared with other definitions. However, as albuminuria in people living with type 1 diabetes is typically a microvascular complication, indicating the early stages of renal disease, we repeated the analysis using the WHO definition of the metabolic syndrome/combined metabolic abnormalities without microalbuminuria. The increase in the prevalence of combined metabolic abnormalities remained when albuminuria was removed from the definition (from 21.8% in 2001 to 38.2% in 2022 in women [p<0.0001] and from 24.7% in 2001 to 46.4% in 2022 in men [p<0.0001]).
In our study, an increase in the prevalence of combined metabolic abnormalities was also observed when using the NCEP-ATPIII and IDF definitions of the metabolic syndrome/combined metabolic abnormalities, which use WC as the key obesity measure [28], but the overall prevalence was higher compared with the WHO definitions of the metabolic syndrome/combined metabolic abnormalities (with and without albuminuria). This may be due to the fact that WC data were only available from 2005 onwards and only for a minority of participants, although the small differences in threshold values for hypertension and HDL-cholesterol between definitions may also have had an effect. A recent Belgian study reported a prevalence of combined metabolic abnormalities in people living with type 1 diabetes of 30% according to the NCEP-ATPIII definition (data collected between 2018 and 2022) [29].
We have shown previously that, in people living with type 1 diabetes, glycaemic and lipid control improved over time due to a combination of provision of technology, education and quality monitoring [14]. HbA1c and LDL-cholesterol levels also decreased over time in the present study in people living with type 1 diabetes. Rates of lipid-lowering and antihypertensive therapy increased, which may be at least partially explained by the European Association for the Study of Diabetes and the American Diabetes Association consensus guidelines recommending more routinely cardiovascular treatment in addition to glucose-lowering treatment in people living with diabetes. As a result, the proportions of people being treated with lipid-lowering or antihypertensive drugs and thus by definition being identified as having dyslipidaemia or hypertension strongly increased, as did the number of people with combined metabolic abnormalities. However, disturbingly, obesity and central obesity became more prevalent with the decrease in HbA1c.
Those with combined metabolic abnormalities had higher glucose levels (HbA1c >58 mmol/mol or >7.5%) and (as per definition) more overweight and hypertension and worse lipid control compared to those without combined metabolic abnormalities. Our results are in line with the findings of Lee et al [10]. In their study, people with combined metabolic abnormalities had higher glucose levels (HbA1c of 68 mmol [8.4%] vs 64 mmol [8.0%]) and a significantly higher prevalence of hypertension (89% vs 29%), dyslipidaemia (combined elevated TG levels and lower HDL-cholesterol levels, 50% vs 9.1%) and obesity (50% vs 7.2%) compared to those without combined metabolic abnormalities. In our population, despite higher use of statins and antihypertensive drugs in those with combined metabolic abnormalities, approximately one-fifth of individuals with combined metabolic abnormalities were not on statins. In addition, one-fifth of those with combined metabolic abnormalities smoked, further accelerating the risk of micro- and macrovascular complications in this population.
We found a strong relationship between combined metabolic abnormalities and the prevalence of CVD, but also eye complications, peripheral neuropathy and chronic kidney disease, corrected for age, diabetes duration and HbA1c. This finding is in line with some previous observations, depending on the definition of the metabolic syndrome/combined metabolic abnormalities used, and highlights the importance of identification of combined metabolic abnormalities and initiation of more aggressive therapeutic approaches in these patients [10, 12, 22, 30].
Our data show that people with combined metabolic abnormalities are less often treated with CSII. The maximum insulin storage capacity of insulin pumps may have influenced treatment decisions for individuals with combined metabolic abnormalities, especially those with higher body weight. However, no patch pumps for which the maximum insulin storage capacity could be a major issue were available in Belgium over the time period of data collection.
In our population, people with combined metabolic abnormalities are more often treated with adjunct therapies such as metformin, SGLT2 inhibitors and GLP1 receptor agonists. Metformin is the most commonly used treatment to increase insulin sensitivity in insulin-resistant conditions. It decreases hepatic glucose production and enhances insulin-stimulated glucose disposal in peripheral tissues [22, 31]. Metformin is an inexpensive and well-established oral glucose-lowering drug, and the first-line treatment in patients with type 2 diabetes. It is frequently used as an adjunct to intensive insulin therapy in people living with type 1 diabetes [32, 33]. It has been shown to have some benefit in reducing insulin doses and weight, although no long-term beneficial effects were observed when patients were followed for 10 years [34]. The recent international study ‘REducing with Metformin Vascular Adverse Lesions’ (REMOVAL) suggests a reduction in cardiovascular risk as a result of metformin use in people with long-standing type 1 diabetes [35]. However, in most countries, including Belgium, there is no official indication for use of metformin in patients with type 1 diabetes. Nevertheless, 11% of our type 1 diabetes population used metformin as an adjunct therapy (2022 data), in line with 8–15% of the population reported in Scotland (2016 data) [35] and 4–7% of the population in the USA (T1D Exchange Registry, 2016–2018 data) [26].
As SGLT2 inhibitors and GLP1 receptor agonists are intended for use in people living with type 2 diabetes, use of these drugs for treatment of people living with type 1 diabetes is rare and they are prescribed on an individual basis. SGLT2 inhibitors reduce blood glucose levels by decreasing the resorption of glucose in the kidneys, and exert nephroprotective and cardioprotective effects [22]. Studies of the use of SGLT2 inhibitors in people living with type 1 diabetes have shown a positive effect on BMI and daily insulin dose, but warn of a potential increased risk of euglycaemic ketoacidosis [4, 36]. While further research is needed on the potential cardiorenal benefits of use of SGLT2 inhibitors in people with type 1 diabetes, for the moment it may be advisable to prescribe them only to compliant patients with a BMI greater than 27 kg/m2, and to interrupt their use in cases of insulin dose reduction and dehydration [37, 38]. GLP1 receptor agonists enhance insulin secretion in a glucose-dependent manner, inhibit the release of glucagon, promote satiety and slow down gastric emptying. In addition to their glucose-lowering effect, GLP1 receptor agonists have a positive effect on BMI and cardiovascular events [4, 22, 39]. Due to their safety profile and demonstrated positive effect on obesity and insulin resistance in people living with type 1 diabetes, these drugs may be used as an adjunct therapy in such patients [39].
Despite important strengths, such as the size and quality of the database as well as the duration of observation, our study has limitations. The cross-sectional nature of the data does not allow individual longitudinal follow-up or investigation of causality. Also, the lack of information on physical activity, ethnicity or socioeconomic status is a weakness.
Conclusion
People living with type 1 diabetes are increasingly affected by overweight, obesity and combined metabolic abnormalities. This co-occurrence of diseases may result in a further elevated risk of microvascular and macrovascular complications. Early identification of the presence of combined metabolic abnormalities should enable therapeutic interventions to be modified towards multifactorial approaches, with attention to education on avoidance of overweight (e.g. dietary counselling) in addition to strict glycaemic control and intensification of use of antihypertensive drugs and statins. The use of adjunct therapies deserve to be explored more thoroughly in this population as a tool to reduce the risk of complications.
Abbreviations
- CSII
Continuous subcutaneous insulin infusion
- eGDRWC
Estimated glucose disposal rate based on waist circumference
- eGDRBMI
Estimated glucose disposal rate based on BMI
- GEE
Generalised estimating equations
- GLP1
Glucagon-like peptide-1
- IQED
Initiative for Quality Improvement and Epidemiology in Diabetes
- NCEP-ATPIII
National Cholesterol Education Program Adult Treatment Panel III
- SGLT2
Sodium–glucose cotransporter 2
- TG
Triglycerides
- WC
Waist circumference
Acknowledgements
This article is written on behalf of the IQED Group of Experts. The members of the IQED Group of Experts are L. Crenier, C. De Block, A.-S. Vanherwegen, A. Lavens, C. Mathieu, F. Nobels, J.-C. Philips, P. Oriot, M. Vandenbroucke and V. Vanelshocht. We would like to thank the staff of all Belgian specialised diabetes centres for the data collection. Some of the data were presented as an abstract at the EASD meeting in 2023.
Data availability
Data cannot be shared publicly because of the use of pseudonymised person-level data. Readers who wish to access some or all of the data require approval from the Belgian Information Security Committee on Social Security and Health. More information about the access procedure may be obtained by contacting iqed@sciensano.be. Metadata (e.g. overview of variables, legal framework) are available on https://fair.healthdata.be/.
Funding
The IQED programme is funded by the Belgian National Institute for Health and Disability Insurance.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
AL, CM, PO and CDB developed the concept and design of this study. Data analysis was performed by AL. All authors made substantial contributions to the interpretation of results. AL, CDB, PO and CM drafted the manuscript, and all authors contributed to critical revision of the manuscript for important intellectual content. All authors approved the final manuscript for publication. AL had full access to the data and accepts the responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nathan DM, Cleary PA, Backlund J-YC et al (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353(25):2643–2653. 10.1056/NEJMoa052187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DCCT Research Group, Nathan DM, Genuth S et al (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329(14):977–986. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group (2016) Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 39(5):686–693. 10.2337/dc15-1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van der Schueren B, Ellis D, Faradji RN, Al-Ozairi E, Rosen J, Mathieu C (2021) Obesity in people living with type 1 diabetes. Lancet Diabetes Endocrinol 9(11):776–785. 10.1016/S2213-8587(21)00246-1 [DOI] [PubMed] [Google Scholar]
- 5.Edqvist J, Rawshani A, Adiels M et al (2019) BMI, mortality, and cardiovascular outcomes in type 1 diabetes: findings against an obesity paradox. Diabetes Care 42(7):1297–1304. 10.2337/dc18-1446 [DOI] [PubMed] [Google Scholar]
- 6.Chillarón JJ, Flores Le-Roux JA, Benaiges D, Pedro-Botet J (2014) Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism 63(2):181–187. 10.1016/j.metabol.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 7.Colom C, Rull A, Sanchez-Quesada JL, Pérez A (2021) Cardiovascular disease in type 1 diabetes mellitus: epidemiology and management of cardiovascular risk. J Clin Med 10(8):1798. 10.3390/jcm10081798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purnell JQ, Braffett BH, Zinman B et al (2017) Impact of excessive weight gain on cardiovascular outcomes in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care 40(12):1756–1762. 10.2337/dc16-2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilpatrick ES, Rigby AS, Atkin SL (2007) Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: ‘double diabetes’ in the Diabetes Control and Complications Trial. Diabetes Care 30(3):707–712. 10.2337/dc06-1982 [DOI] [PubMed] [Google Scholar]
- 10.Lee AS, Twigg SM, Flack JR (2021) Metabolic syndrome in type 1 diabetes and its association with diabetes complications. Diabet Med 38(2):e14376. 10.1111/dme.14376 [DOI] [PubMed] [Google Scholar]
- 11.Helliwell R, Warnes H, Kietsiriroje N et al (2021) Body mass index, estimated glucose disposal rate and vascular complications in type 1 diabetes: beyond glycated haemoglobin. Diabet Med 38(5):e14529. 10.1111/dme.14529 [DOI] [PubMed] [Google Scholar]
- 12.Gingras V, Leroux C, Fortin A, Legault L, Rabasa-Lhoret R (2017) Predictors of cardiovascular risk among patients with type 1 diabetes: a critical analysis of the metabolic syndrome and its components. Diabetes Metab 43(3):217–222. 10.1016/j.diabet.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 13.Sciensano (2001) Initiative for Quality Improvement and Epidemiology in Diabetes (IQED). Available from https://www.sciensano.be/en/projects/initiative-quality-improvement-and-epidemiology-diabetes. Accessed 21 Feb 2024
- 14.Lavens A, Nobels F, De Block C et al (2021) Effect of an integrated, multidisciplinary nationwide approach to type 1 diabetes care on metabolic outcomes: an observational real-world study. Diabetes Technol Ther 23(8):565–576. 10.1089/dia.2021.0003 [DOI] [PubMed] [Google Scholar]
- 15.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106(25):3143–3421. 10.1161/circ.106.25.3143 [PubMed] [Google Scholar]
- 16.Alberti KGMM, Eckel RH, Grundy SM et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–1645. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 17.International Diabetes Foundation (2006) The IDF consensus worldwide definition of the metabolic syndrome. Available from https://idf.org/media/uploads/2023/05/attachments-30.pdf. Accessed 21 Dec 2023
- 18.Nyström T, Holzmann MJ, Eliasson B, Svensson A-M, Sartipy U (2018) Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes Obes Metab 20(3):556–563. 10.1111/dom.13110 [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of LDL cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502 [PubMed] [Google Scholar]
- 20.Mach F, Baigent C, Catapano AL et al (2020) ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 41(1):111–188. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 21.Boutari C, Mantzoros CS (2022) A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 133:155217. 10.1016/j.metabol.2022.155217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karamanakos G, Kokkinos A, Dalamaga M, Liatis S (2022) Highlighting the role of obesity and insulin resistance in type 1 diabetes and its associated cardiometabolic complications. Curr Obes Rep 11(3):180–202. 10.1007/s13679-022-00477-x [DOI] [PubMed] [Google Scholar]
- 23.Sciensano (2020) Weight status. Available from https://www.healthybelgium.be/en/health-status/determinants-of-health/weight-status. Accessed 21 Dec 2023
- 24.Giandalia A, Russo GT, Ruggeri P et al (2023) The burden of obesity in type 1 diabetic subjects: a sex-specific analysis from the AMD Annals Initiative. J Clin Endocrinol Metab 108(11):e1224–e1235. 10.1210/clinem/dgad302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conway B, Miller RG, Costacou T et al (2010) Temporal patterns in overweight and obesity in type 1 diabetes. Diabet Med 27(4):398–404. 10.1111/j.1464-5491.2010.02956.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster NC, Beck RW, Miller KM et al (2019) State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 21(2):66–72. 10.1089/dia.2018.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhee E-J (2022) The influence of obesity and metabolic health on vascular health. Endocrinol Metab 37(1):1–8. 10.3803/EnM.2022.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belete R, Ataro Z, Abdu A, Sheleme M (2021) Global prevalence of metabolic syndrome among patients with type I diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr 13:25. 10.1186/s13098-021-00641-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mertens J, Weyler J, Dirinck E et al (2023) Prevalence, risk factors and diagnostic accuracy of non-invasive tests for NAFLD in people with type 1 diabetes. JHEP Rep 5(7):100753. 10.1016/j.jhepr.2023.100753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Block CEM, De Leeuw IH, Van Gaal LF (2005) Impact of overweight on chronic microvascular complications in type 1 diabetic patients. Diabetes Care 28(7):1649–1655. 10.2337/diacare.28.7.1649 [DOI] [PubMed] [Google Scholar]
- 31.Herman R, Kravos NA, Jensterle M, Janež A, Dolžan V (2022) Metformin and insulin resistance: a review of the underlying mechanisms behind changes in GLUT4-mediated glucose transport. Int J Mol Sci 23(3):1264. 10.3390/ijms23031264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiang JL, Kirkman MS, Laffel LMB, Peters AL, Type 1 Diabetes Sourcebook Authors (2014) Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 37(7):2034–2054. 10.2337/dc14-1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aberer F, Pieber TR, Eckstein ML, Sourij H, Moser O (2022) Glucose-lowering therapy beyond insulin in type 1 diabetes: a narrative review on existing evidence from randomized controlled trials and clinical perspective. Pharmaceutics 14(6):1180. 10.3390/pharmaceutics14061180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staels F, Moyson C, Mathieu C (2017) Metformin as add-on to intensive insulin therapy in type 1 diabetes mellitus. Diabetes Obes Metab 19(10):1463–1467. 10.1111/dom.12948 [DOI] [PubMed] [Google Scholar]
- 35.Petrie JR, Chaturvedi N, Ford I et al (2017) Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 5(8):597–609. 10.1016/S2213-8587(17)30194-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maffei P, Bettini S, Busetto L, Dassie F (2023) SGLT2 inhibitors in the management of type 1 diabetes (T1D): an update on current evidence and recommendations. Diabetes Metab Syndr Obes 16:3579. 10.2147/DMSO.S240903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teng R, Kurian M, Close KL, Buse JB, Peters AL, Alexander CM (2021) Comparison of protocols to reduce diabetic ketoacidosis in patients with type 1 diabetes prescribed a sodium–glucose cotransporter 2 inhibitor. Diabetes Spectr 34(1):42–51. 10.2337/ds20-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musso G, Sircana A, Saba F, Cassader M, Gambino R (2020) Assessing the risk of ketoacidosis due to sodium-glucose cotransporter (SGLT)-2 inhibitors in patients with type 1 diabetes: a meta-analysis and meta-regression. PLoS Med 17(12):e1003461. 10.1371/journal.pmed.1003461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathieu C, Ahmadzai I (2023) Incretins beyond type 2 diabetes. Diabetologia 66(10):1809–1819. 10.1007/s00125-023-05980-x [DOI] [PubMed] [Google Scholar]
- 40.Stevens LA, Coresh J, Feldman HI et al (2007) Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol 18(10):2749–2757. 10.1681/ASN.2007020199 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly because of the use of pseudonymised person-level data. Readers who wish to access some or all of the data require approval from the Belgian Information Security Committee on Social Security and Health. More information about the access procedure may be obtained by contacting iqed@sciensano.be. Metadata (e.g. overview of variables, legal framework) are available on https://fair.healthdata.be/.