Abstract
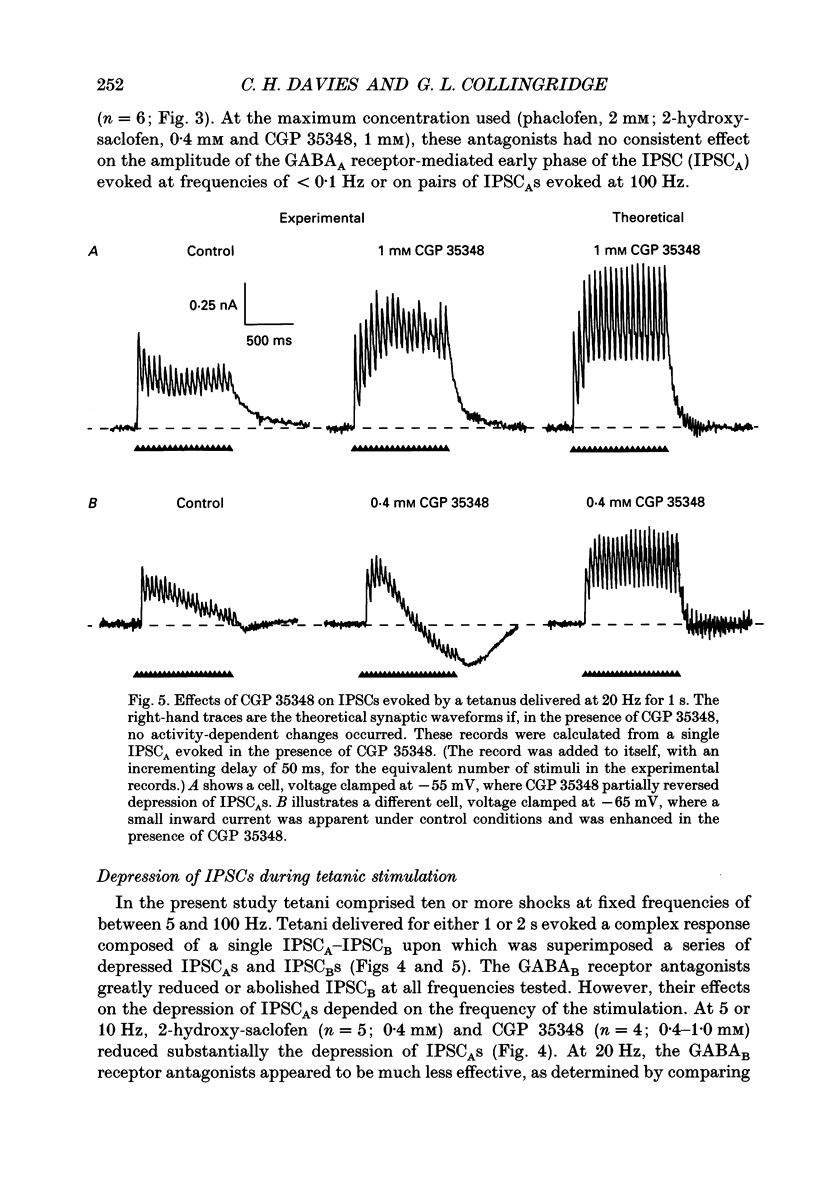
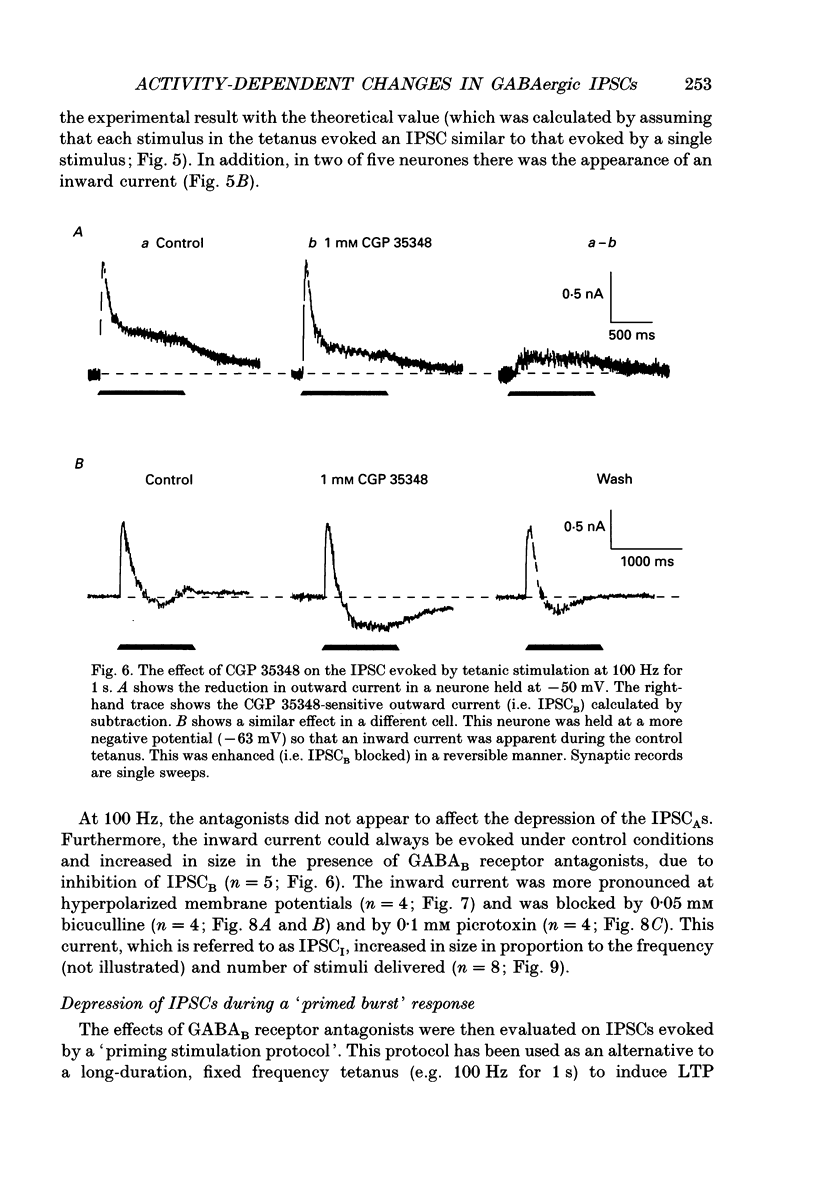
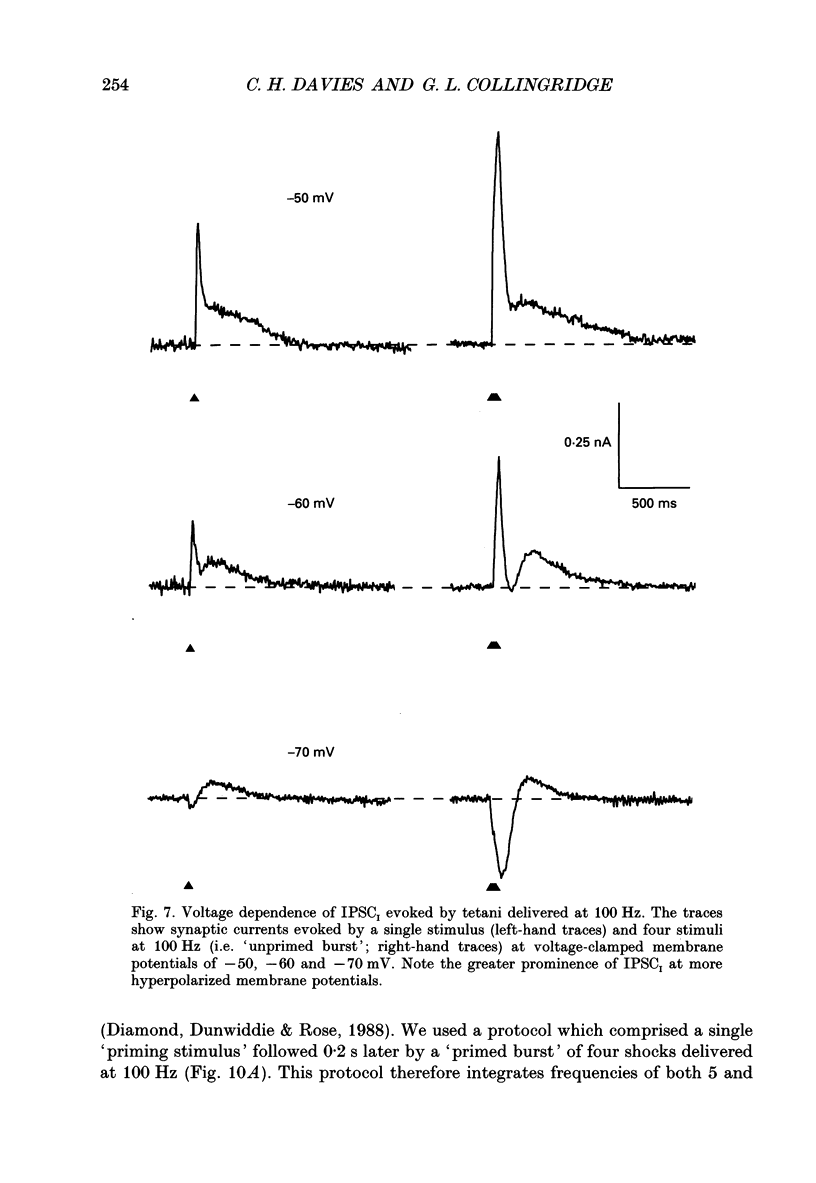
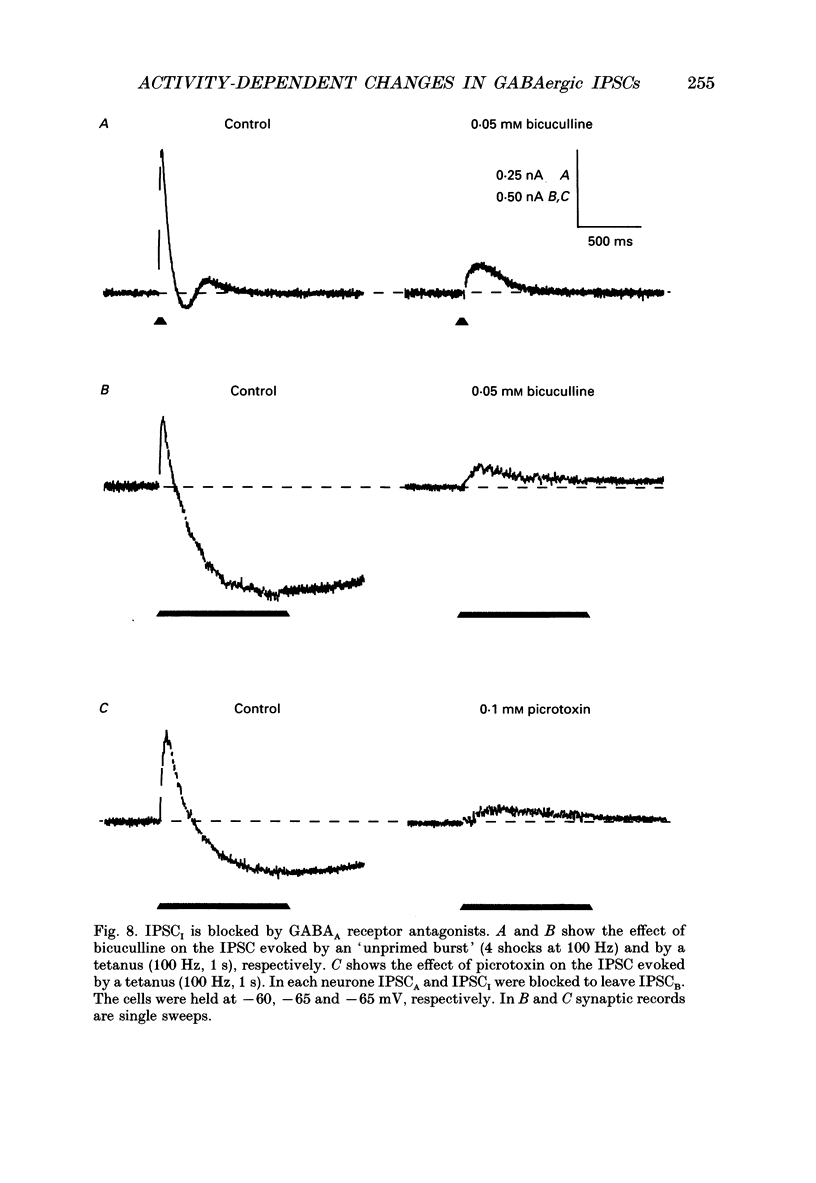
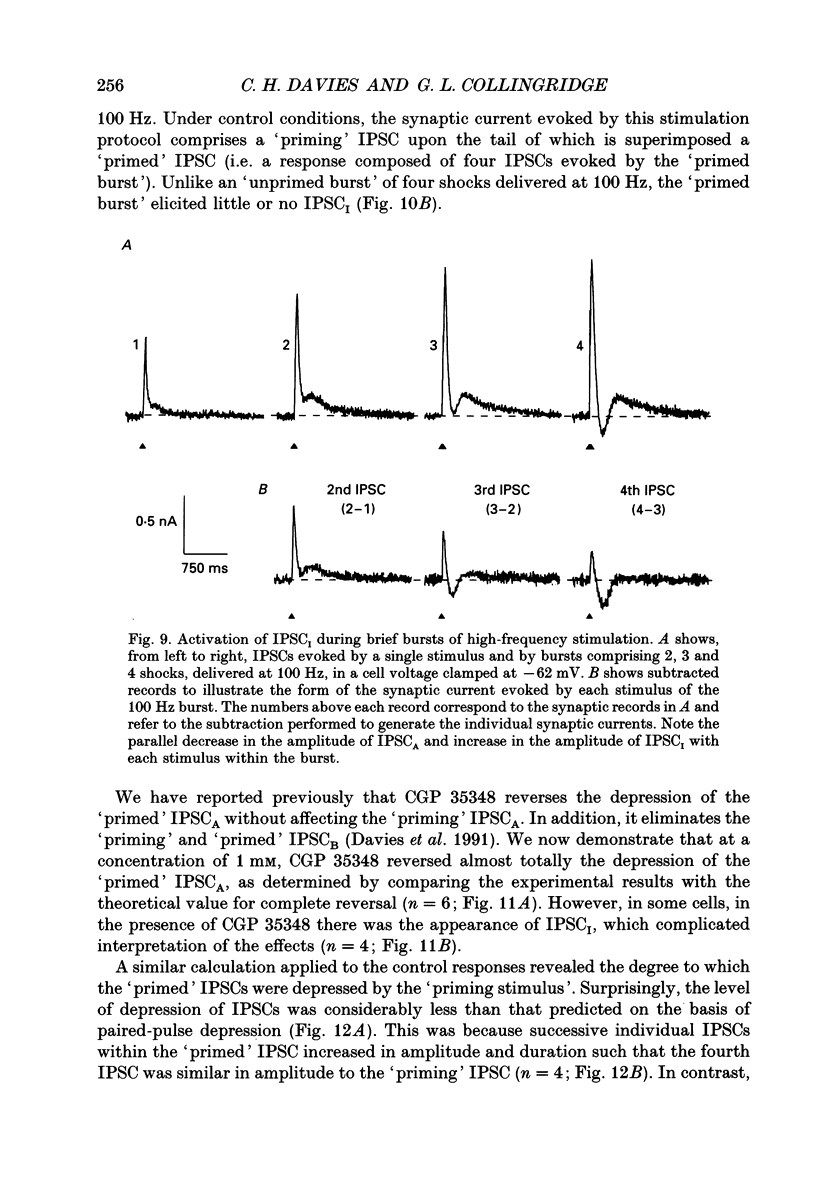
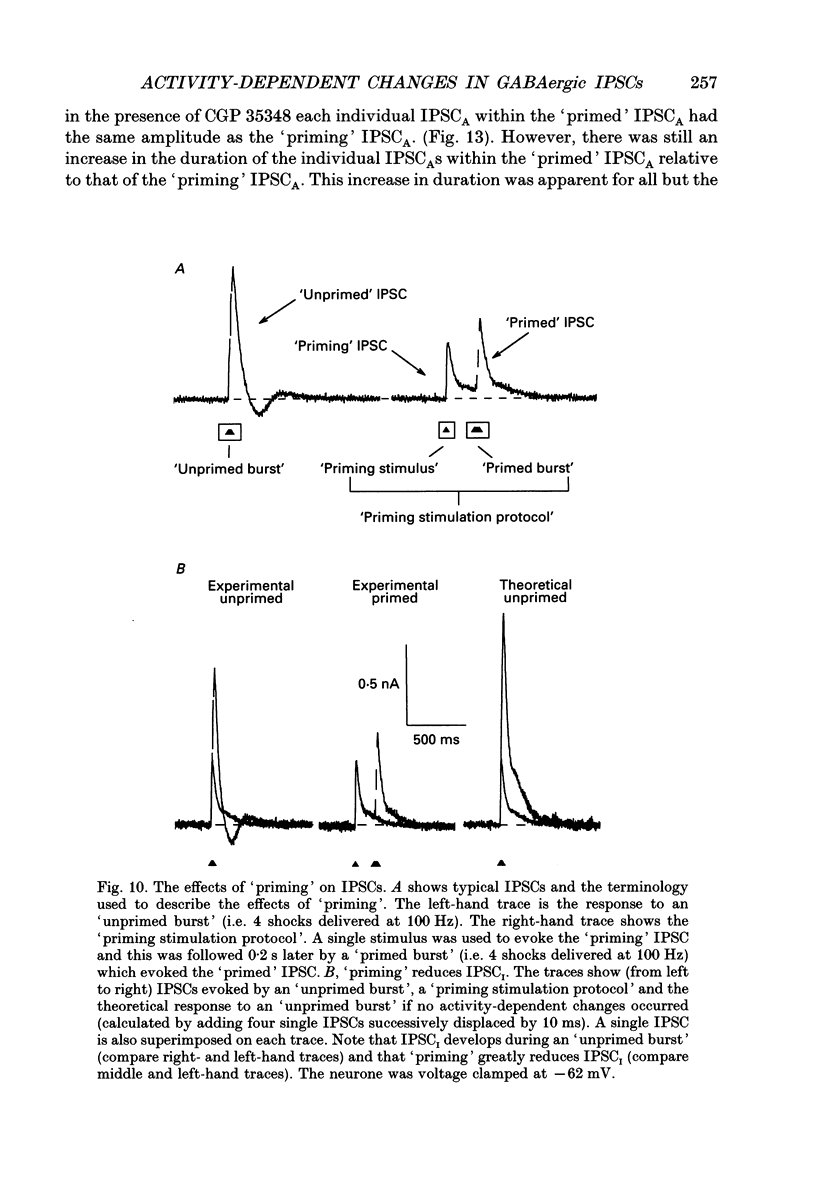
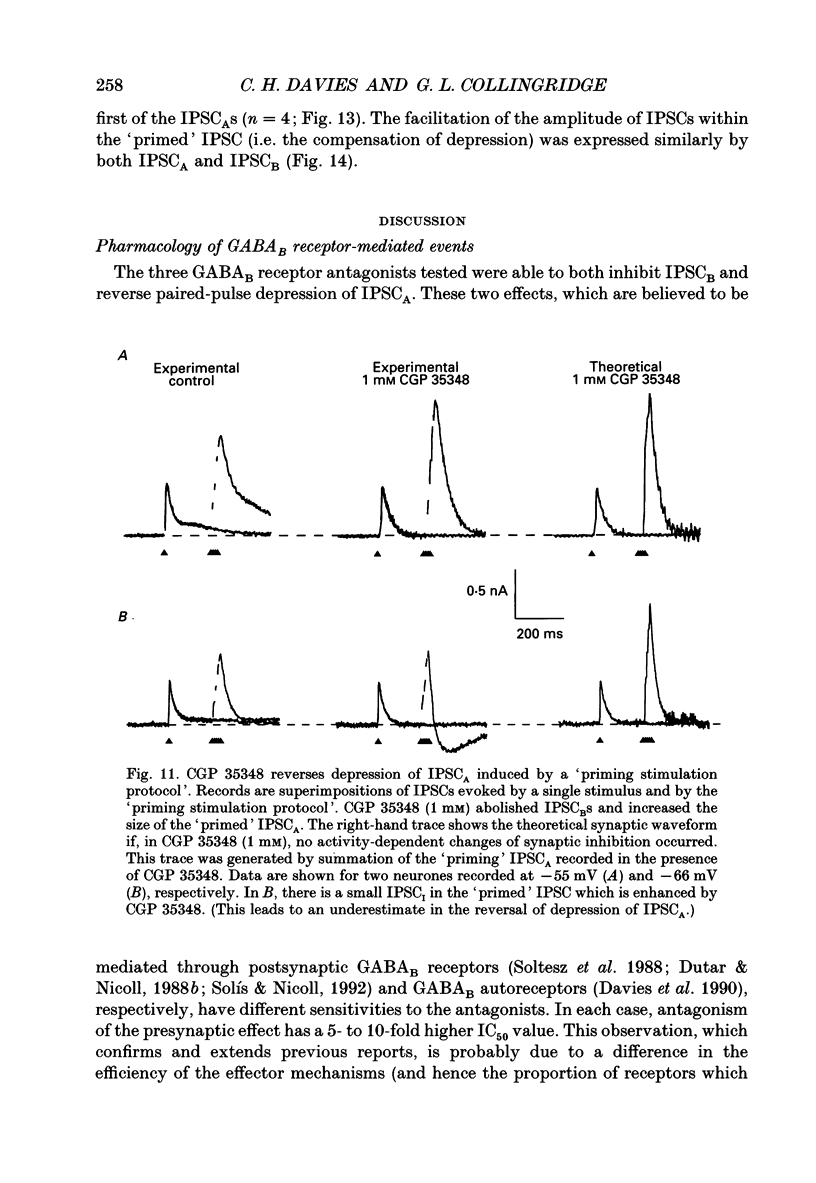
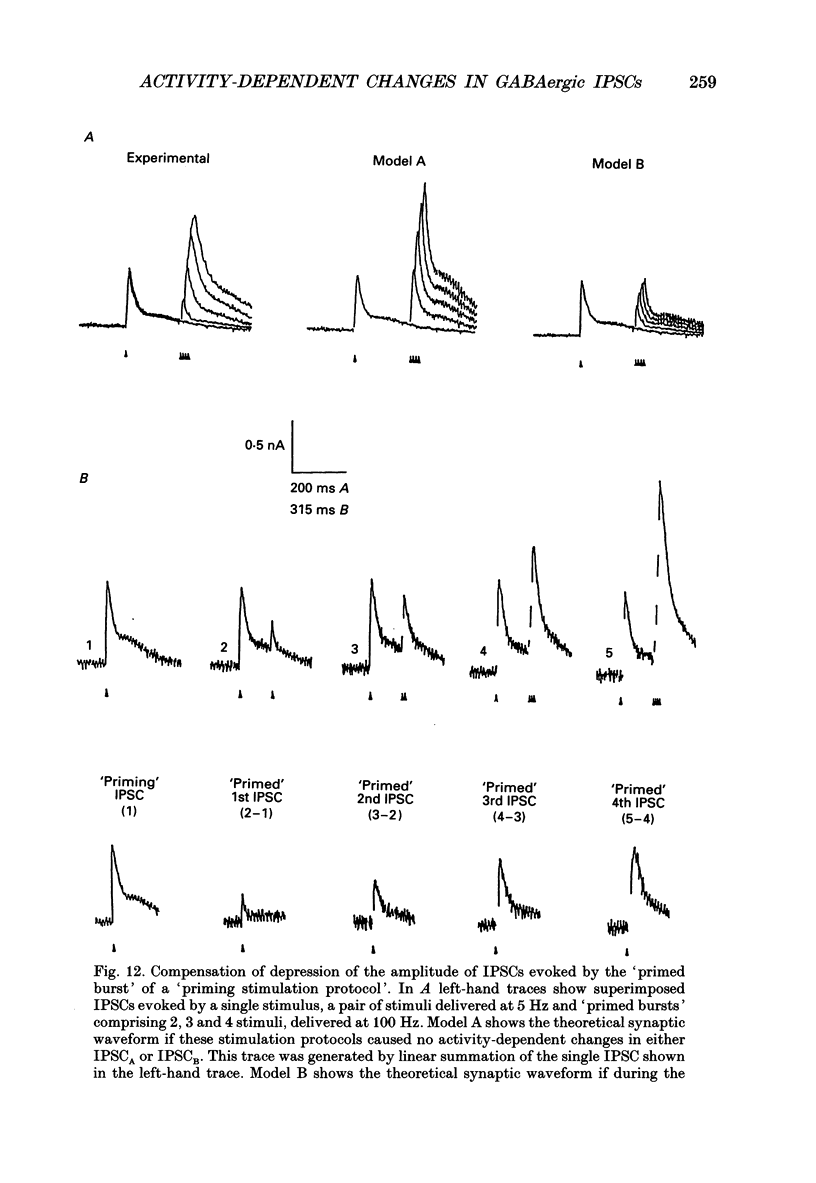
1. Intracellular recording techniques were used to study the effects of repetitive stimulation on monosynaptically activated inhibitory postsynaptic currents (IPSCs) in rat hippocampal slices. This was achieved by stimulation in stratum radiatum close to a recorded CA1 pyramidal neurone after pharmacological blockade of excitatory synaptic responses, using a combination of the N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor antagonists D-2-amino-5-phosphonopentanoate (AP5; 0.04-0.1 mM) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 0.02-0.04 mM), respectively. 2. Fixed-intensity stimulation at frequencies of less than 0.1 Hz evoked biphasic IPSCs of constant amplitude and waveform. In contrast, when two shocks (paired pulse) or longer trains of ten or more stimuli (i.e. tetani) were delivered at frequencies of between 0.2 and 20 Hz there was marked depression of both phases of every IPSC (by 60-100%) relative to the first or 'priming' IPSC evoked. 3. The gamma-aminobutyric acid (GABA)B receptor antagonists phaclofen (0.4-2 mM), 2-hydroxy-saclofen (0.02-0.4 mM) and 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP 35348; 0.01-1 mM) reduced or abolished, in a concentration-dependent and reversible manner, both the late phase of the IPSC (IPSCB) and paired-pulse depression of the early phase of the IPSC (IPSCA). Expressed in terms of IC50 values, all three antagonists were 5-10 times more potent at blocking IPSCB than paired-pulse depression. 4. Paired-pulse depression, at 5 and 10 Hz, has been shown to be mediated by GABA acting on presynaptic GABAB receptors (i.e. GABAB autoreceptors). We now show that GABAB receptor antagonists reverse paired-pulse depression over the entire range of frequencies (0.1-50 Hz) that it occurs. 5. GABAB receptor antagonists reversed substantially the depression of IPSCs during tetani delivered at 5 or 10 Hz. However at 20 Hz, GABAB receptor antagonists appeared to be less effective. At 100 Hz they appeared to be ineffective at reversing the depression of IPSCA; since the antagonists block IPSCB the net effect was to reduce the level of outward current. 6. At frequencies of 20 Hz or more, there was also the appearance of a slow inward current which increased in size in proportion to the frequency and number of shocks in the tetanus. This current (termed here IPSCI) was more pronounced at hyperpolarized membrane potentials and was blocked by picrotoxin (0.1 mM) or bicuculline (0.05 mM). 7. 'Priming' is considered to represent a more physiological pattern of activity than a tetanus.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
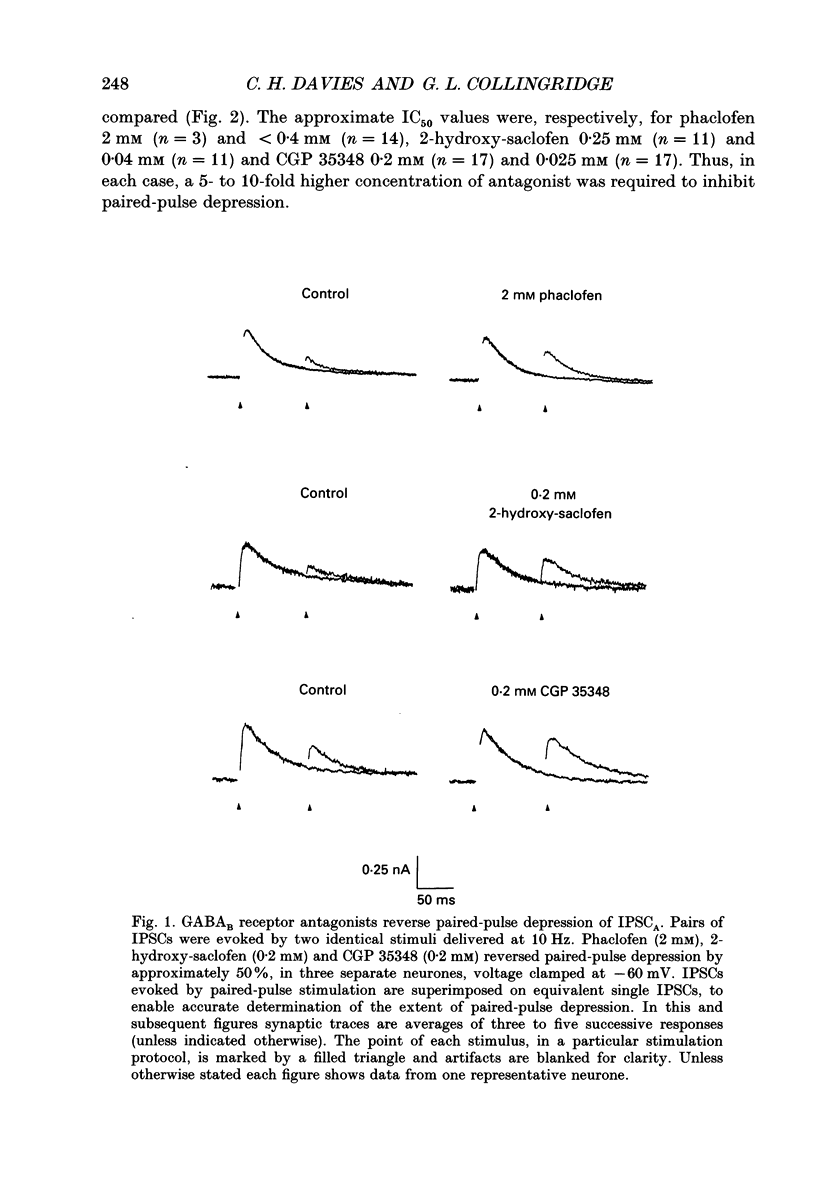
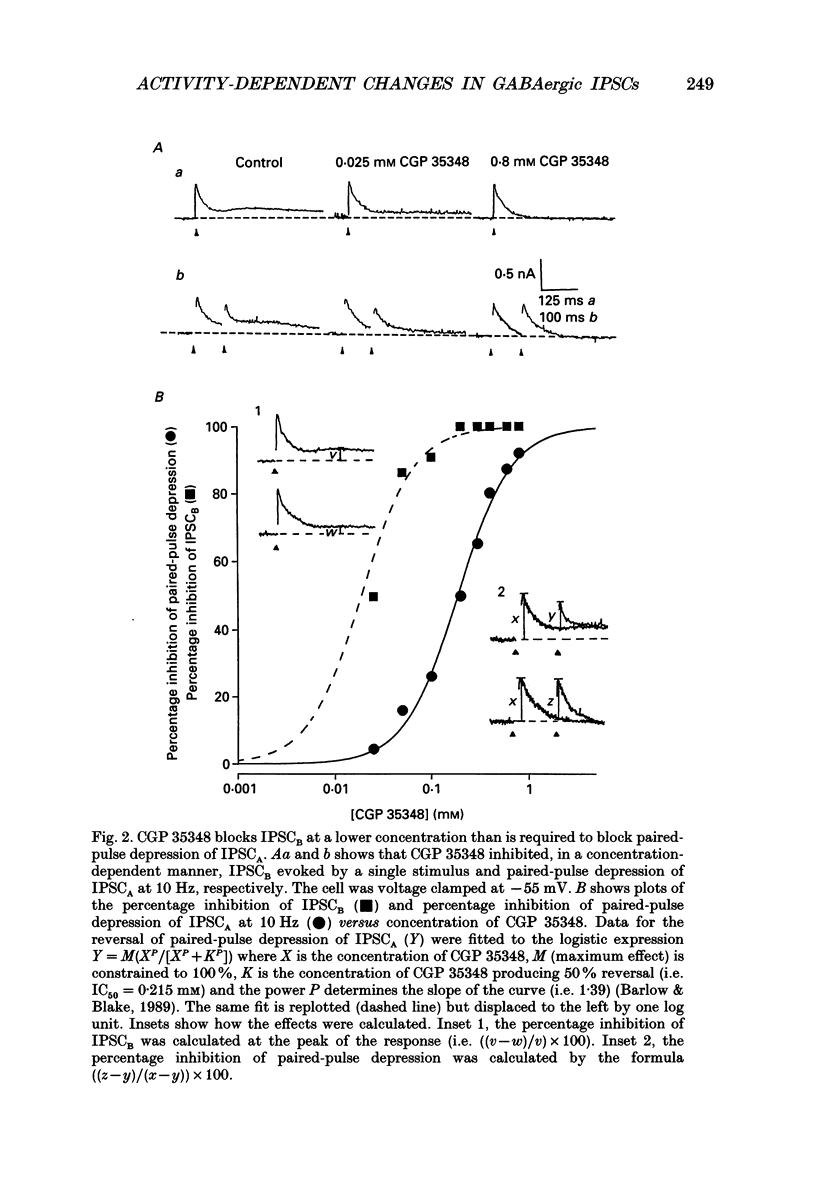
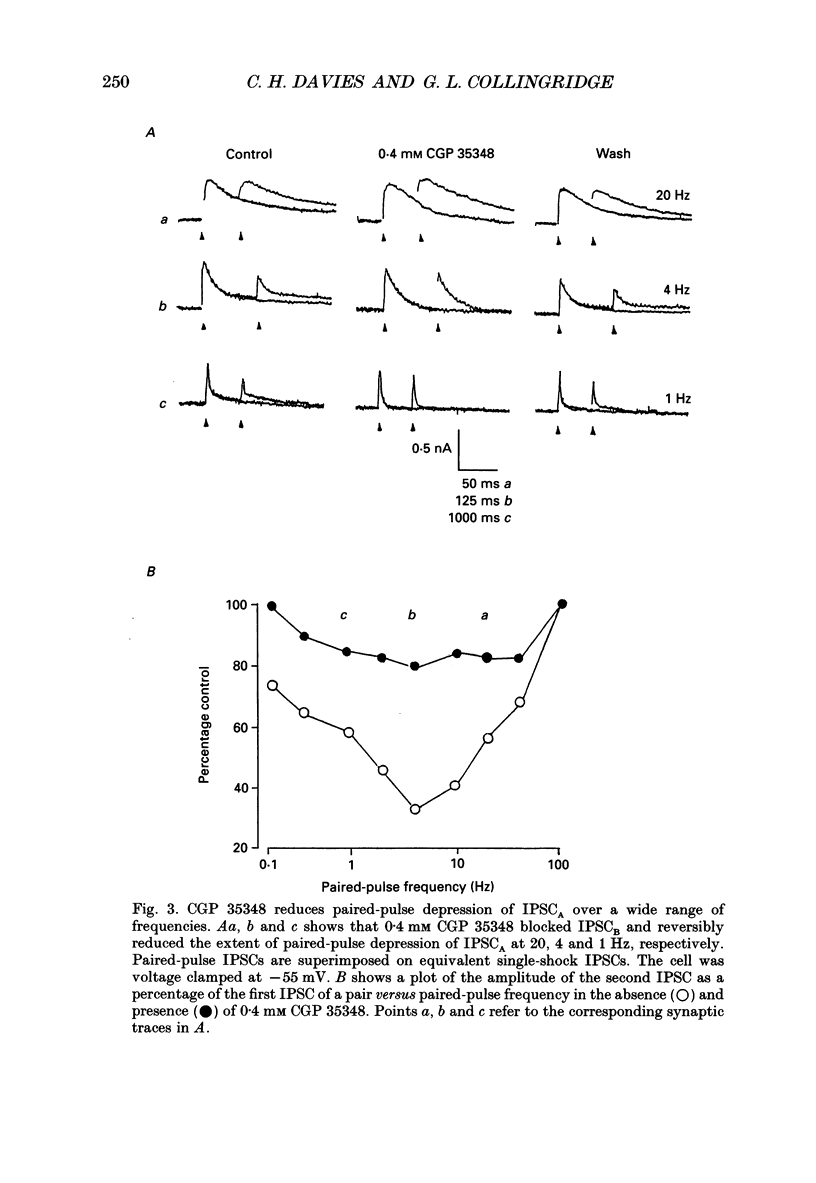
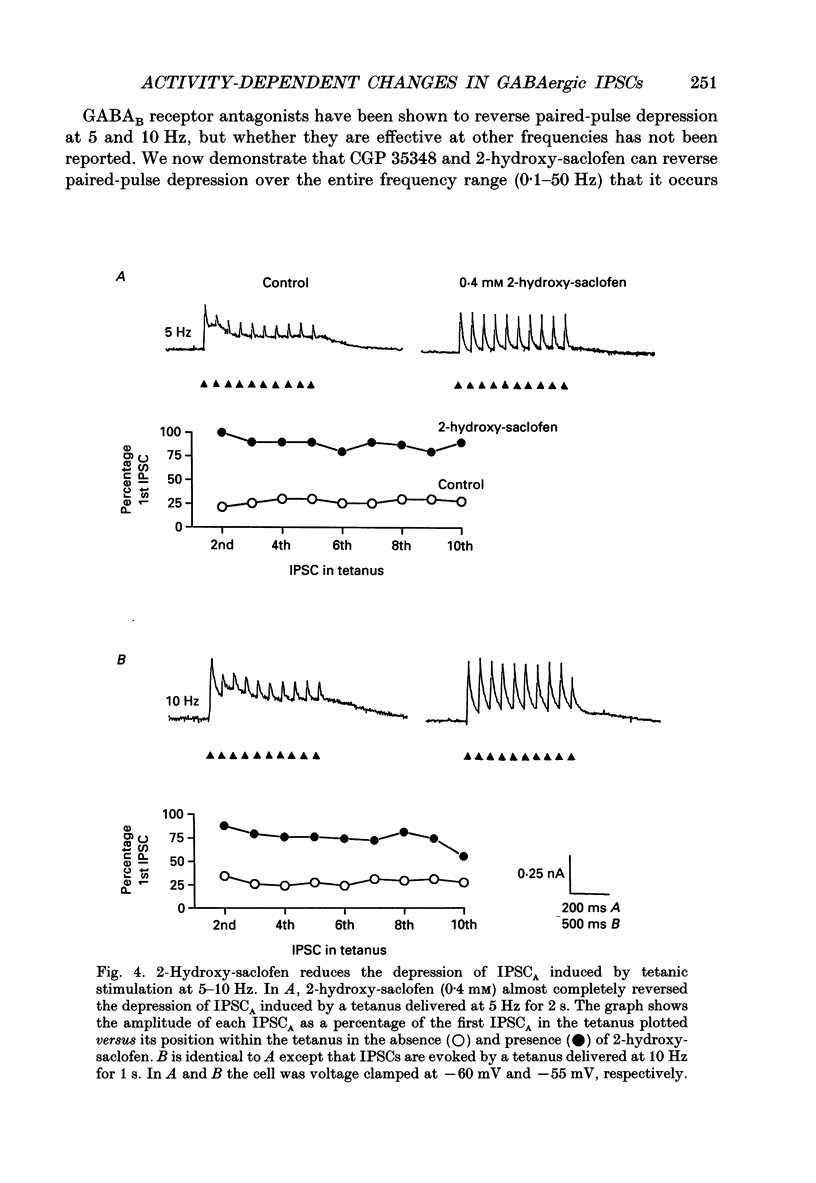
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M., Perreault P. A GABAergic depolarizing potential in the hippocampus disclosed by the convulsant 4-aminopyridine. Brain Res. 1987 Jan 1;400(1):191–195. doi: 10.1016/0006-8993(87)90671-8. [DOI] [PubMed] [Google Scholar]
- Avoli Massimo. Synaptic Activation of GABAA Receptors Causes a Depolarizing Potential Under Physiological Conditions in Rat Hippocampal Pyramidal Cells. Eur J Neurosci. 1992 Oct;4(1):16–26. doi: 10.1111/j.1460-9568.1992.tb00105.x. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. H., Starkey S. J., Pozza M. F., Collingridge G. L. GABA autoreceptors regulate the induction of LTP. Nature. 1991 Feb 14;349(6310):609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Prince D. A. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989 May;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond D. M., Dunwiddie T. V., Rose G. M. Characteristics of hippocampal primed burst potentiation in vitro and in the awake rat. J Neurosci. 1988 Nov;8(11):4079–4088. doi: 10.1523/JNEUROSCI.08-11-04079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R., Hynes M. A., King G. L. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986 Nov;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988 Sep;1(7):585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Lange G. D., Barker J. L. (-)-Baclofen activates presynaptic GABAB receptors on GABAergic inhibitory neurons from embryonic rat hippocampus. Neurosci Lett. 1988 Feb 15;85(1):105–109. doi: 10.1016/0304-3940(88)90437-5. [DOI] [PubMed] [Google Scholar]
- Herron C. E., Lester R. A., Coan E. J., Collingridge G. L. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986 Jul 17;322(6076):265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Isaacson J. S., Solís J. M., Nicoll R. A. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993 Feb;10(2):165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Lambert N. A., Levitin M., Harrison N. L. Induction of giant depolarizing potentials by zinc in area CA1 of the rat hippocampus does not result from block of GABAB receptors. Neurosci Lett. 1992 Feb 3;135(2):215–218. doi: 10.1016/0304-3940(92)90439-e. [DOI] [PubMed] [Google Scholar]
- McCarren M., Alger B. E. Use-dependent depression of IPSPs in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1985 Feb;53(2):557–571. doi: 10.1152/jn.1985.53.2.557. [DOI] [PubMed] [Google Scholar]
- Michelson H. B., Wong R. K. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991 Sep 20;253(5026):1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- Mott D. D., Lewis D. V. Facilitation of the induction of long-term potentiation by GABAB receptors. Science. 1991 Jun 21;252(5013):1718–1720. doi: 10.1126/science.1675489. [DOI] [PubMed] [Google Scholar]
- Nathan T., Lambert J. D. Depression of the fast IPSP underlies paired-pulse facilitation in area CA1 of the rat hippocampus. J Neurophysiol. 1991 Nov;66(5):1704–1715. doi: 10.1152/jn.1991.66.5.1704. [DOI] [PubMed] [Google Scholar]
- Olpe H. R., Karlsson G. The effects of baclofen and two GABAB-receptor antagonists on long-term potentiation. Naunyn Schmiedebergs Arch Pharmacol. 1990 Aug;342(2):194–197. doi: 10.1007/BF00166964. [DOI] [PubMed] [Google Scholar]
- Pacelli G. J., Su W., Kelso S. R. Activity-induced decrease in early and late inhibitory synaptic conductances in hippocampus. Synapse. 1991 Jan;7(1):1–13. doi: 10.1002/syn.890070102. [DOI] [PubMed] [Google Scholar]
- Perreault P., Avoli M. A depolarizing inhibitory postsynaptic potential activated by synaptically released gamma-aminobutyric acid under physiological conditions in rat hippocampal pyramidal cells. Can J Physiol Pharmacol. 1988 Aug;66(8):1100–1102. doi: 10.1139/y88-180. [DOI] [PubMed] [Google Scholar]
- Roepstorff A., Lambert J. D. Comparison of the effect of the GABA uptake blockers, tiagabine and nipecotic acid, on inhibitory synaptic efficacy in hippocampal CA1 neurones. Neurosci Lett. 1992 Nov 9;146(2):131–134. doi: 10.1016/0304-3940(92)90060-k. [DOI] [PubMed] [Google Scholar]
- Seabrook G. R., Howson W., Lacey M. G. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABAB receptors on neurones in rat brain slices. Br J Pharmacol. 1990 Dec;101(4):949–957. doi: 10.1111/j.1476-5381.1990.tb14186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I., Haby M., Leresche N., Crunelli V. The GABAB antagonist phaclofen inhibits the late K+-dependent IPSP in cat and rat thalamic and hippocampal neurones. Brain Res. 1988 May 17;448(2):351–354. doi: 10.1016/0006-8993(88)91275-9. [DOI] [PubMed] [Google Scholar]
- Solís J. M., Nicoll R. A. Pharmacological characterization of GABAB-mediated responses in the CA1 region of the rat hippocampal slice. J Neurosci. 1992 Sep;12(9):3466–3472. doi: 10.1523/JNEUROSCI.12-09-03466.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann R. H., Peck E. J., Ayala G. F. Biphasic response of hippocampal pyramidal neurons to GABA. Neurosci Lett. 1981 Feb 6;21(3):319–324. doi: 10.1016/0304-3940(81)90224-x. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Activity-dependent disinhibition. I. Repetitive stimulation reduces IPSP driving force and conductance in the hippocampus in vitro. J Neurophysiol. 1989 Mar;61(3):501–511. doi: 10.1152/jn.1989.61.3.501. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Activity-dependent disinhibition. II. Effects of extracellular potassium, furosemide, and membrane potential on ECl- in hippocampal CA3 neurons. J Neurophysiol. 1989 Mar;61(3):512–523. doi: 10.1152/jn.1989.61.3.512. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Activity-dependent disinhibition. III. Desensitization and GABAB receptor-mediated presynaptic inhibition in the hippocampus in vitro. J Neurophysiol. 1989 Mar;61(3):524–533. doi: 10.1152/jn.1989.61.3.524. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Effects of the GABA uptake inhibitor tiagabine on inhibitory synaptic potentials in rat hippocampal slice cultures. J Neurophysiol. 1992 Jun;67(6):1698–1701. doi: 10.1152/jn.1992.67.6.1698. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983 Feb 17;301(5901):603–604. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Watkins D. J. Cellular factors influencing GABA response in hippocampal pyramidal cells. J Neurophysiol. 1982 Oct;48(4):938–951. doi: 10.1152/jn.1982.48.4.938. [DOI] [PubMed] [Google Scholar]
- Xie X., Smart T. G. Properties of GABA-mediated synaptic potentials induced by zinc in adult rat hippocampal pyramidal neurones. J Physiol. 1993 Jan;460:503–523. doi: 10.1113/jphysiol.1993.sp019484. [DOI] [PMC free article] [PubMed] [Google Scholar]


