Abstract
To investigate the influencing factors of lymph node detection after minimally invasive radical surgery in patients with colorectal cancer (CRC) and the application value of carbon nanoparticle tracing. A total of 120 patients with CRC who underwent minimally invasive radical surgery were included. They were divided into groups according to whether they were grouped by carbon nano-tracers, and the baseline data were matched by propensity score method. Univariate and multivariate methods were used to evaluate lymph nodes and positive lymph nodes after minimally invasive radical surgery. The number of detected independent factors. After propensity score matching, there were 37 patients in the tracer group and the non-tracer group; there was no significant difference in baseline data between the two groups; the number of positive lymph nodes detected in the tracer group was significantly higher than that in the non-tracer group. The results of univariate analysis showed that gender, lesion location, maximum tumor diameter, and whether to perform nanocarbon tracing were all related to the number of lymph nodes detected after minimally invasive radical surgery. Nanocarbon tracing was an independent influencing factor for the number of lymph nodes detected after minimally invasive radical surgery. Pathological type, T stage, histopathological grade, nerve invasion, and whether or not with cancer nodules were all related to the number of positive lymph nodes detected after minimally invasive radical surgery; multivariate analysis showed that T stage, pathological Histological grade, vascular invasion, and cancer nodules were all independent influencing factors for the number of positive lymph nodes detected after minimally invasive radical surgery. The number of lymph nodes detected in patients with CRC after minimally invasive radical surgery is closely related to the location of the lesion and whether carbon nanoparticle tracing is performed; those with T2-4 stage, histological grade 3–4, combined with vascular invasion and cancer nodules are more likely to Positive lymph nodes were detected.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-024-01582-0.
Keywords: Colorectal cancer, Detection, Metastasis, Lymph node, Nano-carbon tracer
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors of the digestive system, ranking third in morbidity and fifth in mortality among all cancers. In recent years, with the increasing emphasis on early cancer detection and screening, coupled with the widespread use of endoscopy, the early detection rate of CRC has significantly improved [1]. Currently, accurate localization of the lesion is believed to be closely related to cases involving endoscopic non-curative resection, small tumor volume, multiple primary tumors, and postoperative recurrence after radical resection of CRC [2].
While several methods for localizing CRC exist, the error rate for barium enema, CT, and colonoscopy can reach 10% to 35%. Although dye injection under colonoscopy improves localization accuracy, traditional dyes like methylene blue have limitations. Due to its small particle size, methylene blue diffuses quickly, contaminating the surgical field, and its retention time in tissue is short [3, 4]. Indocyanine green (ICG)-based methods for tumor localization have also been reported. While ICG staining primarily aids in intestinal localization, its use in lymph node tracing remains secondary. However, its adoption in clinical practice is limited by factors such as high cost, long preparation time, and short fluorescence penetration depth [5].
Indian ink has been considered a safe dye for lesion marking. However, the presence of potentially hazardous components in its formulation, such as ethylene glycol, phenol, shellac, and gelatin, has been linked to significant complications, including intra-abdominal abscesses, peritonitis, fat necrosis, intestinal infarction, and intestinal perforation [6]. Nanocarbon, based on the same principle, is a newer staining agent composed of carbon-based materials. It has gained wide use in malignant tumor surgeries due to its high biocompatibility, precise localization, long-lasting staining, and ability to trace lymph nodes [7]. Studies have shown that nanocarbon can increase the number of lymph nodes detected in CRC patients after surgery, though its ability to increase the detection of positive lymph nodes remains inconclusive [8].
This study included 120 patients with CRC who underwent minimally invasive radical surgery between October 2019 and May 2021 at the Department of Gastrointestinal Surgery, Longyan First Affiliated Hospital of Fujian Medical University. The aim was to explore the factors influencing lymph node detection after minimally invasive surgery in CRC patients and assess the clinical value of nanocarbon tracers.
Materials and methods
General information
A total of 120 patients with CRC who underwent minimally invasive radical surgery were included in the study. Inclusion criteria: 1- Histopathologically confirmed CRC; 2- Histopathological type belongs to adenocarcinoma; 3- Successfully completed laparoscopic radical resection + D3 lymph node dissection; 4- Elective surgery; 5- The same group of surgeons completed the operation. Exclusion criteria: 1- previous abdominal surgery; 2- emergency surgery; 3- CRC recurrence; 4- received neoadjuvant therapy before surgery; The study design conformed to the requirements of the Declaration of Helsinki. This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Longyan First Hospital of Fujian Province,informed consent was obtained from all subjects and/or their legal guardian(s).
Nanocarbon tracer
The nanocarbon tracer was received from Chongqing Meilai Pharmaceutical Co., Ltd. (Chongqing, China). Indications for the application of nanocarbon tracers were as follows: Imaging is difficult to locate accurately; Radical surgery is performed after non-curative endoscopic biopsy surgery; Mechanical bowel preparation was performed 1–2 days before surgery, and the electronic colonoscope was placed under the microscope.The operation process followed to the protocol prvided by manufacturer strictly. To mark the location of the lesion, we pierced the submucosa at a 45° angle, injected 0.5 mL of 0.9% sodium chloride solution to stimulate the mucosal bulge, and then injected 0.1 mL of nanocarbon stock solution (Chongqing Laimei Pharmaceutical). If the endoscope could pass through the lesion area smoothly, we injected at 1 cm on the side of the lesion and 1 cm on the side of the anus (total 4 points); Each quadrant was injected separately (4 points in total).
Laparoscopic radical surgery
Under general anesthesia and tracheal intubation, the surgical plan was determined according to the location of the lesion, and total mesocolectomy or total mesorectal excision was used to complete laparoscopic radical resection + D3 lymph node dissection;, Complete excision of the intestinal segment, mesentery and mesangial root lymphatic and adipose tissue where the lesion was located, and dissection of the paraintestinal, middle and central lymph nodes at the same time (Fig. 1).
Fig. 1.
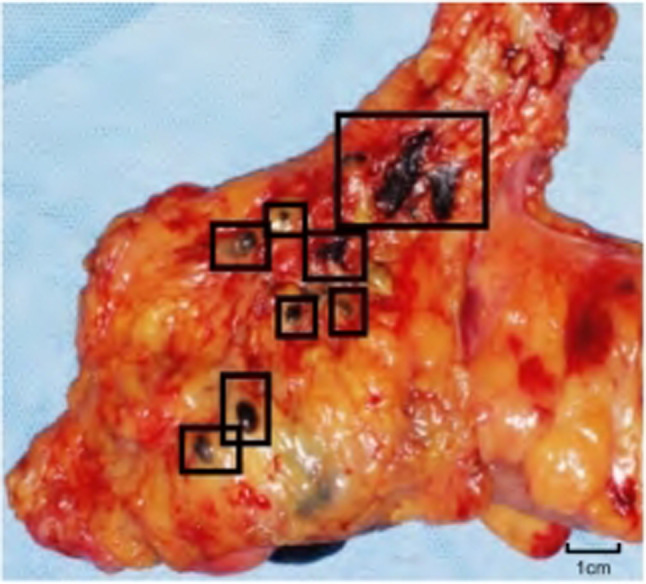
The lymph nodes of the observation group were stained with nano-carbon
Observation indicators
Review the clinicopathological data of the case records, including gender, age, height, age, lesion location, histopathological type, maximum diameter of the lesion, T stage, histological grade, vascular and nerve invasion, cancer nodule and lymph node detection, etc.
Statistical processing
SPSS 24.0 software was used to analyze the data; gender, age, height, lesion location, histopathological type, maximum diameter of the lesion, T stage, histological grade, vascular and nerve invasion, cancer nodules and lymph node detection were included. A fixed-effect variable was used to calculate the propensity score; the propensity score was matched by the nearest neighbor matching method of 1:1, and the matching tolerance was 0.02; the Kolmogorov–Smirnov test was used to complete the normality evaluation, and the paired t test was used to compare the measurement data that conformed to the normal distribution., expressed as (x ± s); Mann–Whitney U test was used for comparison of measurement data that did not conform to the normal distribution, expressed as M (P25, P75); χ2 test was used for comparison of count data, expressed as rate; Poisson regression was used for multivariate analysis Multivariate model; P < 0.05 was considered statistically significant.
Results
Analysis of baseline data after matching
Among the 120 patients, 43 underwent preoperative colonoscopy with nanocarbon tracing, while in control group,no tracer was used. During the operation, black staining of the tumor site was clearly seen (Fig. 1), and no complications occurred. After propensity score matching, there were 37 patients in the tracer group and the non-tracer group (Figure S1), there was no significant difference in baseline data between the two groups (P > 0.05) (Table 1).
Table 1.
Analysis of baseline data after matching
| Index | Non tracer group (n = 37) | Tracer group (n = 37) | χ2 | P Value |
|---|---|---|---|---|
| Gender | 0.48 | 0.42 | ||
| Male | 22 | 25 | ||
| Female | 15 | 12 | ||
| Age (years old) | 0.07 | 0.86 | ||
| < 60 | 17 | 18 | ||
| ≥ 60 | 20 | 19 | ||
| BMI(kg/m2) | 0.08 | 0.84 | ||
| < 24 | 16 | 17 | ||
| ≥ 24 | 21 | 20 | ||
| Lesion location | 2.44 | 0.20 | ||
| Right hemicolon | 7 | 7 | ||
| Left hemicolon | 10 | 16 | ||
| Rectum | 20 | 14 | ||
| Pathological type | 1.35 | 0.33 | ||
| Uplift type | 32 | 27 | ||
| Ulcerative type | 5 | 10 | ||
| Maximum diameter lesion (cm) | 0.25 | 0.69 | ||
| < 4 | 30 | 28 | ||
| ≥ 4 | 7 | 9 | ||
| T stage | 1.19 | 0.39 | ||
| Tis | 6 | 4 | ||
| T1 | 12 | 14 | ||
| T2 | 10 | 9 | ||
| T3 | 7 | 9 | ||
| T4 | 2 | 1 | ||
| Histological grading | 0.23 | 0.71 | ||
| Grade 1 ~ 2 | 30 | 32 | ||
| Grade 3 ~ 4 | 7 | 5 | ||
| Vascular invasion | 0.05 | 0.90 | ||
| No | 33 | 32 | ||
| Yes | 4 | 5 | ||
| Nerve invasion | 0.22 | 0.73 | ||
| No | 36 | 34 | ||
| Yes | 1 | 3 | ||
| Carcinoma nodule | 0.05 | 0.90 | ||
| No | 35 | 34 | ||
| Yes | 2 | 3 |
Lymph node detection and metastasis
The schematic illustrating the observed results is displayed in Fig. 2A. The number of detected lymph nodes and the number of metastasis-positive lymph nodes in the observation group were significantly higher than those in the control group(P < 0.05, Fig. 2B)., and the number of lymph nodes with a diameter of less than 5 mm and the number of metastasis-positive lymph nodes were significantly higher than those in the control group (P < 0.05, Fig. 2C). The black staining rate of lymph nodes with a diameter of less than 3 mm in the observation group was significantly higher than that of lymph nodes with a diameter of more than 3 mm (P < 0.05, Fig. 2D).
Fig. 2.
A schematic of results observed. B chi-square analysis of lymph node visualization based on diameter of 3 mm. C Comparison of number of lymph nodes less than 5 mm in both groups. D Comparison of number of metastasis-positive lymph nodes less than 5 mm in both groups
Lymph node detection after minimally invasive radical surgery
Among the 37 patients in the tracer group, 1 patient was excluded due to staining problems, and 726 lymph nodes were detected in the remaining 36 patients, and the median number of lymph nodes detected was 19 (14, 25); 38 positive lymph nodes were detected, and the median number of lymph nodes detected The number of outputs was 0 (0, 2). In the non-tracer group, 614 lymph nodes were detected, and the median number of lymph nodes was 17 (14, 20); 23 positive lymph nodes were detected, and the median number of positive lymph nodes was 0 (0, 0). The number of positive lymph nodes detected in the tracer group was significantly higher than that in the non-tracer group (P < 0.05); there was no significant difference in the number of positive lymph nodes detected between the two groups (P > 0.05).
Analysis of influencing factors on the number of lymph nodes detected after minimally invasive radical surgery
The results of univariate analysis showed that gender, age, lesion location, maximum tumor diameter, and whether nanocarbon tracing was performed were associated with the number of lymph nodes detected after minimally invasive radical surgery (P < 0.05). The results of multivariate analysis showed that the location of the lesion and whether to perform nanocarbon tracing were independent influencing factors of the number of lymph nodes detected after minimally invasive radical surgery (P < 0.05) (Table 2, 3).
Table 2.
Univariate analysis of influencing factors on the number of lymph nodes detected after minimally invasive radical surgery
| Index | N | Number of lymph nodes [n,M (P25,P75)] |
Z | P Value |
|---|---|---|---|---|
| Gender | 3.72 | 0.02* | ||
| Male | 47 | 17 (15,21) | ||
| Female | 26 | 19 (16,25) | ||
| Age (years old) | 3.22 | 0.03* | ||
| < 60 | 34 | 19 (16,23) | ||
| ≥ 60 | 39 | 17 (15,22) | ||
| BMI(kg/m2) | 0.28 | 0.46 | ||
| < 24 | 34 | 19 (16,23) | ||
| ≥ 24 | 39 | 17 (15,21) | ||
| Lesion location | 4.93 | 0.01* | ||
| Right hemicolon | 13 | 25 (17,32) | ||
| Left hemicolon | 26 | 18 (14,21) | ||
| Rectum | 34 | 17 (15,21) | ||
| Pathological type | 1.09 | 0.45 | ||
| Uplift type | 59 | 18 (15,22) | ||
| Ulcerative type | 14 | 18 (16,25) | ||
| Maximum diameter lesion (cm) | 4.02 | 0.03* | ||
| < 4 | 60 | 18 (15,22) | ||
| ≥ 4 | 13 | 20 (16,25) | ||
| T stage | 0.85 | 0.58 | ||
| Tis | 9 | 16 (12,20) | ||
| T1 | 26 | 18 (15,22) | ||
| T2 | 20 | 17 (15,21) | ||
| T3 | 16 | 19 (16,24) | ||
| T4 | 2 | 20 (13,27) | ||
| Histological grading | 1.15 | 0.26 | ||
| Grade 1 ~ 2 | 62 | 18 (16,22) | ||
| Grade 3 ~ 4 | 11 | 19 (14,22) | ||
| Vascular invasion | 0.57 | 0.56 | ||
| No | 64 | 18 (15,22) | ||
| Yes | 9 | 20 (16,27) | ||
| Nerve invasion | 0.93 | 0.33 | ||
| No | 70 | 18 (15,22) | ||
| Yes | 3 | 25 (11,30) | ||
| Carcinoma nodule | 1.36 | 0.61 | ||
| No | 69 | 18 (15,22) | ||
| Yes | 4 | 16 (13,28) | ||
| Nano carbon tracer | 2.9 | 0.03* | ||
| No | 37 | 17 (14,20) | ||
| Yes | 36 | 19 (14,25) |
Table 3.
Multivariate analysis of influencing factors on the number of lymph nodes detected after minimally invasive radical surgery
| Index | B | SE | χ2 | OR | 95%CI | P |
|---|---|---|---|---|---|---|
| Left hemicolon | − 0.45 | 0.07 | 68.54 | 0.77 | 0.50 ~ 0.96 | < 0.001 |
| Rectum | − 0.30 | 0.05 | 35.96 | 0.85 | 0.62 ~ 0.99 | < 0.001 |
| Nano carbon tracer | 0.28 | 0.03 | 32.10 | 1.29 | 1.03 ~ 1.85 | < 0.001 |
Analysis of influencing factors on the number of positive lymph nodes detected after minimally invasive radical surgery
The results of univariate analysis showed that the pathological type, T stage, histopathological grade, vascular invasion, nerve invasion and whether it was associated with cancer nodules [no vs yes: 0 (0, 0) vs 4 (2, 9)] All were related to the number of positive lymph nodes detected after minimally invasive radical surgery (P < 0.05); multivariate analysis showed that T stage, histopathological grade, vascular invasion and combined cancer nodules were all positive after minimally invasive radical surgery The number of lymph nodes detected was an independent factor (P < 0.05) (Table 4–5).
Table 4.
Univariate analysis of influencing factors on the number of positive lymph nodes after minimally invasive radical surgery
| Index | N | Number of lymph nodes [n,M (P25,P75)] |
Z | P Value |
|---|---|---|---|---|
| Gender | 6.35 | 0.68 | ||
| Male | 47 | 0 (0,0) | ||
| Female | 26 | 0 (0,1) | ||
| Age (years old) | 5.93 | 0.54 | ||
| < 60 | 34 | 0 (0,1) | ||
| ≥ 60 | 39 | 0 (0,0) | ||
| BMI(kg/m2) | 3.76 | 0.81 | ||
| < 24 | 34 | 0 (0,1) | ||
| ≥ 24 | 39 | 0 (0,0) | ||
| Lesion location | 2.90 | 0.55 | ||
| Right hemicolon | 13 | 0 (0,0) | ||
| Left hemicolon | 26 | 0 (0,0) | ||
| Rectum | 34 | 0 (0,1) | ||
| Pathological type | 0.87 | 0.02 | ||
| Uplift type | 59 | 0 (0,0) | ||
| Ulcerative type | 14 | 1 (0,4) | ||
| Maximum diameter lesion (cm) | 3.39 | 0.52 | ||
| < 4 | 60 | 0 (0,0) | ||
| ≥ 4 | 13 | 0 (0,1) | ||
| T stage | 1.01 | 0.01* | ||
| Tis | 9 | 0 (0,0) | ||
| T1 | 26 | 0 (0,0) | ||
| T2 | 20 | 0 (0,1) | ||
| T3 | 16 | 0 (0,2) | ||
| T4 | 2 | 0 (0,4) | ||
| Histological grading | 2.93 | 0.03* | ||
| Grade 1 ~ 2 | 62 | 0 (0,0) | ||
| Grade 3 ~ 4 | 11 | 0 (0,4) | ||
| Vascular invasion | 2.64 | 0.03* | ||
| No | 64 | 0 (0,0) | ||
| Yes | 9 | 2 (0,4) | ||
| Nerve invasion | 5.77 | 0.02* | ||
| No | 70 | 0 (0,0) | ||
| Yes | 3 | 2 (1,6) | ||
| Carcinoma nodule | 4.47 | 0.01* | ||
| No | 69 | 0 (0,0) | ||
| Yes | 4 | 4 (2,9) | ||
| Nano carbon tracer | 0.74 | 0.61 | ||
| No | 37 | 0 (0,0) | ||
| Yes | 36 | 0 (0,2) |
Table 5.
Multivariate analysis of influencing factors on the number of positive lymph nodes after minimally invasive radical surgery
| Index | B | SE | χ2 | OR | 95%CI | P |
|---|---|---|---|---|---|---|
| Stage T2 | 21.77 | 0.49 | 110.55 | 5.74 | 1.56 ~ 11.90 | < 0.001 |
| Stage T3 | 24.29 | 0.41 | 137.40 | 6.27 | 1.19 ~ 18.99 | < 0.001 |
| Stage T4 | 23.82 | 0.63 | 125.68 | 3.96 | 1.07 ~ 7.94 | < 0.001 |
| Histological grade 3 ~ 4 | 1.69 | 0.36 | 50.47 | 4.82 | 1.42 ~ 9.98 | < 0.001 |
| Vascular invasion | 0.83 | 0.39 | 24.31 | 3.14 | 1.60 ~ 12.98 | < 0.001 |
| Nano carbon tracer | 2.04 | 0.30 | 62.14 | 7.59 | 1.33 ~ 16.81 | < 0.001 |
Discussion
Accurate localization of lesions is the key to laparoscopic radical resection of CRC patients [9]; studies [10] have shown that additional surgical radical surgery after non-curative endoscopic resection of CRC patients can effectively prolong the overall survival time of patients. Another report confirmed [11] that the preoperative nanocarbon localization has a high accuracy rate for patients with early CRC in additional surgery, which is of great significance for improving the accuracy and safety of resection. Through the positioning of carbon nano-staining under colonoscopy 1–2 days before operation, the patient does not need to undergo mechanical bowel preparation again, which is helpful for the elimination of gas in the intestinal lumen before operation. Avoid the problem of increased difficulty in exploration due to the staining of mesangial lesions [12, 13].
The results of multivariate analysis in this study showed that the location of the lesion and whether to perform nanocarbon tracing were independent influencing factors of the number of lymph nodes detected after minimally invasive radical surgery (P < 0.05). Cancer nodules were all independent influencing factors of the number of positive lymph nodes detected after minimally invasive radical surgery (P < 0.05), confirming that nanocarbon tracing can increase the total number of detected lymph nodes in CRC patients after laparoscopic radical surgery, but did not affect the number of positive lymph nodes detected. out of the situation. Nanocarbons have been confirmed to have high lymphatic system tropism, with relatively uniform particle diameters, with an average size of around 150 nm [14]. Because the hydrostatic pressure of the interstitial fluid is higher than the pressure in the lymphatic capillaries, nanocarbons can quickly enter the lymphatic channels after local injection into the human body; at the same time, they can also be phagocytosed by macrophages and migrate into the lymphatic system, staining the lymph node tissue in the tumor area. [15, 16]. It has been reported that the lymph node staining rate of CRC patients after nanocarbon staining can reach 57%, which can significantly improve the detection rate of lymph nodes with a diameter of less than 5 mm compared with the past, and this result has been further confirmed by follow-up studies [17]. Pathologists mainly search for lymph nodes through a combination of direct vision and touch, and nanocarbon staining can improve the efficiency of pathologists in finding lymph nodes, increase the number of lymph nodes detected, especially those with smaller diameters, thereby maximizing the accuracy of pathological N staging. However, some scholars believe that although carbon nano-tracers can increase the operation of patients with digestive tract tumors. The total number of positive lymph nodes detected in the later period did not significantly increase the number of positive lymph nodes detected [18], which is also supported by this study.
In recent years, nanocarbons have also been used for sentinel lymph node tracing in patients with CRC; a study of T1-2 stage CRC patients showed that after preoperative injection of nanocarbons under digestive endoscopy, the median number of sentinel lymph nodes detected is 3, and the sensitivity and specificity for lymph node metastasis prediction can reach 91.2% and 100%, respectively [19]. However, the clinical value of sentinel lymph node tracing in CRC patients remains controversial, and whether selective lymph node dissection should be used in CRC patients remains unclear. Some scholars believe that the main purpose of sentinel lymph node detection in CRC patients is to detect lymph nodes with a higher risk of metastasis, rather than to control the extent of surgical resection [20].
This study also has certain limitations: (1) Although the baseline data was balanced by propensity score matching, the number of cases after matching was small, and it was a single-center retrospective report, so the conclusions could not completely rule out the influence of confounding factors; (2) In surgery, the right hemicolon is tend to have a longer resected bowel length than the left hemicolon, which may result in the detection of more lymph nodes. A multicenter prospective study conducted in Japan indicates that the optimal margin may be 10 cm, as metastasis to paracolic lymph nodes more than 10 cm distal to the tumor is rare[21], which suggests the number of lymph nodes retrieved may be influenced not by bowel length but other factors such as tracers like Indian ink[22] or indocyanine green (ICG) fluorescence[23]. In other words, positive lymph nodes matters more compared with the length of intestinal tracts. Follow-up studies are still needed to further analyze the impact of nanocarbon tracers on the survival of patients.
Conclusion
In conclusion, the number of lymph nodes detected after minimally invasive radical surgery in CRC patients is related to the location of the lesions and whether nanocarbons are used. In addition, T2-4, histological grade 3–4, combined with vascular invasion and cancer nodules are more likely to detect positive lymph nodes. The application of nanocarbon tracer technology can help detect more lymph nodes, but its value in improving the detection of positive lymph nodes remains to be proven.
Supplementary Information
Author contributions
BX,GL,ZW contributed to the study design, statistical analysis; DX, JC, SL drafted the manuscript. All authors contributed to the interpretation of the results and critically revised the manuscript. All authors approve the final version of the manuscript and take responsibility for the integrity of the work.
Funding
This study didn’t receive any funding.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
This study was performed in compliance with all relevant ethical regulations, and all participants provided informed consent. The protocols for the collection of human samples in the study were approved by the Ethical Committee of Longyan First Affiliated Hospital of Fujian Medical University. All participants provided written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ge W, Li Q, Liu WJ, et al. Carbon nanoparticle suspension could help get a more accurate nodal staging for patient with rectal cancer. Sci Rep. 2021;11(1):9933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li K, Chen D, Chen W, et al. A case-control study of using carbon nanoparticles to trace decision-making lymph nodes around inferior mesenteric artery in rectal cancer. Surg Endosc. 2019;33(3):904–10. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Chen M, Lv L, et al. Injection of carbon nanoparticles for lymph node detection after laparoscopic colorectal cancer surgery. J Nanosci Nanotechnol. 2021;21(2):886–94. [DOI] [PubMed] [Google Scholar]
- 4.Martín MJ, Gentili C, Lassalle V. In vitro biological tests as the first tools to validate magnetic nanotheranostics for colorectal cancer models. Chem Med Chem. 2020;15(12):1003–17. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Fu Y, Wang J, Yang H, Zhang X. Advantages of contrast-enhanced ultrasound in the localization and diagnostics of sentinel lymph nodes in breast cancer. J Zhejiang Univ Sci B. 2023;24(11):985–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Wang XD, Du SY. Production of gold/silver doped carbon nanocomposites for effective photothermal therapy of colon cancer. Sci Rep. 2020;10(1):7618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ünal S, Akta ç Y, Benito JM, et al. Cyclodextrin nanoparticle bound oral camptothecin for colorectal cancer: formulation development and optimization. Int J Pharm. 2020;584(6):119468. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Deng X, Wang L, et al. Clinical application of carbon nanoparticles in lymphatic mapping during colorectal cancer surgeries: a systematic review and meta-analysis. Dig Liver Dis. 2020;7(7):1590–7. [DOI] [PubMed] [Google Scholar]
- 9.Bhattarai DP, Kim BS. NIR-triggered hyperthermal effect of polythiophene nanoparticles synthesized by surfactant-free oxidative polymerization method on colorectal carcinoma cells. Cells. 2020;9(9):E2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin KT, Yao JY, Ying XJ, et al. Nanomedicine and early cancer diagnosis: molecular imaging using fluorescence nanoparticles. L Curr Top Med Chem. 2020;22(9):1124–9. [DOI] [PubMed] [Google Scholar]
- 11.Rajpoot K, Jain SK. (99m)Tc-labelled and pHawakened microbeads entrapping surface-modified lipid nanoparticles for the augmented effect of oxaliplatin in the therapy of colorectal cancer. J Microencapsul. 2020;7(10):1–15. [DOI] [PubMed] [Google Scholar]
- 12.Maffioli A, Danelli P. Carbon nanoparticles application during colorectal cancer surgery: updates from China. Dig Liver Dis. 2020;52(12):1443–4. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Deng X, Wang L, et al. Clinical application of carbon nanoparticles in lymphatic mapping during colorectal cancer surgeries: a systematic review and meta-analysis. Dig Liver Dis. 2020;52(12):1445–54. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Xu H, Zhang X, et al. Effect of carbon nanoparticle tracer combined with laparoscopy in the treatment of colon cancer. J Nanosci Nanotechnol. 2020;20(10):6007–12. [DOI] [PubMed] [Google Scholar]
- 15.Liu P, Tan J, Tan Q, et al. Application of carbon nanoparticles in tracing lymph nodes and locating tumors in colorectal cancer: a concise review. Int J Nanomed. 2020;15(12):9671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh R, Dumlupinar G, Andersson-Engels S, et al. Emerging applications of upconverting nanoparticles in intestinal infection and colorectal cancer. Int J Nanomedicine. 2019;14(2):1027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin N, Qiu J, Wu W, et al. Preoperative carbon nanoparticles and titanium clip combined labeling method for transverse colon tumor surgery. Asian J Surg. 2019;42(8):844–5. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, Mo S, Liu Q, et al. The safety and effectiveness of carbon nanoparticles suspension in tracking lymph node metastases of colorectal cancer: a prospective randomized controlled trial. Jpn J Clin Oncol. 2020;50(5):535–42. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Wu Y, She J, et al. 3D nitrogen-doped carbon nanofoam arrays embedded with PdCu alloy nanoparticles: assembling on flexible microelectrode for electrochemical detection in cancer cells. Anal Chim Acta. 2021;8(5):1158–65. [DOI] [PubMed] [Google Scholar]
- 20.Kim EJ, Chung JW, Kim SY, et al. Autologous blood, a novel agent for preoperative colonic localization: a safety and efficacy comparison study. Surg Endosc. 2019;33:1080–6. [DOI] [PubMed] [Google Scholar]
- 21.Ueno H, Hase K, Shiomi A, et al. Optimal bowel resection margin in colon cancer surgery: prospective multicentre cohort study with lymph node and feeding artery mapping. Lancet Reg Health West Pac. 2023;33: 100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iguchi K, Watanabe J, Suwa Y, et al. The effect of preoperative endoscopic tattooing using India ink on lymph node yield in laparoscopic colectomy for stage I right-sided colon cancer. Int J Colorectal Dis. 2023;38(1):77. [DOI] [PubMed] [Google Scholar]
- 23.Lakatos L, Illyes I, Budai A, et al. Feasibility of indocyanine green (ICG) fluorescence in ex vivo pathological dissection of colorectal lymph nodes-a pilot study. Pathol Oncol Res. 2024;30:1611853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.



