Abstract
Tumor energy metabolism plays a crucial role in the occurrence, progression, and drug resistance of tumors. The study of tumor energy metabolism has gradually become an emerging field of tumor treatment. Recent studies have shown that epigenetic regulation is closely linked to tumor energy metabolism, influencing the metabolic remodeling and biological traits of tumor cells. This review focuses on the primary pathways of tumor energy metabolism and explores therapeutic strategies to target these pathways. It covers key areas such as glycolysis, the Warburg effect, mitochondrial function, oxidative phosphorylation, and the metabolic adaptability of tumors. Additionally, this article examines the role of the epigenetic regulator SWI/SNF complex in tumor metabolism, specifically its interactions with glucose, lipids, and amino acids. Summarizing therapeutic strategies aimed at these metabolic pathways, including inhibitors of glycolysis, mitochondrial-targeted drugs, exploitation of metabolic vulnerabilities, and recent developments related to SWI/SNF complexes as potential targets. The clinical significance, challenges, and future directions of tumor metabolism research are discussed, including strategies to overcome drug resistance, the potential of combination therapy, and the application of new technologies.
Keywords: Tumor energy metabolism, Glycolysis, Mitochondrial phosphorylation, Therapeutic targets, Drug resistance, SWI/SNF complex
Introduction
Tumor energy metabolism is the process by which tumor cells convert and utilize energy for growth and survival, involving the reprogramming of metabolic pathways. Tumor cells metabolize energy differently from normal cells by reprogramming various pathways, such as aerobic respiration, anaerobic fermentation, and fatty acid oxidation. The metabolic characteristics of tumors are closely related to their biological behavior. Tumor cells frequently show uncontrolled growth and proliferation, necessitating metabolic reprogramming to satisfy their increased energy and biosynthetic demands. This reprogramming also helps reduce oxidative stress, enabling tumor cells to adapt to varying energy requirements in different physiological and pathological conditions. For instance, normal cells generate energy via oxidative phosphorylation when oxygen is available, whereas tumor cells rely on glycolytic pathways for energy. Glycolysis produces energy at a faster rate and also produces a variety of intermediate metabolites that can be used to synthesize nucleotides and amino acids to support the rapid proliferation of cells [1]. Lactic acid generated by glycolysis can inhibit the effector functions of immune cells in the tumor microenvironment (TME), thereby facilitating tumor evasion and progression [2]. These metabolic changes support tumor cell survival in harsh conditions, facilitate tumor development, and offer potential targets for early diagnosis and targeted therapy.
Otto Warburg’s observations were the starting point for the study of cancer metabolism. He discovered the famous “Warburg effect” in the early twentieth century, in which tumor cells metabolize glucose to lactate even under aerobic conditions, and he believes that impaired respiration of cancer cells is a prerequisite for cells to become cancerous [3–5]. Over the next few decades, researchers tried to explain how the Waberg effect promotes tumor growth. Two key findings indicate that glycolysis and TCA cycle metabolites promote tumor growth. They do this by facilitating biosynthesis and acting as signaling molecules. One of the key findings is that ATP produced by glycolysis can support primary tumor growth after the electron transport chain (ETC) in cancer cells is disrupted [6]. The TCA circulating metabolite oxaloacetate has been shown to be required for primary and metastatic tumor growth [7, 8]. Another finding is that reactive oxygen species (ROS) produced by oxidative metabolism can support tumorigenesis [9]. And ROS metabolites can act as signaling molecules to promote tumor growth by controlling gene expression [10]. As research progressed, scientists began to explore how tumor cells alter metabolic pathways. It includes multiple metabolic pathways such as glycolysis, oxidative phosphorylation, and fatty acid metabolism. Signaling pathways such as MYC, AKT, mTOR and HIFs discovered in the 90 s of the twentieth century increase glycolysis through transcriptional regulation and phosphorylation.
Recently, the study of tumor energy metabolism has become one of the hot spots in tumor biology and treatment research [11–14]. A variety of metabolism-based antitumor drugs have shown good results in experiments. For example, small molecule inhibitors developed for the glycolytic pathway, such as the glucose transporter 1 (GLUT1) inhibitor Bay-876 and the hexokinase (HK2) inhibitor Benserazide (Benz) [15–17], as well as Ab3-810, a monoclonal antibody targeting glutamine transporter 5 (SLC1A5), and metformin, a drug targeting mitochondrial complex I, can activate AMPK, thereby inhibiting the growth, survival, and metastasis of various tumor cells such as breast cancer, liver cancer, and pancreatic cancer [18–20].
Despite these advances, translating these findings into effective clinical interventions remains challenging, particularly due to issues such as drug resistance stemming from the metabolic adaptations of tumors and variability in their responses to treatment. Previous studies have focused more on the metabolic pathways of cells and their contribution to energy production, and the energy metabolism of tumor cells is usually regarded as a relatively static process, and it is difficult to describe the dynamic change process of metabolism and the interaction between metabolites and tumors. Recent studies have focused on the dynamic properties of tumor energy metabolism, especially the bidirectional relationship between epigenetics and tumor metabolism [21]. Epigenetic modifications affect the metabolic state and biological behavior of tumors by regulating the expression of metabolism-related genes without altering the DNA sequence [22, 23]. Tumor metabolites regulate gene expression through epigenetic modifications. For example, metabolites such as lactate are not only a result of energy metabolism, but can also affect the growth and metastatic ability of tumor cells by regulating epigenetic states [24].
Therefore, understanding how tumor energy metabolism relates to epigenetic regulation can help develop new treatment strategies. This review starts with an introduction to key processes in tumor metabolism, including glycolysis and mitochondrial function, as well as tumor adaptation and plasticity. Specially, we focus on the epigenetic factor-SWI/SNF chromatin remodeling complex, which showed closely relationship to tumor metabolism but lack in-depth enough knowledge, in order to provide new ideas for the development of tumor metabolism therapy and targeted drugs. Finally, strategies targeting metabolic pathways was discussed, not only the latest clinical trails but also future research directions. This will provide innovative approaches to cancer treatment based on tumor energy metabolism.
The main pathways of energy metabolism in tumors
Tumor cells require significant energy and materials for rapid growth, and their metabolism is regulated through aerobic glycolysis and mitochondrial oxidative phosphorylation. This adaptation helps cells thrive in the low-oxygen tumor microenvironment. It also provides the energy and materials needed for biological activities like tumor growth, invasion, and migration. Tumor metabolism is regulated at both the gene and protein levels. Additionally, genomic instability can alter metabolic pathways, impacting how cells produce and utilize energy. For instance, the elevated expression of the proto-oncogene MYC enhances the levels of key enzymes in both glycolysis and fatty acid synthesis, leading to increased energy resource production [25]. The activity of proteins, such as hexokinase (HK) and phosphofructokinase (PFK), which are key enzymes in the glycolytic pathway, directly affects the rate of glucose metabolism [26].
Glycolysis and the Waburg effect
Transition of tumor cells to aerobic glycolysis
Glycolysis is an important process in cellular metabolism, and tumor cells often exhibit high levels of glycolysis known as the "Warburg effect" or "aerobic glycolysis". This process occurs in the cytoplasm and consists of two phases: the first stage converts glucose into pyruvate, NADH, and 2 ATP molecules, while the second phase involves fermentation, in which pyruvate is reduced and metabolized into organic acids or alcohols, such as lactic acid.
The special needs of tumor cells for energy metabolism are closely related to their ability to grow and metastasize. Aerobic glycolysis produces ATP more quickly and maintains high levels of glycolytic intermediates to support the anabolic reactions necessary for synthesizing macromolecules like DNA, RNA, proteins, and lipids, thus facilitating rapid growth and proliferation [27]. Lactic acid produced by glycolysis leads to the acidification of the tumor microenvironment, which helps inhibit the immune response and promotes the proliferation and invasion of tumor cells [28, 29]. Glycolysis generates significantly fewer reactive oxygen species (ROS) than oxidative phosphorylation, which protects tumor cells from oxidative stress and contributes to their resistance to apoptosis [30]. Aerobic glycolysis does not require mitochondria to maintain redox balance, as NADH produced by glycolysis is oxidized back to NAD + when pyruvate is converted to lactic acid, allowing NAD + to continue supporting the glycolytic cycle [31].
The role of key enzymes and their regulatory mechanisms
Glycolysis involves 10 enzymatic steps. The three key enzymes that catalyze irreversible reactions—HK, phosphofructokinase-1 (PFK1), and pyruvate kinase (PK)—along with glucose transporter (GLUT), PFK, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), pyruvate dehydrogenase (PDH), and lactate dehydrogenase (LDH). Studies have found that overexpression of these enzymes can enhance the metabolic activity of tumor cells, which in turn promotes their proliferation and survival.
HK promotes the growth and proliferation of cells primarily by converting glucose to glucose-6-phosphate. Different subtypes of HK (e.g., HK1, HK2, HK3, and HK4) exhibit different expression patterns in different types of tumors. The overexpression of HK2 in a variety of tumors is closely related to the aggressiveness and metastasis of tumors [32].
PKM2 is responsible for the conversion of phosphoenolpyruvate to pyruvate and, in the process, ATP is generated. It is usually highly expressed in tumor cells and is present in both active tetrameric and inactive dimer states [33]. In the dimer state, PKM2 has low catalytic activity, which allows cells to accumulate glycolytic intermediates that can not only enter the glycolytic pathway, but also be converted into substrates for other metabolic pathways, thus providing cells with the necessary biosynthetic precursors and energy requirements to promote rapid proliferation and growth of tumor cells [34].
PFK1 is the primary regulatory point in response to cellular energy levels and allosteric effectors, responsible for the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate. PFK activity is regulated by a variety of metabolites, including ATP, ADP, and fructose-2,6-bisphosphate. In tumor cells, PFK expression and activity are often significantly increased, promoting enhanced glycolysis [35]. The overexpression of PFK is closely related to cell proliferation, migration and survival [36].
LDH is primarily responsible for the reduction of pyruvate to lactate. The expression level of LDH is closely related to the aggressiveness and prognosis of tumors. Especially in malignant tumors such as pancreatic cancer and lung cancer, the high expression of LDH-A is positively correlated with the degree of malignancy of the tumor. LDH-A affects cancer cell metabolism by controlling the NADH/NAD ratio, and elevated LDH-A levels lead to tumor metastasis by promoting EMT [37]. The production of lactate during glycolysis and the release of tumor microenvironment (TME) acidosis are major factors, which lead to high tumor invasion, migration, and immune cell suppression. Inhibition of LDH can effectively reduce the glucose uptake and proliferation of tumor cells, and at the same time improve the function of tumor infiltrating T cells, thereby enhancing anti-tumor immunity [38].
Many studies have shown that key enzymes are regulated by transcription, metabolite feedback, and cell signaling pathways. Expression of almost all glycolytic enzymes are upregulated by transcription factors c-MYC (MYC) and Hypoxia-inducible factor-1 (HIF-1). Including hexokinase II (HK II), phosphofructokinase (PFK), Enolase 1 (ENO1), pyruvate dehydrogenase (PDH), pyruvate kinase (PKM2) and lactate dehydrogenase (LDHA). c-MYC and HIF-1 also are the driver of the metabolic transition from oxidation to glycolysis. They cooperation in activating HK2 further enhances the retention of glucose for its glycolytic conversion to lactate, thereby contributing to the Warburg effect [39]. After c-MYC is reduced, the expression of HK2, PFKM, PKM2 and LDHA in colon cancer cells is reduced, glycolysis is inhibited, and intracellular ATP is reduced, resulting in endoplasmic reticulum stress and immunogenic cell death in colorectal cancer cells [40]. The activity of PFK-1 is regulated by a variety of metabolites. For example, PFKFB3 is a potent PFK-1 activator. Studies have shown that PFKFB3 is upregulated in a variety of cancers, and PFKFB3 overexpression can enhance glycolysis in ovarian cancer cells, increase lactate production, and reduce cellular oxygen consumption, supporting tumor growth, chemoresistance, metastasis and stemness in ovarian cancer [41]. Activation of cell signaling pathways, such as the PI3K-AKT-mTOR pathway, increases the amount of GLUT1 and GLUT12 on the cell membrane, increases glucose uptake by tumor cells, and produces the required ATP through aerobic glycolysis for proliferation, migration, and invasion. As a consequence of the aerobic glycolysis, extracellular acidification also provides GLUT12 with the electrochemical gradient of H + to cotransport and accumulate glucose inside the cell, further feeding the aerobic glycolysis [42].
Mitochondrial function and the role of oxidative phosphorylation in tumor metabolism
Mitochondria are the main energy production centers in cells, and tumor cells often adapt to their living environment by changing the function and metabolic pathways of mitochondria, thereby promoting the growth and metastasis of tumors. For example, tumor cells enhance their proliferation and survival by increasing their uptake of pyruvate and glutamate. This process promotes mitochondrial energy production [43]. ATP synthesis and metabolite production in mitochondria are crucial for the viability of tumor cells. If mitochondria malfunction, it can lead to insufficient energy supply, negatively impacting tumor cell proliferation and metastasis [44]. Understanding the function of mitochondria and their role in tumor metabolism is important for the development of new cancer treatment strategies.
Oxphosphorylation (OXPHOS) is a key function of mitochondria that primarily takes place in the inner mitochondrial membrane. This process transfers electrons from nutrients like glucose and fatty acids to oxygen via electron transport chains. This reaction forms water and releases energy, which is then used to synthesize ATP. Additionally, mitochondria regulate metabolic pathways including glycolysis, fatty acid oxidation (FAO), and amino acid metabolism [45]. Within the mitochondria, pyruvate and fatty acids are converted to acetyl-CoA, which then enters the tricarboxylic acid cycle (TCA cycle), where NADH and FADH2 are further generated. These reduced equivalents are eventually converted to ATP via the electron transport chain (ETC). The electron transport chain consists of several complexes, including complex I (NADH dehydrogenase), complex II (succinate dehydrogenase), complex III (cytochrome bc1 complex), and complex IV (cytochrome c oxidase). The individual proteins in the complex ensure efficient transport of electrons through their specific conformational changes and binding capacity. Research indicates that dysfunction in the electron transport chain is closely linked to various metabolic diseases and the aging process [46, 47].
Oxygen phosphorylation (OXPHOS) is a key function of mitochondria and occurs primarily in the inner mitochondrial membrane. This process transfers electrons from nutrients such as glucose and fatty acids to oxygen through the electron transport chain. This reaction forms water and releases energy, which is then used to synthesize ATP. In ovarian cancer cells, higher OXPHOS activity is linked to increased tumor cell growth [48]. Estrogen receptor-positive breast cancer cells exhibit increased OXPHOS, which is associated with tumor cell proliferation and viability [49]. OXPHOS influences immune cell function and tumor immune escape mechanisms by regulating the metabolic states within the tumor microenvironment [50]. Mitochondrial dysfunction in specific cancer subtypes is closely related to their clinical features. For instance, glioma stem cells demonstrate higher mitochondrial activity and expression of OXPHOS proteins compared to isogenetically differentiated glioma cells [51]. Cancer stem cells in cholangiocarcinoma rely on mitochondrial oxidative metabolism and peroxisome proliferator-activated receptor γ coactivator (PGC-1α) to maintain their characteristics [52]. Breast cancer cell subtypes exhibit different metabolic phenotypes, and these metabolic signatures are directly related to their clinical classification [53]. Triple-negative breast cancer is highly dependent on OXPHOS, especially when chemotherapy is suppressed, and inhibition of OXPHOS can enhance the effect of chemotherapy [54]. Osteogenic differentiation in breast cancer cells activates both the classical TGF-β/Smad signaling pathway and the non-canonical MAPK pathway. This process enhances OXPHOS, promoting the epithelial-mesenchymal transition (EMT). The EMT phenotype can be reversed by inhibiting OXPHOS with rotenone [55]. Estrogen upregulation in breast cancer cells increases the content of mitochondrial biogenesis (PGC1-α, TFAM) and transcription factors associated with OXPHOS (OCTN2, ND1, COX-IV) [56]. In gastric cancer, patients with the microsatellite instability (MSI) subtype have been found to be positively correlated with the activation of oxidative stress-related pathways [57].
Mitochondria also play a role in regulating glycolysis, fatty acid oxidation (FAO), and amino acid metabolism. In the mitochondrial matrix, pyruvate and fatty acids convert to acetyl-CoA. This acetyl-CoA then enters the tricarboxylic acid cycle (TCA cycle), where it is oxidized to produce NADH and FADH2. Glutamine is another important source of energy for tumors, participating in the TCA cycle to produce energy [58]. Without glucose, tumor cells sustain growth and survival by increasing fatty acid uptake and oxidation [59].
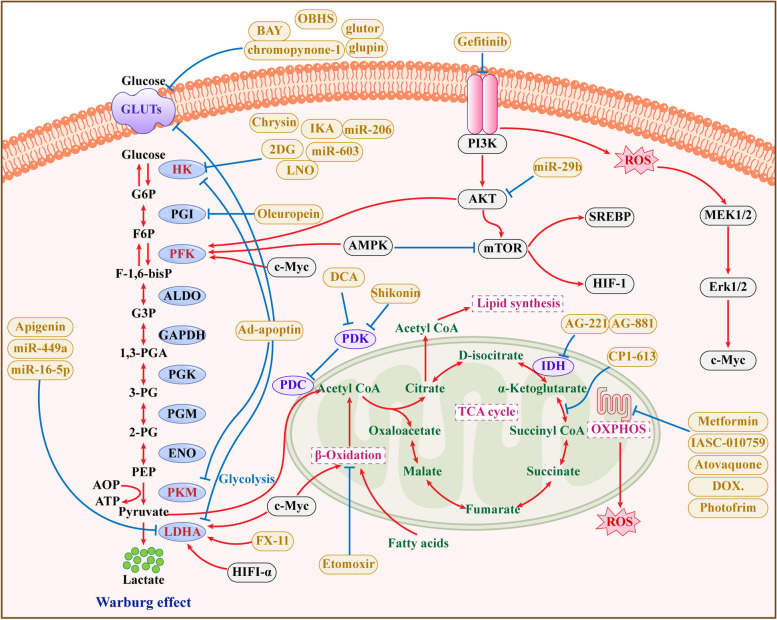
There is a complex interaction between glycolysis and mitochondrial oxidative phosphorylation, and their mutual regulation is essential for maintaining cellular energy balance and metabolic fitness. The interaction between glycolysis and oxidative phosphorylation is primarily evident in the flow of metabolites and energy demands. Under hypoxia or increased energy demands, glycolysis rates rise significantly to quickly provide energy. In contrast, when oxygen is plentiful, mitochondrial oxidative phosphorylation serves as the primary energy source. Metabolite feedback regulation is crucial for the mutual regulation of glycolysis and oxidative phosphorylation. The accumulation of lactate, a glycolytic byproduct, can further influence glycolysis rates by affecting pH and intracellular signaling pathways. At the same time, metabolites such as ATP and NADH produced by mitochondria can inhibit the activity of glycolysis-related enzymes through a negative feedback mechanism to maintain intracellular energy homeostasis [60, 61]. Activating signaling pathways like AMPK promotes glycolysis while inhibiting oxidative phosphorylation, leading to increased ATP production [62]. Warburg posits that 'aerobic glycolysis' in tumor cells indicates mitochondrial dysfunction. The contemporary view suggests that when mitochondria are overworked, glycolysis produces NADH faster than NAD can be regenerated, leading to saturated aerobic glycolysis. Consequently, tumor cells increase glycolysis for more energy, resulting in lactic acid production [63]. Mitochondrial dysfunction is closely related to the improvement of tumor drug resistance and malignancy, and targeting oxidative phosphorylation and the electron transport chain can effectively reduce the energy metabolism of tumor cells, thereby inhibiting their proliferation and metastasis [64] ( Fig. 1).
Fig. 1.
Inhibitors that target mitochondrial metabolism and Warburg effect
The blue circles in the figure are the enzymes in the glycolysis and mitochondrial metabolism pathways, the yellow circles are the targeted inhibitors, and the white circles are the relevant regulatory factors
Tumor metabolic plasticity is key to survival and drug resistance mechanisms
Tumor metabolic plasticity refers to the ability of tumor cells to flexibly adjust their metabolic pathways to adapt to survival and proliferation under different microenvironments and metabolic needs. This characteristic not only plays an important role in the occurrence and development of tumors, but also brings challenges to the treatment of tumors. Metabolic reprogramming involves activating transcription factors, abnormal signaling pathways, and altering the expression of metabolic enzymes. For instance, HIF-1α is activated in hypoxic conditions, increasing the expression of LDHA and enhancing glycolysis in tumor cells, which boosts their energy production and promotes proliferation and survival [65]. The increased expression and half-life of c-Myc protein can activate the β-TrCP/c-Myc/HK2 pathway, enhance glycolysis in colorectal cancer, significantly increase intracellular lactate, and promote the progression of colorectal cancer [66]. Additionally, tumor cells can be reprogrammed for rapid proliferation by modifying their fatty acid, amino acid, and nucleotide metabolism.
Cancer cells enhance their proliferation and survival by increasing fatty acid uptake and synthesis. They can synthesize up to 95% of saturated and monounsaturated fatty acids de novo. These fatty acids serve not only as energy sources but also play crucial roles in signal transduction and regulating cell functions. High levels of FASN are linked to a more aggressive tumor phenotype. Knocking out key enzymes related to fatty acid synthesis, such as FASN, ACC, and ACLY, can reduce cell proliferation and induce apoptosis in tumor cells [67–71]. Glutamate and leucine are highly utilized in cancer cells, and glutamine not only meets the energy needs of tumor cells but also provides carbon and nitrogen sources for macromolecular biosynthesis. For example, pancreatic cancer cells rely on glutamine metabolism to survive in a hypoxic environment [72, 73]. Colorectal cancer cells proliferate rapidly by remodeling the glutamine metabolic pathway [18]. Pancreatic cancer cells use the non-canonical KRAS-induced glutamine pathway to maintain redox homeostasis, supporting cell growth by increasing the nicotinamide adenine dinucleotide phosphate (NADPH/NADP +) ratio [74].
During cancer therapy, cancer cells often resist drug effects through metabolic reprogramming. For example, bladder cancer cells, when treated with cisplatin chemotherapy, rapidly shift to a metabolic pattern dependent on oxidative phosphorylation, thereby evading drug inhibition [75]. Pancreatic cancer's resistance to conventional treatment is partly due to the reprogramming of multiple metabolic pathways by the mutant KRAS gene. This mutation up-regulates glycolytic flux and enhances both the hexosamine biosynthesis pathway and the pentose phosphate pathway by increasing the expression of glycolytic enzymes such as GLUT1, HK 1/2, PFK, and LDHA. Researchers remodel glutamine metabolism in pancreatic cancer by increasing the expression of aspartate aminotransferase (GOT1) and decreasing glutamate dehydrogenase (GLUD1), thereby increasing flux through GOT1-dependent pathways. Additionally, researchers enhance lipid metabolism in pancreatic cancer cells by increasing the expression of carnitine palmitoyltransferase 1a (CPT1a), acyl-CoA oxidase (AOX), and peroxisome proliferator-activated receptor γ (PPARγ), which helps protect against metabolic stress caused by drugs. Furthermore, mutant KRAS expression can also increase NRF2 transcription, activate the ROS detoxification program, and help pancreatic cancer cells resist ROS [76, 77]. The TIAM2 gene, which is involved in lipid metabolism, increases lung adenocarcinoma (LAUD) cells' tolerance to osimertinib and enhances their motility [78]. ACLY promotes MITF-PGC1α axis transcription by increasing histone acetylation of the MITF locus, making melanoma resistant to MAPK inhibitors [79].
Epigenetics and tumor energy metabolism
Epigenetic modifications regulate gene expression without changing the DNA sequence through mechanisms such as chromatin remodeling, DNA methylation, histone modifications, RNA modifications, and regulation of non-coding RNAs. These modifications can inhibit DNA repair mechanisms and increase genomic instability. It also impacts cell cycle regulation, allowing cells with unstable genomes to proliferate. This further exacerbates genomic damage and instability, which may significantly contribute to tumorigenesis and its progression [80, 81]. It also impacts cell cycle regulation, allowing cells with unstable genomes to proliferate. This further exacerbates genomic damage and instability, which may significantly contribute to tumorigenesis and its progression [82].
In addition, numerous studies have shown that tumor energy metabolism is closely related to epigenetic regulation. In the past few years, the study of m6A in tumor glycolysis has experienced an explosion of research, with numerous studies revealing the molecular mechanism by which m6A modification regulates tumor glycolysis and its key role in this metabolic process. m6A methylation affects the metabolic profile and biological behavior of tumors by affecting the stability of key enzyme genes [83]. Liu et al.’s study shows that m6A can modify the GLUT1 gene to enhance the stability of GLUT1 mRNA, thereby promoting the glycolysis and cell proliferation of Colorectal cancer [84]. Enolase (ENO1) promotes LAUD development in an m6A-dependent manner. Liu and colleagues found the combination of YTHDF1, a m6A “reader”, with methylated ENO1 mRNA leads to increased expression of ENO1 and promotes glycolysis of LAUD [85]. They also found m6A modification may enhance glycolytic capacity and development of LUAD by enhancing the stability of NPM1 to promote the expression of glycolytic enzymes (e.g., ENO1, HK2, LDHA, LDHB, and GLUT1) in LUAD [86]. Li et al. found that METTL3 promotes the expression of HK2 in an m6A-dependent manner, enhancing glycolysis of pancreatic ductal adenocarcinoma cells, thereby promoting their perineural invasion [87].
Currently, the most researched epigenetic mechanism in tumor metabolism is the regulation of non-coding RNAs. Including lncRNA、miRNA、circRNA and piRNA. LncRNAs are non-coding RNAs that are more than 200 nucleotides in length. LncRNA KCNQ1OT1 can stabilize HK2 in osteosarcoma, colorectal cancer, and ovarian cancer, enhancing aerobic glycolysis and promoting cancer progression [88, 89]. LncRNA LINC00671 is inversely correlated with LDHA levels, and inhibition of its expression can increase LDHA content in thyroid cancer cells and promote glycolysis, growth, and lung metastasis both in vitro and in vivo [90]. Circular RNA (circRNA) can specifically adsorb miRNA, and CircRNA microarray sequencing has shown that miR-487a carried by CircRNF20 in breast cancer can promote the binding of HIF-1α to the HK2 promoter, thereby enhancing the transcription and expression of HK2 and promoting the proliferation of breast cancer cells through the Warburg effect [91]. In gastric cancer, miR-3-515p carried by CircCUL3 can activate signal transducer and activator of transcription 3 (STAT3). This activation accelerates the transcription of HK2, ultimately triggering the Warburg effect and promoting gastric cancer development [91].
Another regulatory mechanism, chromatin remodeling, has emerged as an important factor in tumor metabolism. The mammalian SWI/SNF chromatin remodeling complex acts as an important epigenetic regulator, with genetic aberrations found in 25% of cancers [92, 93]. Numerous studies indicate a link between mutations in the SWI/SNF subunit and tumor metabolism. For instance, BRG1 can bind to the promoters of key fatty acid synthesis enzymes to regulate their transcription, affecting the expression of these key enzymes, such as fatty acid synthase, ATP citrate lyase, and acetyl-CoA carboxylase. This regulation impacts fatty acid synthesis in breast cancer cells, subsequently influencing their proliferation. Notably, BRG1’s role in regulating proliferation through fatty acid metabolism is unique to breast cancer [94, 95]. Drug development targeting SWI/SNF complexes, including small molecule inhibitors and immunotherapy strategies, has shown promising results in clinical trials, with patients responding well to these targeted therapies [96, 97]. Therefore, exploring the relationship between SWI/SNF complexes and tumor metabolism may provide important targets and ideas for new therapeutic strategies.
The basic structure and function of the SWI/SNF complex
The SWI/SNF chromatin remodeling complex is a heterogeneous collection of related protein complexes required for gene regulation and genome integrity. It is an ATP-dependent chromatin reconstructor that regulates the accessibility of transcription factors to DNA by changing the assembly of nucleosomes, and reshapes the structure of chromatin by using the energy generated by ATP hydrolysis [98]. SWI/SNF complex is involved in the processes of cell mitosis, DNA replication, DNA damage repair, etc., and plays a role in cell differentiation and maintenance of cell state [99]. ARID1A subunit mutations are present in more than 50% of ovarian clear cell carcinomas and 40% of endometrioid carcinomas [100–102]. Therefore, its potential as a tumor biomarker is of increasing concern. Specific subunits of the SWI/SNF complex, such as ARID1A and SMARCA4 expression levels, mutation status, and copy number variation, can be used as diagnostic and prognostic markers for tumors. For example, ARID1A deletion is considered a characteristic hallmark of clear cell carcinoma of the ovary and is closely related to patient prognosis [103].
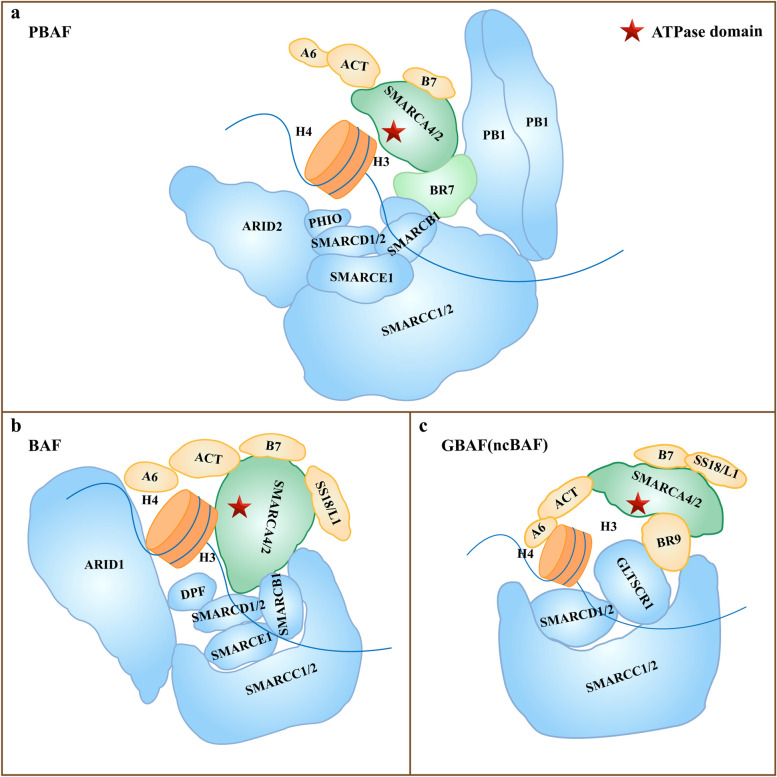
The complex can be divided into three modules: the motor module, the substrate recruitment module (SRM) and the regulatory ARP module (actin-related proteins, ARPs). The motor module is the BRG1 (gene name SMARCA4) or BRM (gene name SMARCA2) subunit, which is the site where DNA translocation reactions occur. BRM and BRG1 have ATPase activity and can directly participate in chromatin remodeling by using ATP hydrolysis energy to reposition nucleosomes, exchange nucleosome histone dimers, or remove entire nucleosome octamers regulated by the ARP module [104–106]. The SRM encompasses a nucleosome-binding lobe (NBL), a DNA-binding lobe (DBL) and a histone tail (HBL). All three SWI/SNF complexes have an NBL and a DBL, while the HBL is unique to RSC and PBAF. The specific subunits in the SRM module are used to categorize SWI/SNF complexes into three types: BAF (BRG1 or BRM-related factor), PBAF (polybrominated related factor) and ncBAF/GBAF (GLTSCR1 or a complex containing GLTSCR1L and BRD9 [107, 108]. The SRM encompasses a nucleosome-binding lobe (NBL), a DNA-binding lobe (DBL) and a histone tail (HBL). All three SWI/SNF complexes have an NBL and a DBL, while the HBL is unique to RSC and PBAF [104, 109]. The purpose of the SRM is to adjust chromatin structure (via transcription factors and histone modifications) and target the motor module to regulate chromatin structure and gene transcription. Actin, ARP4, and BCL7A are the main components of the ARP module. These proteins function to connect the SRM and the motor module and regulate the activity of the motor module [110] (Fig. 2).
Fig. 2.
The structure of three SWI/SNF complexes
There are three types of complexes: PBAF (a), BAF (b) and ncBAF/GBAF(c). The subunits of the SWI complex can be divided into motor module (green), substrate recruitment module (blue), and regulatory actin-related protein module (yellow) according to function. The ARID2, PBRM1,BAF45D, and BRD7 subunits are exclusive PBAF (a). GLTSCR1 subunit is unique to ncBAF/GBAF (c). The star is ATPase domain
Effect of SWI/SNF complex on glucose metabolism in tumors
The PBRM1 (PB1) subunit is a specific subunit of the PBAF SWI/SNF complex. Research has shown that approximately 46% of chronic kidney cell carcinomas have PBRM1 subunit mutations [111]. It has been found that in kidney cancer cells 786-O and SN12C, PBRM1 deficiency not only increases the levels of Akt and mTOR in the cells and their phosphorylated forms, activates AKT-mTOR signaling, but also increases the expression of mRNA and protein, which are key enzymes in glycolysis, such as phosphofructokinase (PFKP), enolase (ENO1), pyruvate kinase (PKM), and LDHA. Together with HIF1α, it promotes glycolysis of kidney cancer cells, and the researchers also observed that PBRM1 deficiency can promote the proliferation, migration and invasion of 786-O and SN12C cells, and high levels of PB1 can prolong the life expectancy of kidney cancer patients [112].
BRM is the ATPase subunit of the SWI/SNF complex. The expression of BRM, which is encoded by the SMARCA2 gene, is closely related to the expression of the glycolytic enzyme pyruvate kinase M2 (PKM2) and the AMPKα1-encoding gene (PRKAA1) in bladder cancer cells. The PRKAA1 gene encodes AMP-activated protein kinase (AMPK), and inactivation of AMPK promotes cellular metabolic reprogramming. BRM may be a negative regulator of PKM2 and AMPK-dependent metabolic processes in bladder cancer. Furthermore, BRM has different molecular characteristics in different bladder cancer cell lines. After the overexpression of BRM, the expression of almost all glycolytic genes (including PKM2), except fructose-1,6-bisphosphatase (FBP1), has been shown to increase in T24 bladder cancer cells, leading to increased glucose uptake. Compared to that in T24 bladder cancer cells, the expression of FBP1 is greater at both the protein and transcript levels in 5637 bladder cancer cells, resulting in decreased glucose uptake. The difference in FBP1 expression between the two cell lines may be the reason for the observed phenotypic differences. T24 bladder cancer cells are larger, spindle-shaped, and more basal-like, while 5637 bladder cancer cells are more tubular-like. Low expression of FBP1 is associated with the clinical stages of bladder cancer and is closely related to lower survival rates in bladder cancer patients (in whom there is more frequent urinary epithelial recurrence and metastasis) [113]. BRM plays an important role in glycolysis in bladder cancer cells by affecting the glycolytic process in bladder cancer cells through the influence of the key enzyme of glycolysis, FBP1, which in turn affects tumor phenotype. Differences in FBP1 expression also exist in different types of breast cancer; for example, basal-like breast cancer cells have a lower level of FBP1 expression than luminal breast cancer cells do, and FBP1 deficiency induces glycolysis and leads to an increase in glucose uptake, which is critical for EMT and basal-like breast cancer [114, 115].
The ARID1A gene is the most commonly mutated gene in 10%-15% of hepatocellular carcinomas (HCCs). Studies have shown that ARID1A deficiency leads to metabolic reprogramming in HCC cells by inhibiting the expression of the key glycolysis gene PKM. When glycolysis is suppressed, HCC cells shift their glucose metabolism toward the TCA cycle and oxidative phosphorylation. Additionally, HCC cells become more sensitive to copper, and the use of the copper ionophore elesclomol effectively treats ARID1A-deficient HCC in vivo. Researchers have proposed TCA cycle-targeted copper therapy as a promising and effective approach for treating ARID1A-deficient HCC patients [116].
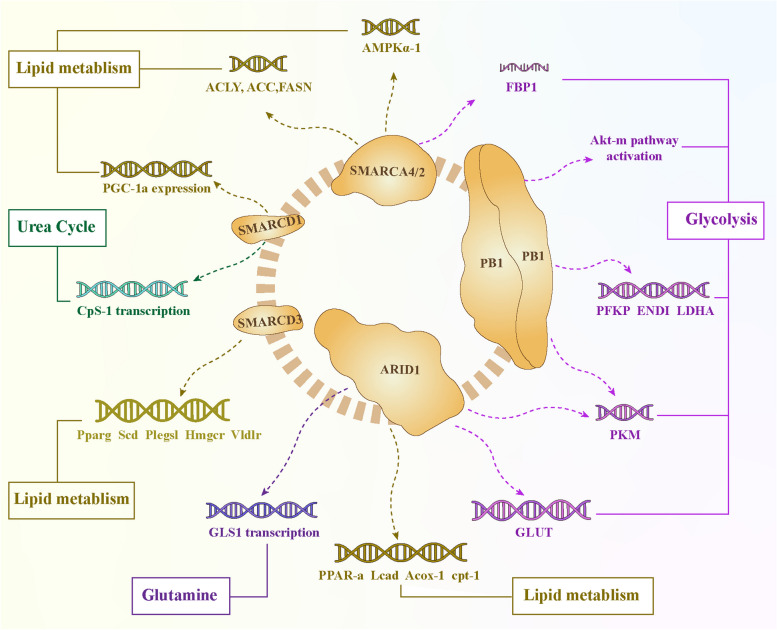
In addition, there is a potential link between the subcellular localization of the SWI/SNF complex subunit and glycolysis of tumor cells. The ARID1B (BAF250b) subunit is a typical nuclear tumor suppressor; however, ARID1B localized in the cytoplasm can interact with c-RAF (RAF1) and PPP1CA, thereby stimulating RAF-ERK signaling and β-catenin transcriptional activity. It is also associated with increased levels of ERK1 and ERK2 and the active form of β-catenin, which are closely related to the activation of the RAF-ERK signaling pathway and the Warburg effect. The activation of the RAF-ERK signaling pathway not only is closely related to the Warburg effect but also stimulates the expression of glucose transporters and glycolytic enzymes through the transcription factors MYC and HIF1α, thereby increasing glucose levels, glycolytic flux, and lactate production in cancer cells. Moreover, ERK can upregulate MYC expression through PKM2 phosphorylation to increase the phosphorylation of the glycolytic enzyme PFKFB2 and enhance glycolytic flux [117, 118] (Fig. 3).
Fig. 3.
Schematic diagram of SWI/SNF complex regulating tumor metabolism
The purple gene and arrow represent glucose metabolism. PB1 deficiency can increase the expression of PFKP, ENO1, PKM and LDHA, activate AKT-mTOR signaling, and significantly increase the concentration of lactate in cells. The coding gene SMARCA2 of BRM negatively regulates PKM2 to inhibit glycolysis, negatively regulates the expression of AMPKα1 coding gene PRKAA1 to inhibit the transport of glucose by GLUT-1, and overexpression of BRM can also increase the expression of FBP1 to promote cellular glycolysis. Deletion of ARID1A inhibits the expression of the key glycolysis gene PKM, leading to suppression of glycolysis. Cytoplasmic ARID1B interacts with c-RAF, stimulating the RAF-ERK signaling pathway. The activated transcription factors MYC and HIF1α then jointly promote the expression of GLUT and glycolytic enzymes, increasing glucose levels, glycolytic flux, and lactate production in cancer cells. Orange genes and arrows are associated with lipid metabolism. Silencing SMARCD1 can inhibit the activity and transcriptional activation ability of PGC-1α, downregulate the expression levels of fatty acid oxidation genes, and lead to significant accumulation of lipid droplets in liver cancer cells. SMARCD3 and FOXA1 cooperatively regulate the core genes involved in lipid and fatty acid metabolism, including Pparg, Scd1, Hmgcr, Ptgs1, and Vldlr, leading to an increase in the cellular content of fatty acids. Deletion of the ARID1A gene significantly reduces the expression of lipid beta-oxidation-related genes such as PPAR-alpha, Lcad, Acox-1, and cpt-1, leading to decreased lipid consumption and passive lipid accumulation. The blue gene and arrow represent glutamine metabolism. ARID1A is the first enzyme in glutamine breakdown and a transcriptional repressor of the rate-limiting enzyme GLS1, inhibiting glutamine metabolism through GLS1. The green gene and the arrow represent urea metabolism. Baf60a (SMARCD1) cooperates with Y-box binding protein-1 to inhibit the transcription of carbamoyl-phosphate synthetase 1 (CPS1), thereby suppressing urea synthesis
Effect of SWI/SNF complex on tumor lipid metabolism
Studies have shown that BAF60a is involved in the accumulation of cell lipid droplets and palmitic acid-induced cell senescence, in hepatoma cells HepG2, BAF60a (SMARCD1) is associated with PGC-1α activity and its transcriptional activation ability, FAO gene expression, when BAF60a (SMARCD1) is silenced, the expression of PGC-1α and FAO is down-regulated, and the lipid droplets of hepatocytes are significantly accumulated, on the contrary, when BAF60a is overexpressed, the expression of PGC-1α and FAO is up-regulated. Lipid droplet accumulation is reduced. Moreover, BAF60a overexpression can also inhibit palmitic acid-induced HepG2 cell senescence by inhibiting SA-β-Gal activity, p16/p21 expression, p-p38 expression, and γH2AX expression [119]. It has been found through ChIP-seq and RNA-seq that BAF60c (SMARCD3) is an epigenetic regulator of fatty acid metabolism, and its deletion will weaken fatty acid metabolism in vivo, and the study found that SMARCD3 co-regulate the transcription of lipid metabolism genes (arachidonic acid metabolism, fatty acid metabolism, cholesterol synthesis) with FOXA1, and several core genes of lipid metabolism Pparg, Scd1, Hmgcr, Ptgs1 and Vldlr are directly related to it Baf60c-BAF binds to FOXA1 and SMARCD3 loss down-regulates lipid transport, storage genes, and major transcriptional regulators of lipid metabolism. Tamoxifen-mediated SMARCD3 loss in tumor cells leads to a threefold decrease in the total free fatty acid content, which includes the monounsaturated fatty acid oleic acid, the polyunsaturated fatty acid docosahexaenoic acid, and the long-chain saturated fatty acid tridecanoic acid; these fatty acids are involved in the synthesis of complex lipids and play roles in cancer cell signaling and survival. The results of studies also suggest that the overexpression of Baf60c is sufficient to drive tumor growth in the absence of KRAS [120, 121].
Studies have shown that ARID1A is closely related to the expression of lipid β oxidation-related genes PPAR-α, Lcad, Acox-1, cpt-1 and other genes. When ARID1A is deficient, the chromatin accessibility of PPAR-α promoter and CPT1α is significantly reduced, and the expression of two key rate-limiting enzymes, CPT1α and ACOX1, is reduced for fatty acid oxidation. In this way, it can reduce lipid consumption, passively increases lipid accumulation, and promote the occurrence and development of liver cancer [122, 123].
Effect of SWI/SNF complex on tumor alternative metabolic processes
The SWI/SNF complex can directly interact with the first enzyme and rate-limiting enzyme of glutamine metabolism, glutaminase 1 (GLS1) gene. Inactivation of the SWI/SNF complex increases the expression of GLS1. In a study of ovarian clear cell carcinoma (OCCC) cells, researchers mined published ChIP-seq data and found that SWI/SNF subunits such as ARID1A, SNF5, SMARCA4, and BAF155 can directly bind to GLS1. They further analyzed the relationship between the ARID1A subunit and OCCC glutamine metabolism and demonstrated that ARID1A is a transcriptional suppressor of GLS1. Knocking out ARID1A enhances the binding of RNA polymerase II to the GLS1 promoter. The glutaminase inhibitor CB-839 is effective at treating ARID1A-inactivated OCCC [124]. Furthermore, studies have shown that the SWI/SNF complex subunit Baf60a can participate in the maintenance of liver urea homeostasis. Baf60a, in conjunction with Y box-binding protein-1, inhibits the transcription of carbamoyl phosphate synthetase 1, thereby suppressing urea synthesis [125].
Therapeutic strategies for tumor energy metabolism
Tumor metabolic fragility provides a novel way to develop therapeutic strategies, create personalized treatments, and tackle chemoresistance. Identifying the metabolic profile of tumor cells is crucial. For example, pancreatic cancer cells produce and release large amounts of lactate through glycolysis. This lactate inhibits immune cells in the tumor microenvironment and is linked to the cancer's high aggressiveness [126]. Experimental studies have demonstrated that targeting the glycolytic enzyme ENO1 can delay the progression of pancreatic cancer in mice and significantly extend survival [127]. Additionally, treatments that target metabolic pathways can be integrated with traditional chemotherapy, leading to the development of novel treatment strategies. In a recent study, drugs that target the SWI/SNF complex have effectively inhibited cancer cell growth, offering a promising new direction for tumor metabolism therapy [128].
Therapeutic strategies targeting the glycolytic process
Inhibiting rate-limiting enzymes in glycolysis is a promising strategy for cancer treatment. The effects of rate-limiting enzyme inhibitors on tumor cells are generally similar: they primarily act on these enzymes to reduce glycolysis and ATP production, which induces apoptosis in the tumor cells. Recently, researchers have developed inhibitors that target key enzymes in the glycolytic pathway, including PKM2, and these have entered clinical trials. For instance, PKM2 is frequently overexpressed in several types of cancer, and inhibiting its activity can effectively slow tumor growth while enhancing sensitivity to chemotherapy. Studies indicate that PKM2 inhibitors enhance the response of tumor cells to targeted therapies. They also reduce drug resistance, which in turn improves patient prognosis [129]. Clinical trials have also explored the effects of other glycolysis inhibitors, such as lactate dehydrogenase A inhibitors and pyruvate dehydrogenase kinase inhibitors, which have shown promising anti-tumor activity in various cancer models, including non-small cell lung cancer (NSCLC) and HCC [130, 131].
While glycolytic inhibitors may theoretically reduce tumor growth, their clinical effectiveness is frequently inadequate. Many inhibitors face clinical limitations because they can be cytotoxic, leading to liver damage, cardiovascular issues, and immune system suppression. For instance, 2-deoxyglucose (2-DG) disrupts normal glycolysis by competitively inhibiting hexokinase (HK) and promotes apoptosis in various tumor cells [132, 133]. Dichloroacetic acid (DCA) is a DK inhibitor that specifically inhibits PDK1 in breast and prostate cancer cells. However, it is dependent on the cancer subtype and can be cytotoxic [134]. Drugs that block glucose metabolism in tumor cells can weaken tumor-infiltrating T cells, diminishing their effectiveness and aiding tumor growth [135]. Furthermore, when glycolysis is inhibited, tumor cells can adapt their metabolism to use alternative energy pathways, leading to less effective treatment outcomes [136].
Therefore, the development of novel inhibitors targeting specific molecules is particularly urgent. Researchers have discovered more drugs that have long half-lives, good solubility, and fewer side effects. These include chemotherapy drugs, targeted therapies, biologics, and natural products. For example, the HK oral inhibitor chlorinidamine (LND) selectively targets various tumors, and its adverse effects do not overlap when combined with other chemotherapy drugs. Consequently, LND is frequently utilized as a chemosensitizer to enhance the efficacy of chemotherapy drugs against tumors. Consequently, LND is frequently utilized as a chemosensitizer to enhance the efficacy of chemotherapy drugs against tumors [137]. Ikarugamycin (IKA) inhibits the glycolysis of pancreatic cancer cells by selectively binding to HK2, demonstrating a good anticancer effect without significant cytotoxicity in mice with pancreatic cancer xenograft models [138]. Gefitinib, a chemotherapy drug, slows the growth of NSCLC cells by disrupting their glycolysis process and inhibiting the PI3K-Akt-mTOR signaling pathway [139]. Biologics like the oncolytic adenovirus Ad-apoptin reduce glucose uptake and lactate production in NSCLC by blocking the AMPK/mTOR signaling pathway [140]. Several miRNAs target key glycolysis enzymes: miR-206 targets HK2, while miR-449a and miR-16-5p target LDHA [141–143]. Overexpression of miR-603 reduces glucose uptake and lactate production, and it also inhibits the proliferation, migration, and invasion of ovarian cancer cells by targeting HK2 [144]. miR-29b negatively regulates the expression of AKT2 and AKT3, downregulates HK2 and PKM2, and reduces the Warburg effect, thereby slowing ovarian cancer progression [145]. Natural products like Shikonin, which binds to and inhibits pyruvate kinase (PK) while regulating glycolysis in LAUD [146]. The plant-derived flavonoid apigenin reduces GLUT1 and GLUT3 levels, decreases glucose metabolic activity, and increases apoptosis in thyroid cancer cells [147]. Chrysin, a natural flavonoid, inhibits glycolysis and promotes apoptosis in HCC cells by downregulating HK-2 expression [148]. Oleuropein, an iridoid ether terpene glycoside found in plants, targets glucose-6-phosphate isomerase (GPI) to inhibit glycolysis and tumor growth in liver cancer [149]. Bendicycloheptene sulfonate (OBHS) inhibits glycolysis in breast cancer cells by downregulating GLUT1 expression, thus slowing cancer progression and proliferation [150].
Therapeutic strategies targeting mitochondrial metabolism
Therapeutic strategies targeting mitochondrial metabolism are also gaining traction. TCA cycle enzyme inhibitors, such as the mutant isocitrate dehydrogenase (IDH) inhibitors AG-221 and AG-881, are already in clinical trials for the treatment of IDH2 or IDH1/2-mutated acute myeloid leukemia [151, 152]. CPI-613, which targets the α-ketoglutarate dehydrogenase complex and pyruvate dehydrogenase, is being investigated for the treatment of leukemia, lymphoma, and small cell lung cancer [153, 154].
Oxidative phosphorylation inhibitors, including mitochondrial complex I (NADH) inhibitors and metformin, are currently in clinical trials for treating breast, colorectal, and prostate cancers [155, 156]. IACS-010759 is being investigated for the treatment of brain cancer and acute myeloid leukemia, and it has been shown to effectively reduce the growth rate of tumor cells [157]. Additionally, atovaquone has been shown to inhibit cancer cell proliferation by targeting complex III. It also exhibits antitumor activity in animal models of acute myeloid leukemia, acute lymphoblastic leukemia, cervical cancer, breast cancer, and gastric cancer [158–161]. Doxorubicin and photofrin sodium, which is approved for esophageal cancer and non-small cell lung cancer, can inhibit complex IV [162]. The complex V inhibitor niclosamide is in phase I/II clinical trials for prostate and colon cancer, and nitazoxanide is in phase II clinical trials for different types of advanced cancer [163].
Etomoxir, an inhibitor that targets CPT1, stops tumor progression in a model of high-grade serous ovarian cancer during fatty acid oxidation (FAO) [164]. However, etomoxir is toxic. Excessive etomoxir can promote macrophage polarization and inhibit T cell differentiation, resulting in anti-tumor immune-related off-target effects [165, 166]. In contrast, inhibitors of other enzymes in FAO, such as trimetazidine and ranolazine, which inhibit 3-ketoyl-CoA thiolase, were not observed in cell lines, primary cells, or mice [167]. There is a need to develop FAO-targeted inhibitors that possess high specificity and low toxicity.
In glutamine metabolism, the GLS inhibitor CB-839 has entered clinical trials for treating hematologic malignancies, colorectal cancer, melanoma, NSCLC, TNBC, and others [168]. Studies have shown that R162, an inhibitor of high glutamate dehydrogenase (GDH), can inhibit the growth of glioma by causing an imbalance in redox homeostasis [169].
Additionally, mitochondrial dynamics, including fusion and fission processes, are closely linked to tumor resistance [170]. Chemotherapy-resistant ovarian cancer cells contain numerous interconnected mitochondria [171]. Strategies targeting mitochondrial dynamics, such as intervening in the processes of mitochondrial fusion and fission, are considered potential therapeutics. GTPase dynein-associated protein 1 (DRP1) is crucial for mitochondrial fission. Its inhibitor, Mdivi-1, can reduce mitochondrial fission, lower oxidative metabolism in colorectal cancer cells, and impede cell proliferation [172]. Atractylolactone I can block DRP1-mediated mitochondrial fission and disrupt mitochondrial membrane integrity, thereby inducing apoptosis [173]. Bromodomain inhibitors (BETi) downregulate the proteins involved in mitochondrial fission in triple-negative breast cancer cells and increase the expression of fusion mitochondria, which impairs mitochondrial fission, affects OXPHOS, and promotes cell death [174]. Research indicates that knocking down ephrin type A receptor 2 (EPHA2) enhances mitochondrial fusion, reduces mitochondrial fission, and promotes mitophagy and autophagy. Additionally, the drug sesamol targets EPHA2 to improve cisplatin sensitivity in cervical cancer by regulating mitochondrial dynamics [175]. The androgen receptor antagonist apalutamide (ARN) and the complex I inhibitor IACS-010759 (IACS) regulate the process of mitochondrial fission and fusion in prostate cancer, and the combination of the two can increase androgen-sensitive prostate cancer cell death and mitochondrial oxidative stress [176]. Moreover, several naturally derived compounds, including inauhzin, matrine, aloe vera gel glucomannan, as well as chemotherapy drugs like triptolide and camptothecin, influence mitochondrial dynamics by stimulating DRP1 phosphorylation and dephosphorylation, showing promise for colorectal cancer treatment and clinical application [177] (Table 1).
Table 1.
Therapeutic targeting of metabolic pathways
| Metabolism | Tagrgets | Inhibits | Ref |
|---|---|---|---|
| Glycolysis | HK | 2-DG | [132, 133] |
| HK | LND | [137] | |
| HK | IKA | [133] | |
| HK | miR-206 | [141] | |
| HK | miR-603 | [138] | |
| HK | Chrysin | [148] | |
| GPI | Oleuropein | [149] | |
| LDHA | miR-449a | [142] | |
| LDHA | miR-16-5p | [143] | |
| PDK1 | DCA | [134] | |
| PK | Shikonin | [146] | |
| GLUT-1 | BAY-876 | [15] | |
| GLUT-1 | OBHS | [150] | |
| GLUT-1/3 | Apigenin | [147] | |
| PI3K-Akt-mTOR | Ad-apoptin | [140] | |
| PI3K-Akt-mTOR | miR-29b | [145] | |
| Mitochondrial metabolism | IDH | AG-221, AG-881 | [151, 152] |
| OGDC | CPI-613 | [153, 154] | |
| PDH | CPI-613 | ||
| Respiratory chain complexI | metformin | [155, 156] | |
| Respiratory chain complex I | IACS-010759 | [157] | |
| Respiratory chain complex I | IACS-010759 | [158] | |
| Respiratory chain complex III | Atovaquone | [158–161] | |
| Respiratory chain complex IV | Doxorubicin, Photofrin | [162] | |
| Respiratory chain complex V | Niclosamide, Nitazoxanide | [163] | |
| Fatty acid oxidation | CPT1 | etomoxir | [164–166] |
| DRP1 | Mdivi-1 | [172] | |
| DRP1 | Atractylenolide I | [173] | |
| Glutamine metabolism | GLS | CB-839 | [124] |
| GDH | R162 | [169] |
Exploiting the metabolic vulnerabilities of tumors
Metabolic vulnerability describes how tumor cells rely on specific metabolic pathways, creating new opportunities for targeted therapy [178, 179]. Researchers can develop therapeutic strategies by identifying cancer cells’ dependence on specific nutrients or metabolic pathways. For example, targeted therapy for glutamine metabolism can effectively reduce the proliferation of acute myeloid leukemia cells by blocking glutamine uptake [180]. Dependence on the mTOR pathway often causes drug resistance to targeted therapies; thus, targeting this pathway may help overcome resistance to drugs like PI3K inhibitors (alpelisib and pictilisib) [181]. In imatinib-resistant gastrointestinal stromal tumors (GIST), the GIST 882 cell line exhibits elevated glycolysis and OXPHOS levels, making it more susceptible to glycolysis inhibition, while the GIST-T1 cell line shows reduced mitochondrial respiration and is more vulnerable to OXPHOS inhibition [182]. Many tumors depend heavily on fatty acid synthesis. Acetyl-CoA carboxylase 1 (ACC1), the rate-limiting enzyme in this process, has emerged as a target for treating acute myeloid leukemia, breast cancer, ovarian cancer, non-small cell lung cancer, and liver cancer [183, 184]. AG-120, a mutant isocitrate dehydrogenase (IDH)1 inhibitor, has been approved by the United States Food and Drug Administration as a first-line treatment for IDH1-mutant acute myeloid leukemia [185]. These targeted therapies can be used alone or in combination with other treatments to enhance their effectiveness.
Synthetic lethality is a method that selectively kills tumor cells while sparing normal cells by simultaneously blocking two or more interdependent metabolic pathways. For example, the GLS1 inhibitor BPTES works synergistically with olaparib to inhibit the growth of VHL-deficient renal cell carcinoma (RCCC) [186]. CB-839, a small molecule inhibitor of GLS1, is used in combination with erlotinib to inhibit the growth of EGFR-mutated NSCLC and with Glutor, an inhibitor of the glucose transporter GLUT1/2/3, to suppress colon cancer cell growth [187, 188]. Milk thistle silibinin directly interacts with the GLUT4 subtype to inhibit glucose uptake. When combined with paclitaxel, it significantly reduces ovarian cancer cell growth, proving more effective than paclitaxel alone [189]. The ATP citrate lyase (ACLY) inhibitor SB-204990, in combination with glutamine deprivation, causes KRAS-driven cancer cell death [190]. Tumors deficient in SMARCA4/2 depend on OXPHOS for survival and are highly sensitive to inhibitors targeting either OXPHOS or glutamine metabolism. At clinically relevant doses, alanine supplementation has a synergistic effect with OXPHOS inhibition or conventional chemotherapy, leading to a significant reduction in tumor activity [191]. Combining inhibitors that target multiple metabolic pathways can enhance therapeutic efficacy. For instance, when the mitochondrial complex I and fission inhibitor MDIVI-1 is used alone, breast cancer cells maintain their energy requirements for growth by enhancing glycolysis. However, when combined with the glycolysis inhibitor 2DG, breast cancer cell death is significantly increased [192]. OXPHOS metabolism inhibitors, when used in combination with glucose metabolism inhibitors, such as Marizomib (a dual inhibitor of proteasomes and OXPHOS), synergistically inhibit the activity of triple-negative breast cancer alongside the glycolysis inhibitor 2DG [193]. Studies have shown that the combination of mitochondrial complex III inhibitors resveratrol and tamoxifen can overcome tamoxifen resistance in breast cancer cells [194].
"Metabolic checkpoints" refer to important enzymes or receptors in metabolic pathways whose activity levels can affect immune cell function [195]. Tumor cell metabolism alters the microenvironment, making it acidic and hypoxic. In this environment, tumor cells compete with immune cells for nutrients like glutamine, which impacts immune cell function and the efficacy of immunotherapy. Accumulation of lactate and amino acid metabolites in the tumor microenvironment inhibits T cell activation and promotes the survival of Treg cells [196]. Lactate plays a key role in transforming macrophages into tumor-associated macrophages (TAMs) [197]. Most TAMs are considered the M2 phenotype, which is anti-inflammatory and pro-tumor [198]. High lactate concentrations hinder NK cell immune surveillance, allowing tumor cells to evade attack by the immune system [199]. Sodium chloride in the tumor microenvironment can enhance T cell metabolic adaptation, thereby enhancing the activation status and effector function of CD8 T cells [200]. Modulating the various metabolic functions of immune cells is another important branch of immunotherapy [201].
Indoleamine 2,3-dioxygenase (IDO) inhibitors are a type of immunotherapy drug developed based on "metabolic checkpoints." These inhibitors can block tryptophan metabolism in cancer cells and activate T cells [202]. Initially, the IDO inhibitor Epacadostat, when combined with Keytruda (a PD-1 antibody), failed in a phase III clinical trial for malignant melanoma. This failure was due to increased side effects and did not extend the patients' progression-free survival (PFS) or overall survival (OS) [203]. In the most recent clinical trial, the combination of IDO inhibitors with chemotherapy and radiotherapy was successfully tested in phase I for pediatric brain tumors and is expected to enter phase II/III trials soon [204]. Additionally, studies indicate that quinolineate, a product of tryptophan metabolism, serves as a metabolic checkpoint in glioblastoma. It does this by inducing NMDA receptor activation and activating Foxo1/PPARγ signaling in macrophages [205].
Avasimibe, an inhibitor of acetyl-CoA acetyltransferase 1 (ACAT1), has demonstrated significant anti-tumor effects in a mouse model of melanoma. Additionally, combining avasimibe with an anti-PD-1 antibody has improved anti-tumor immunotherapy[206]. Glutamine metabolism serves as another potential "metabolic checkpoint" in cancer immunotherapy. Glutamine inhibitors can reduce tumor cell activity, while activated CD8T cells can adapt to glutamine blockade by increasing acetate metabolism [195]. The glutamine antagonist 6-diazo 5-oxo-l-norleucine (DON) inhibits various glutamine-utilizing enzymes and reduces tumor cell viability, but it also has toxic side effects. To address this, Leone et al. developed a novel DON prodrug that activates only in the tumor microenvironment, thereby avoiding toxic off-target effects on other tissues [207]. Zheng et al. developed a hypoxia-activated prodrug, HDON, which, when combined with combretastatin A4 nanoparticles, selectively kills tumor cells in animal models and significantly increases tumor suppression rates [208]. Research has shown that glucose-6-phosphate dehydrogenase (G6PD) serves as a metabolic checkpoint in tumor-activated cytotoxic T lymphocytes. It regulates the expression of granzyme B, which is positively correlated with the response of mesothelioma patients to immune checkpoint inhibitor immunotherapy [209]. G6PD also serves as a critical metabolic checkpoint for the metabolism and functional reprogramming of regulatory T cells [210]. Therefore, an in-depth study of the metabolic characteristics of tumor cells and their relationship with immune responses can enhance the overall effectiveness of tumor therapies. Additionally, this research is significant for developing new targeted therapies that enable more precise and individualized treatment.
Recent advances in the use of SWI/SNF complexes in targeted drug therapy
The SWI/SNF complex plays a crucial role in tumor metabolism, making it an attractive target for therapy. Currently, clinical studies are evaluating drugs that target this complex to assess their potential in various cancer types, which could lead to new therapeutic strategies. The POU2F3-POU2AF2/3 transcription factor complex is a key regulator of small cell lung cancer (SCLC). The POU2F3 subtype (SCLC-P) relies specifically on the activity of the mSWI/SNF chromatin remodeling complex. Notably, orally available mSWI/SNF ATP enzyme degraders significantly inhibit tumor growth in preclinical models of SCLC-P and multiple myeloma without causing toxicity [211]. Furthermore, recent studies indicate that the SWI/SNF complex influences the effectiveness of targeted therapies by regulating tumor lipid metabolism. In patients with advanced LAUD harboring mEGFR mutations, lipid metabolism and co-mutations in genes such as ARID1A, ARID1B, BCR, and RBM10 are linked to the efficacy of icotinib (an epidermal growth factor receptor-tyrosine kinase inhibitor, EGFR-TKI). These four co-mutated genes are significantly associated with shorter progression-free survival. Additionally, these findings are closely related to the glycerophospholipid and sphingolipid metabolism pathways [212].
Clinical significance and translational potential, challenges, and future directions
Research on metabolism-related targets has led to new therapeutic strategies, particularly for cancer and metabolic diseases. Drugs that target specific metabolic enzymes and metabolites, like the glycolytic pathway, have been clinically used for tumor imaging and treatment. An example is difluorodeoxyglucose (FDG) [213]. Additionally, advancements in nanomedicine offer innovative approaches to drug delivery systems, increasing drug concentration in tumor tissues while minimizing damage to normal tissues [214]. Furthermore, researchers can quickly identify new compounds with potential therapeutic effects using computational chemistry and high-throughput screening techniques, which accelerates the drug development process.
Overcoming drug resistance in metabolically targeted therapies
Tumor cells survive and proliferate due to metabolic reprogramming; however, this adaptation also results in resistance to therapies that target metabolism. Drug resistance can occur through several mechanisms, such as changes in metabolic pathways, increased drug efflux pump expression, altered intracellular signaling, and modifications to the tumor microenvironment [215]. For instance, melanoma cells with the BRAF V600 mutation develop resistance to BRAF inhibitors through metabolic reprogramming after undergoing targeted therapy [216]. Changes in lipid metabolism significantly contribute to resistance against anti-tumor therapies. Tumor cells can improve their survival by regulating lipid synthesis and degradation, which helps them resist drug treatments [217].
Researchers have proposed several strategies to combat these resistance mechanisms. These include combining drugs that work through different mechanisms and reversing resistance by targeting key enzymes in metabolic pathways [218]. For example, inhibiting targeted purine metabolism significantly improves glioblastoma's radiosensitivity [219]. Disrupting mitochondrial metabolism in rectal cancer cells can inhibit their growth and increase their sensitivity to chemotherapy [220]. Combining glutamine inhibitors with prostate chemotherapy can enhance treatment efficacy [221]. Blocking glutamine metabolism in EGFR-mutated non-small cell lung cancer significantly increases sensitivity to almonertinib [222]. The fatty acid transporter CD36 plays a crucial role in lapatinib resistance in breast cancer; inhibiting CD36 expression reduces fatty acid uptake by lapatinib-resistant breast cancer cells [223].
Metabolic therapy combined with other therapies
The combination of metabolic therapy with other treatment modalities has become an important strategy to improve the efficacy of cancer treatment. Research indicates that combining metabolic inhibitors with immune checkpoint inhibitors can greatly improve the anti-tumor immune response. For example, therapies targeting glucose or glutamine metabolism are combined with PD-1/PD-L1 checkpoint inhibitors [224]. Moreover, metabolic interventions can enhance the effectiveness of chemotherapy and radiotherapy. They also increase the sensitivity of tumor cells to these treatments [225]. For instance, HK2-mediated glycolysis influences the efficacy of crizotinib, an ALK inhibitor. Additionally, 2DG enhances the sensitivity of non-small cell lung cancer to crizotinib by inhibiting both HK2-mediated glycolysis and the AKT/mTOR signaling pathway [226]. Knocking down the GLUT3 gene in combination with radiotherapy can significantly inhibit glioblastoma proliferation and help address radiation resistance [227].
Recently, advancements in nanobiomaterials have led to the development of various nanomaterials, including organic, inorganic, lipid, polysaccharide compounds, and synthetic polymers. These nanomaterial-based drug delivery systems enhance the efficacy of therapies like photothermal therapy, photodynamic therapy, radiotherapy, chemotherapy, and immunotherapy [228, 229]. Nanomedicine that targets mitochondrial metabolism offers a promising therapeutic approach [230]. Studies have shown that 3-O-β-D-galactosylated resveratrol polydopamine nanoparticles significantly inhibit tumor growth in liver cancer model mice without causing noticeable toxicity to major organs [231].
However, combining metabolic therapy with other treatments comes with challenges. These include metabolic adaptation and the complexity of the microenvironment [232]. Therefore, future research should focus on optimizing the timing and dosage of combination therapies to enhance synergies and improve treatment efficacy.
Innovations in targeted tumor metabolism
In recent years, the rise of single-cell metabolomics has enabled researchers to analyze the metabolic characteristics of tumor cells at the single-cell level, revealing the metabolic heterogeneity of different tumor cell subsets [233]. This lays the groundwork for improved diagnosis, patient classification, personalized treatment, and targeting specific metabolic pathways and cell types [234]. Single-cell and spatial protein transcriptomics have identified RE1 silencing factor (REST) as a potential biomarker for small cell lung cancer, associated with low neuroendocrine features, increased anti-tumor immunity, and prolonged survival [235]. Additionally, single-cell sequencing technology has shown that the expression of the metabolic enzyme 6-phosphogluconate dehydrogenase (PGD) is significantly up-regulated in LUAD. PGD promotes glycolysis and fatty acid synthesis in LUAD cells by reducing p-AMPK levels, suggesting that PGD could serve as a prognostic biomarker and therapeutic target for LUAD [236]. Metabolomics can identify specific metabolites resulting from the metabolic reprogramming of tumor cells, which may act as potential biomarkers for early cancer detection and monitoring. For instance, clear cell renal cell carcinoma exhibits higher levels of glutamine compared to normal kidney cells [237]. A biomarker panel for the diagnosis of early lung adenocarcinoma was identified through metabolomics of serum from lung adenocarcinoma patients, with a sensitivity of 70%-90% and a specificity of 90%-93%, including seven metabolites (histamine, cysteine, fatty acids (18:2), uracil, uric acid, 3-hydroxypicolinic acid (HPA), indoleacrylic acid (IA)) and related pathways [238]. Liquid biopsy techniques can detect tumor-specific metabolites through blood samples [239]. For example, a specific combination of metabolites in the serum of patients with hepatocellular carcinoma can distinguish between early and advanced tumors [240].
Furthermore, advancements in Deuterium Metabolic Imaging (DMI) technology enable real-time monitoring of tumor metabolic status, offering a novel method for diagnostic and therapeutic monitoring in clinical practice. For instance, 18F-FDG PET imaging technology allows for the assessment of glucose metabolism in tumor cells and can predict patient responses to immunotherapy [241]. DMI can dynamically track the entire cellular metabolism process in tumor cells, including glucose transport, the pentose phosphate pathway, glycolysis, and citric acid cycling, through exogenous 2H labeling. This capability helps identify the optimal time window for immunotherapy and enhances the precision of clinical interventions [242, 243]. Another type of DMI can quantify key metabolites in oncology, such as glucose, lactate, glutamate, and glutamine, to assist in diagnostic analysis, treatment response monitoring, and the identification of new therapeutic targets for various cancer subtypes [244]. Future research should integrate advanced metabolomics and imaging technologies to investigate the mechanisms of metabolic reprogramming, identify new targets, and facilitate the clinical translation of metabolic targeted therapies for tumors.
The influence of SWI/SNF complex in drug resistance has also been gradually emphasized. Research has shown that the combined use of SWI/SNF complex subunit-related inhibitors during tumor treatment can enhance the sensitivity of tumors to drugs. The stable knockdown of the SWI/SNF catalytic subunits BRG1 and BRM in lung cancer and head/neck cancer cells can enhance the sensitivity of cells to cisplatin because the downregulation of BRG1 and BRM hinders the repair of DNA intrastrand adducts and interstrand crosslinks (ICLs) [245]. In the ovary, high expression of SMARCA2 affects the expression of cell cycle- and apoptosis-related proteins in ovarian cancer cells, increasing their resistance to cisplatin [246]. According to the latest research, low SMARCA4 expression and SMARCA2 overexpression are associated with platinum resistance in high-grade serous ovarian cancer (HGSC) cells, possibly related to substantial changes in chromatin accessibility, the activation of fibroblast growth factor (FGF) signaling, the activation of the MAPK pathway, and reduced apoptosis [247]. Research has shown that the glutamate-cysteine ligase catalytic subunit (GCLC) could serve as a new therapeutic target for ARID1A-deficient ovarian cancer cells. BLC7A11 expression is impaired in ARID1A-deficient cancer cells, leading to decreased cellular levels of the antioxidant glutathione (GSH). As a result, these cells are more sensitive to GSH and GCLC [248]. In pancreatic cancer, pancreatic cells with SWI/SNF dysfunction exhibit significantly increased sensitivity to DNA-damaging agents, particularly DNA cross-linking agents (such as cisplatin and oxaliplatin) [249]. However, the knockout of the ARID1A subunit of the SWI/SNF complex can lead to gemcitabine resistance in pancreatic cancer cells. Mechanistically, ARID1A directly binds to the promoter of RRM2 (a gemcitabine resistance-related gene), and the knockout of ARID1A reduces the transcription of RRM2 [250]. In breast cancer, the knockout of the ATPase subunit SMARCA4 of the SWI/SNF chromatin remodeling complex is associated with cisplatin resistance in triple-negative breast cancer cells. After SMARCA4 knockout, the epithelial–mesenchymal transition (EMT) pathway and Hippo-YAP/TAZ target genes are activated in cells. The YAP1 inhibitor verteporfin can reduce the viability and invasiveness of SMARCA4-knockout cells and may serve as a new therapeutic strategy for cisplatin-resistant patients [251]. Therefore, in-depth understanding of the mechanism of drug resistance and the development of corresponding countermeasures will be an important direction for future research (Table 2).
Table 2.
The SWI/SNF complex submits targets in tumor therapy
| Cancer | Submits | Antineoplastic drugs | Outcome | Ref |
|---|---|---|---|---|
| Lung adenocarcinoma with mEGFR mutations | ARID1A, ARID1B, BCR, BM10 | Icotinib | Potential predictive value of serum targeted metabolites and concurrently mutated genes for EGFR-TKI therapeutic efficacy in lung adenocarcinoma patients with EGFR sensitizing mutations | [212] |
| Lung cancer, head/neck cancer | BRG1, BRM | Cisplatin | Downregulation of SWI/SNF chromatin remodeling factor subunits modulates cisplatin cytotoxicity | [245] |
| Ovarian cancer | SMARCA2/4 | Cisplatin | Overexpression of SMARCA2 or CAMK2D is associated with cisplatin resistance in human epithelial ovarian cancer | [246] |
| Ovarian high-grade serous carcinoma cells with low SMARCA4 expression and high SMARCA2 expression contribute to platinum resistance | [244] | |||
| ARID1A | GSH, GCLC | Targeting the Vulnerability of Glutathione Metabolism in ARID1A-Deficient Cancers | [248] | |
| Pancreatic cancer | ARID1A | Cisplatin, Oxaliplatin, Gemcitabine | ARID1A promotes chemosensitivity to gemcitabine in pancreatic cancer through epigenetic silencing of RRM2 | [249] |
| Breast Cancer | SMARCA4 | Cisplatin | SMARCA4 Depletion Induces Cisplatin Resistance by Activating YAP1-Mediated Epithelial-to-Mesenchymal Transition in Triple-Negative Breast Cancer | [251] |
Conclusion
This review examines the key pathways of tumor energy metabolism and their regulatory mechanisms, focusing on glycolysis and mitochondrial oxidative phosphorylation. Identifying key enzymes offers clear targets for drug development and establishes a foundation for new therapeutic strategies. Currently, many new drugs targeting tumor-metabolizing enzymes have issues like low specificity, immune cell suppression, and side effects. In contrast, natural products from various plants show better specificity and fewer side effects, offering new avenues for developing key metabolic enzyme inhibitors. Additionally, we discuss the crucial role of the SWI/SNF complex in tumor energy metabolism. Studies show that the SWI/SNF complex significantly impacts the metabolic pathways of tumor cells by regulating gene expression and chromatin remodeling. Therapeutic strategies targeting the SWI/SNF complex and its downstream metabolic pathways are expected to yield new breakthroughs in tumor treatment. Future research must prioritize the role of SWI/SNF complexes in various tumor types and their microenvironments to gain a comprehensive understanding of their function in tumor energy metabolism.
As clinical trials advance, targeted therapies for metabolism show great promise. Combining metabolic targeted therapy with immunotherapy and chemotherapy will be crucial for developing cancer treatments and may create new opportunities. Meanwhile, as personalized medicine grows, utilizing advanced technologies like metabolomics will become a key research area. High-throughput transcriptomics, metabolomics, and single-cell sequencing technologies will enhance our understanding of tumor metabolism. This knowledge will help us identify new therapeutic targets and biomarkers and optimize treatment options for individualized therapies.
Acknowledgements
Not applicable.
Authors’ contributions
Youwu Hu, Conceptualization, Writing—Original Draft. Wanqing Liu, Revision of the manuscript. WanDi Fang, Revision of the manuscript. Yudi Dong, Review & Editing, Funding Acquisition. Hong Zhang, Revision of the manuscript. Qing Luo, Conceptualization, Review & Editing, Funding Acquisition. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No.32360144 and No.81960532) and the Science and Technology Foundation of Basic Research Program of Guizhou Province grant numbers: ZK[2023]554.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The publication of the article was made by all authors.
Competing interests
No potential conflict of interest was reported by the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klepinin A, Zhang S, Klepinina L, Rebane-Klemm E, Terzic A, Kaambre T, et al. Adenylate kinase and metabolic signaling in cancer cells. Front Oncol. 2020;10:660. 10.3389/fonc.2020.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kondo M, Kumagai S, Nishikawa H. Metabolic advantages of regulatory T cells dictated by cancer cells. Int Immunol. 2024;36(2):75–86. 10.1093/intimm/dxad035. [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21(10):669–80. 10.1038/s41568-021-00378-6. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Reyes I, Cardona LR, Kong H, Vasan K, McElroy GS, Werner M, et al. Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature. 2020;585(7824):288–92. 10.1038/s41586-020-2475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christen S, Lorendeau D, Schmieder R, Broekaert D, Metzger K, Veys K, et al. Breast cancer-derived lung metastases show increased pyruvate carboxylase-dependent anaplerosis. Cell Rep. 2016;17(3):837–48. 10.1016/j.celrep.2016.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Sellers K, Fox MP, Bousamra M 2nd, Slone SP, Higashi RM, Miller DM, et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest. 2015;125(2):687–98. 10.1172/jci72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris IS, DeNicola GM. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020;30(6):440–51. 10.1016/j.tcb.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Liu JY, Wellen KE. Advances into understanding metabolites as signaling molecules in cancer progression. Curr Opin Cell Biol. 2020;63:144–53. 10.1016/j.ceb.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu L, Zhu X, Wu Y. Effects of glucose metabolism, lipid metabolism, and glutamine metabolism on tumor microenvironment and clinical implications. Biomolecules. 2022;12(4). 10.3390/biom12040580. [DOI] [PMC free article] [PubMed]
- 12.Xiao-Yan W, Xiao-Xia Y, Peng-Fei S, Zong-Xue Z, Xiu-Li G. Metabolic reprogramming of glutamine involved in tumorigenesis, multidrug resistance and tumor immunity. Eur J Pharmacol. 2023;940:175323. 10.1016/j.ejphar.2022.175323. [DOI] [PubMed] [Google Scholar]
- 13.Kreuzaler P, Panina Y, Segal J, Yuneva M. Adapt and conquer: metabolic flexibility in cancer growth, invasion and evasion. Mol Metab. 2020;33:83–101. 10.1016/j.molmet.2019.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng L, Hu S, Ma J, Shu Y, Chen Y, Zhang B, et al. Long noncoding RNA RP11–241J12.3 targeting pyruvate carboxylase promotes hepatocellular carcinoma aggressiveness by disrupting pyruvate metabolism and the DNA mismatch repair system. Mol Biomed. 2022;3(1):4. 10.1186/s43556-021-00065-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Q, Ba-Alawi W, Deblois G, Cruickshank J, Duan S, Lima-Fernandes E, et al. GLUT1 inhibition blocks growth of RB1-positive triple negative breast cancer. Nat Commun. 2020;11(1):4205. 10.1038/s41467-020-18020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Zheng M, Wu S, Gao S, Yang M, Li Z, et al. Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J Exp Clin Cancer Res. 2017;36(1):58. 10.1186/s13046-017-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu S, Guo Y, Zhang X, Liu H, Yin M, Chen X, et al. Pyruvate kinase M2 (PKM2) in cancer and cancer therapeutics. Cancer Lett. 2021;503:240–8. 10.1016/j.canlet.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Hara Y, Minami Y, Yoshimoto S, Hayashi N, Yamasaki A, Ueda S, et al. Anti-tumor effects of an antagonistic mAb against the ASCT2 amino acid transporter on KRAS-mutated human colorectal cancer cells. Cancer Med. 2020;9(1):302–12. 10.1002/cam4.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018;27(2):299–313. 10.1016/j.cmet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Lv Z, Guo Y. Metformin and Its benefits for various diseases. Front Endocrinol (Lausanne). 2020;11:191. 10.3389/fendo.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izzo LT, Affronti HC, Wellen KE. The bidirectional relationship between cancer epigenetics and metabolism. Annu Rev Cancer Biol. 2021;5(1):235–57. 10.1146/annurev-cancerbio-070820-035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Peng Q, Jiang X, Tan S, Yang Y, Yang W, et al. Metabolic reprogramming and epigenetic modifications in cancer: from the impacts and mechanisms to the treatment potential. Exp Mol Med. 2023;55(7):1357–70. 10.1038/s12276-023-01020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge T, Gu X, Jia R, Ge S, Chai P, Zhuang A, et al. Crosstalk between metabolic reprogramming and epigenetics in cancer: updates on mechanisms and therapeutic opportunities. Cancer Commun (Lond). 2022;42(11):1049–82. 10.1002/cac2.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma NK, Pal JK. Metabolic ink lactate modulates epigenomic landscape: a concerted role of pro-tumor microenvironment and macroenvironment during carcinogenesis. Curr Mol Med. 2021;21(3):177–81. 10.2174/1566524020666200521075252. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Golinska M, Griffiths JR. HIF-1-independent mechanisms regulating metabolic adaptation in hypoxic cancer cells. Cells. 2021;10(9). 10.3390/cells10092371. [DOI] [PMC free article] [PubMed]
- 26.Littleflower AB, Parambil ST, Antony GR, Subhadradevi L. The determinants of metabolic discrepancies in aerobic glycolysis: providing potential targets for breast cancer treatment. Biochimie. 2024;220:107–21. 10.1016/j.biochi.2024.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64. 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Xu Y, Zhuo W, Zhang L. The emerging role of lactate in tumor microenvironment and its clinical relevance. Cancer Lett. 2024;590:216837. 10.1016/j.canlet.2024.216837. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Zhao Y, Song H, Li Y, Liu Z, Ye Z, et al. Metabolic reprogramming in tumor immune microenvironment: Impact on immune cell function and therapeutic implications. Cancer Lett. 2024;597:217076. 10.1016/j.canlet.2024.217076. [DOI] [PubMed] [Google Scholar]
- 30.Wu W, Zhao S. Metabolic changes in cancer: beyond the Warburg effect. Acta Biochim Biophys Sin (Shanghai). 2013;45(1):18–26. 10.1093/abbs/gms104. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal RK, Luchtel RA, Machha V, Tischer A, Zou Y, Pradhan K et al. Functional succinate dehydrogenase deficiency is a common adverse feature of clear cell renal cancer. Proc Natl Acad Sci U S A. 2021;118(39). 10.1073/pnas.2106947118. [DOI] [PMC free article] [PubMed]
- 32.Li X, Xie L, Zhou L, Gan Y, Han S, Zhou Y, et al. Bergenin inhibits tumor growth and overcomes radioresistance by targeting aerobic glycolysis. Am J Chin Med. 2023;51(7):1905–25. 10.1142/s0192415x23500842. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem. 1986;261(29):13807–12. [PubMed] [Google Scholar]
- 34.Amin S, Yang P, Li Z. Pyruvate kinase M2: a multifarious enzyme in non-canonical localization to promote cancer progression. Biochim Biophys Acta Rev Cancer. 2019;1871(2):331–41. 10.1016/j.bbcan.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Shen J, Jin Z, Lv H, Jin K, Jonas K, Zhu C, et al. PFKP is highly expressed in lung cancer and regulates glucose metabolism. Cell Oncol (Dordr). 2020;43(4):617–29. 10.1007/s13402-020-00508-6. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, Xu F, Yang Z, Cao J, Hu L, She Y. The mechanism of PFK-1 in the occurrence and development of bladder cancer by regulating ZEB1 lactylation. BMC Urol. 2024;24(1):59. 10.1186/s12894-024-01444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comandatore A, Franczak M, Smolenski RT, Morelli L, Peters GJ, Giovannetti E. Lactate Dehydrogenase and its clinical significance in pancreatic and thoracic cancers. Semin Cancer Biol. 2022;86(Pt 2):93–100. 10.1016/j.semcancer.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Verma S, Budhu S, Serganova I, Dong L, Mangarin LM, Khan JF et al. Pharmacologic LDH inhibition redirects intratumoral glucose uptake and improves antitumor immunity in solid tumor models. J Clin Invest. 2024;134(17). 10.1172/jci177606. [DOI] [PMC free article] [PubMed]
- 39.Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8(1):51–6. 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- 40.Lei J, Zhou Z, Fang J, Sun Z, He M, He B, et al. Aspirin induces immunogenic cell death and enhances cancer immunotherapy in colorectal cancer. Int Immunopharmacol. 2023;121:110350. 10.1016/j.intimp.2023.110350. [DOI] [PubMed] [Google Scholar]
- 41.Jiang YX, Siu MKY, Wang JJ, Leung THY, Chan DW, Cheung ANY, et al. PFKFB3 regulates chemoresistance, metastasis and stemness via IAP proteins and the NF-κB signaling pathway in ovarian cancer. Front Oncol. 2022;12:748403. 10.3389/fonc.2022.748403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgos M, Gil-Iturbe E, Idoate-Bayón A, Castilla-Madrigal R, Moreno-Aliaga MJ, Lostao MP. The glucose transporter GLUT12, a new actor in obesity and cancer. J Physiol Biochem. 2024. 10.1007/s13105-024-01028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matés JM, Campos-Sandoval JA, Santos-Jiménez JL, Márquez J. Dysregulation of glutaminase and glutamine synthetase in cancer. Cancer Lett. 2019;467:29–39. 10.1016/j.canlet.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Scheurlen KM, Billeter AT, O’Brien SJ, Galandiuk S. Metabolic dysfunction and early-onset colorectal cancer - how macrophages build the bridge. Cancer Med. 2020;9(18):6679–93. 10.1002/cam4.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchetti P, Fovez Q, Germain N, Khamari R, Kluza J. Mitochondrial spare respiratory capacity: mechanisms, regulation, and significance in non-transformed and cancer cells. Faseb J. 2020;34(10):13106–24. 10.1096/fj.202000767R. [DOI] [PubMed] [Google Scholar]
- 46.Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med. 2019;44(1):3–15. 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molinié T, Cougouilles E, David C, Cahoreau E, Portais JC, Mourier A. MDH2 produced OAA is a metabolic switch rewiring the fuelling of respiratory chain and TCA cycle. Biochim Biophys Acta Bioenerg. 2022;1863(3):148532. 10.1016/j.bbabio.2022.148532. [DOI] [PubMed] [Google Scholar]
- 48.Agostini M, Mancini M, Candi E. Long non-coding RNAs affecting cell metabolism in cancer. Biol Direct. 2022;17(1):26. 10.1186/s13062-022-00341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan Y, Li S, Su L, Tang X, Chen X, Gu X, et al. Mitochondrial inhibitors: a new horizon in breast cancer therapy. Front Pharmacol. 2024;15:1421905. 10.3389/fphar.2024.1421905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiu X, Li Y, Zhang Z. Crosstalk between oxidative phosphorylation and immune escape in cancer: a new concept of therapeutic targets selection. Cell Oncol (Dordr). 2023;46(4):847–65. 10.1007/s13402-023-00801-0. [DOI] [PubMed] [Google Scholar]
- 51.Kuramoto K, Yamamoto M, Suzuki S, Sanomachi T, Togashi K, Seino S, et al. Verteporfin inhibits oxidative phosphorylation and induces cell death specifically in glioma stem cells. Febs J. 2020;287(10):2023–36. 10.1111/febs.15187. [DOI] [PubMed] [Google Scholar]
- 52.Raggi C, Taddei ML, Sacco E, Navari N, Correnti M, Piombanti B, et al. Mitochondrial oxidative metabolism contributes to a cancer stem cell phenotype in cholangiocarcinoma. J Hepatol. 2021;74(6):1373–85. 10.1016/j.jhep.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 53.Ripoll C, Roldan M, Ruedas-Rama MJ, Orte A, Martin M. Breast cancer cell subtypes display different metabolic phenotypes that correlate with their clinical classification. Biology (Basel). 2021;10(12). 10.3390/biology10121267. [DOI] [PMC free article] [PubMed]
- 54.Evans KW, Yuca E, Scott SS, Zhao M, Paez Arango N, Cruz Pico CX, et al. Oxidative phosphorylation is a metabolic vulnerability in chemotherapy-resistant triple-negative breast cancer. Cancer Res. 2021;81(21):5572–81. 10.1158/0008-5472.Can-20-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu Y, Xu W, Zeng H, He Z, Lu X, Zuo D, et al. OXPHOS-dependent metabolic reprogramming prompts metastatic potential of breast cancer cells under osteogenic differentiation. Br J Cancer. 2020;123(11):1644–55. 10.1038/s41416-020-01040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pacheco-Velázquez SC, Ortega M II, Vargas-Navarro JL, Padilla-Flores JA, Robledo-Cadena DX, Tapia-Martínez G, et al. 17-β Estradiol up-regulates energy metabolic pathways, cellular proliferation and tumor invasiveness in ER+ breast cancer spheroids. Front Oncol. 2022;12:1018137. 10.3389/fonc.2022.1018137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai L, Sun Y, Wang K, Guan W, Yue J, Li J, et al. The better survival of msi subtype is associated with the oxidative stress related pathways in gastric cancer. Front Oncol. 2020;10:1269. 10.3389/fonc.2020.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, Ren B, Ren J, Gu M, You L, Zhao Y. The significant role of amino acid metabolic reprogramming in cancer. Cell Commun Signal. 2024;22(1):380. 10.1186/s12964-024-01760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fidelito G, De Souza DP, Niranjan B, De Nardo W, Keerthikumar S, Brown K, et al. Multi-substrate metabolic tracing reveals marked heterogeneity and dependency on fatty acid metabolism in human prostate cancer. Mol Cancer Res. 2023;21(4):359–73. 10.1158/1541-7786.Mcr-22-0796. [DOI] [PubMed] [Google Scholar]
- 60.Sun J, Lin Z, Liao Z, Wu Z, Li H, Wang H. Small extracellular vesicles derived from human adipose-derived stem cells regulate energetic metabolism through the activation of YAP/TAZ pathway facilitating angiogenesis. Cell Biol Int. 2023;47(2):451–66. 10.1002/cbin.11938. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Liu S, Tomar A, Yen FS, Unlu G, Ropek N, et al. Autoregulatory control of mitochondrial glutathione homeostasis. Science. 2023;382(6672):820–8. 10.1126/science.adf4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo L, Zhang B, Zhang W, Xie Y, Chen X, Sun X et al. Inhibition of Carbohydrate Metabolism Potentiated by the Therapeutic Effects of Oxidative Phosphorylation Inhibitors in Colon Cancer Cells. Cancers (Basel). 2024;16(7). 10.3390/cancers16071399. [DOI] [PMC free article] [PubMed]
- 63.Wang Y, Patti GJ. The Warburg effect: a signature of mitochondrial overload. Trends Cell Biol. 2023;33(12):1014–20. 10.1016/j.tcb.2023.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greene J, Segaran A, Lord S. Targeting OXPHOS and the electron transport chain in cancer; Molecular and therapeutic implications. Semin Cancer Biol. 2022;86(Pt 2):851–9. 10.1016/j.semcancer.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Vaupel P, Schmidberger H, Mayer A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95(7):912–9. 10.1080/09553002.2019.1589653. [DOI] [PubMed] [Google Scholar]
- 66.Zhao L, Yu N, Zhai Y, Yang Y, Wang Y, Yang Y, et al. The ubiquitin-like protein UBTD1 promotes colorectal cancer progression by stabilizing c-Myc to upregulate glycolysis. Cell Death Dis. 2024;15(7):502. 10.1038/s41419-024-06890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763–77. 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 68.Mukherjee A, Wu J, Barbour S, Fang X. Lysophosphatidic acid activates lipogenic pathways and de novo lipid synthesis in ovarian cancer cells. J Biol Chem. 2012;287(30):24990–5000. 10.1074/jbc.M112.340083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 2012;72(15):3709–14. 10.1158/0008-5472.Can-11-4112. [DOI] [PubMed] [Google Scholar]
- 70.Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8(4):311–21. 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 71.Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64(6):2070–5. 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- 72.Guillaumond F, Leca J, Olivares O, Lavaut MN, Vidal N, Berthezène P, et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci U S A. 2013;110(10):3919–24. 10.1073/pnas.1219555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karunakaran S, Ramachandran S, Coothankandaswamy V, Elangovan S, Babu E, Periyasamy-Thandavan S, et al. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J Biol Chem. 2011;286(36):31830–8. 10.1074/jbc.M111.229518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liang C, Qin Y, Zhang B, Ji S, Shi S, Xu W, et al. Metabolic plasticity in heterogeneous pancreatic ductal adenocarcinoma. Biochim Biophys Acta. 2016;1866(2):177–88. 10.1016/j.bbcan.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Xu T, Junge JA, Delfarah A, Lu YT, Arnesano C, Iqbal M, et al. Bladder cancer cells shift rapidly and spontaneously to cisplatin-resistant oxidative phosphorylation that is trackable in real time. Sci Rep. 2022;12(1):5518. 10.1038/s41598-022-09438-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bryant KL, Mancias JD, Kimmelman AC, Der CJ. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci. 2014;39(2):91–100. 10.1016/j.tibs.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uddin MH, Zhang D, Muqbil I, El-Rayes BF, Chen H, Philip PA, et al. Deciphering cellular plasticity in pancreatic cancer for effective treatments. Cancer Metastasis Rev. 2024;43(1):393–408. 10.1007/s10555-023-10164-5. [DOI] [PubMed] [Google Scholar]
- 78.Liang L, He H, Jiang S, Liu Y, Huang J, Sun X et al. TIAM2 Contributes to Osimertinib Resistance, Cell Motility, and Tumor-Associated Macrophage M2-like Polarization in Lung Adenocarcinoma. Int J Mol Sci. 2022;23(18). 10.3390/ijms231810415. [DOI] [PMC free article] [PubMed]
- 79.Guo W, Ma J, Yang Y, Guo S, Zhang W, Zhao T, et al. ATP-Citrate lyase epigenetically potentiates oxidative phosphorylation to promote melanoma growth and adaptive resistance to MAPK inhibition. Clin Cancer Res. 2020;26(11):2725–39. 10.1158/1078-0432.Ccr-19-1359. [DOI] [PubMed] [Google Scholar]
- 80.Heydari Z, Moeinvaziri F, Mirazimi SMA, Dashti F, Smirnova O, Shpichka A, et al. Alteration in DNA methylation patterns: Epigenetic signatures in gastrointestinal cancers. Eur J Pharmacol. 2024;973:176563. 10.1016/j.ejphar.2024.176563. [DOI] [PubMed] [Google Scholar]
- 81.Yang K, Liang X, Wen K. Long non‑coding RNAs interact with RNA‑binding proteins to regulate genomic instability in cancer cells (Review). Oncol Rep. 2022;48(4). 10.3892/or.2022.8390. [DOI] [PMC free article] [PubMed]
- 82.Han H, Feng F, Li H. Research advances on epigenetics and cancer metabolism. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021;50(1):1–16. 10.3724/zdxbyxb-2021-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yue SW, Liu HL, Su HF, Luo C, Liang HF, Zhang BX, et al. m6A-regulated tumor glycolysis: new advances in epigenetics and metabolism. Mol Cancer. 2023;22(1):137. 10.1186/s12943-023-01841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu XS, Yang JW, Zeng J, Chen XQ, Gao Y, Kui XY, et al. SLC2A1 is a diagnostic biomarker involved in immune infiltration of colorectal cancer and associated with m6A modification and ceRNA. Front Cell Dev Biol. 2022;10:853596. 10.3389/fcell.2022.853596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma L, Xue X, Zhang X, Yu K, Xu X, Tian X, et al. The essential roles of m(6)A RNA modification to stimulate ENO1-dependent glycolysis and tumorigenesis in lung adenocarcinoma. J Exp Clin Cancer Res. 2022;41(1):36. 10.1186/s13046-021-02200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li F, He C, Yao H, Zhao Y, Ye X, Zhou S, et al. Glutamate from nerve cells promotes perineural invasion in pancreatic cancer by regulating tumor glycolysis through HK2 mRNA-m6A modification. Pharmacol Res. 2023;187:106555. 10.1016/j.phrs.2022.106555. [DOI] [PubMed] [Google Scholar]
- 87.Liu XS, Zhou LM, Yuan LL, Gao Y, Kui XY, Liu XY, et al. NPM1 is a prognostic biomarker involved in immune infiltration of lung adenocarcinoma and associated with m6A modification and glycolysis. Front Immunol. 2021;12:724741. 10.3389/fimmu.2021.724741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu H, Chen R, Kang F, Lai H, Wang Y. KCNQ1OT1 promotes ovarian cancer progression via modulating MIR-142-5p/CAPN10 axis. Mol Genet Genomic Med. 2020;8(2):e1077. 10.1002/mgg3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen C, Wei M, Wang C, Sun D, Liu P, Zhong X, et al. Long noncoding RNA KCNQ1OT1 promotes colorectal carcinogenesis by enhancing aerobic glycolysis via hexokinase-2. Aging (Albany NY). 2020;12(12):11685–97. 10.18632/aging.103334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huo N, Cong R, Sun ZJ, Li WC, Zhu X, Xue CY, et al. STAT3/LINC00671 axis regulates papillary thyroid tumor growth and metastasis via LDHA-mediated glycolysis. Cell Death Dis. 2021;12(9):799. 10.1038/s41419-021-04081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao L, Wang M, Dong Y, Xu B, Chen J, Ding Y, et al. Circular RNA circRNF20 promotes breast cancer tumorigenesis and Warburg effect through miR-487a/HIF-1α/HK2. Cell Death Dis. 2020;11(2):145. 10.1038/s41419-020-2336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mittal P, Roberts CWM. The SWI/SNF complex in cancer - biology, biomarkers and therapy. Nat Rev Clin Oncol. 2020;17(7):435–48. 10.1038/s41571-020-0357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W, Côté J, Xue Y, Zhou S, Khavari PA, Biggar SR, et al. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. Embo j. 1996;15(19):5370–82. [PMC free article] [PubMed] [Google Scholar]
- 94.Nickerson JA, Wu Q, Imbalzano AN. Mammalian SWI/SNF Enzymes and the Epigenetics of Tumor Cell Metabolic Reprogramming. Front Oncol. 2017;7:49. 10.3389/fonc.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu Q, Madany P, Dobson JR, Schnabl JM, Sharma S, Smith TC, et al. The BRG1 chromatin remodeling enzyme links cancer cell metabolism and proliferation. Oncotarget. 2016;7(25):38270–81. 10.18632/oncotarget.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dreier MR, Walia J, de la Serna IL. Targeting SWI/SNF complexes in cancer: pharmacological approaches and implications. Epigenomes. 2024;8(1). 10.3390/epigenomes8010007. [DOI] [PMC free article] [PubMed]
- 97.Malone HA, Roberts CWM. Chromatin remodellers as therapeutic targets. Nat Rev Drug Discov. 2024;23(9):661–81. 10.1038/s41573-024-00978-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang W. The SWI/SNF family of ATP-dependent chromatin remodelers: similar mechanisms for diverse functions. Curr Top Microbiol Immunol. 2003;274:143–69. 10.1007/978-3-642-55747-7_6. [DOI] [PubMed] [Google Scholar]
- 99.Hargreaves DC, Crabtree GR. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011;21(3):396–420. 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jones S, Wang TL, Shih Ie M, Mao TL, Nakayama K, Roden R, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330(6001):228–31. 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mao TL, Ardighieri L, Ayhan A, Kuo KT, Wu CH, Wang TL, et al. Loss of ARID1A expression correlates with stages of tumor progression in uterine endometrioid carcinoma. Am J Surg Pathol. 2013;37(9):1342–8. 10.1097/PAS.0b013e3182889dc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wiegand KC, Lee AF, Al-Agha OM, Chow C, Kalloger SE, Scott DW, et al. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol. 2011;224(3):328–33. 10.1002/path.2911. [DOI] [PubMed] [Google Scholar]
- 103.Takahashi K, Takenaka M, Okamoto A, Bowtell DDL, Kohno T. Treatment Strategies for ARID1A-Deficient Ovarian Clear Cell Carcinoma. Cancers (Basel). 2021;13(8). 10.3390/cancers13081769. [DOI] [PMC free article] [PubMed]
- 104.Phelan ML, Sif S, Narlikar GJ, Kingston RE. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol Cell. 1999;3(2):247–53. 10.1016/s1097-2765(00)80315-9. [DOI] [PubMed] [Google Scholar]
- 105.Kassabov SR, Zhang B, Persinger J, Bartholomew B. SWI/SNF unwraps, slides, and rewraps the nucleosome. Mol Cell. 2003;11(2):391–403. 10.1016/s1097-2765(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 106.Tang L, Nogales E, Ciferri C. Structure and function of SWI/SNF chromatin remodeling complexes and mechanistic implications for transcription. Prog Biophys Mol Biol. 2010;102(2–3):122–8. 10.1016/j.pbiomolbio.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009;78:273–304. 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- 108.Alpsoy A, Dykhuizen EC. Glioma tumor suppressor candidate region gene 1 (GLTSCR1) and its paralog GLTSCR1-like form SWI/SNF chromatin remodeling subcomplexes. J Biol Chem. 2018;293(11):3892–903. 10.1074/jbc.RA117.001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mashtalir N, D’Avino AR, Michel BC, Luo J, Pan J, Otto JE, et al. Modular organization and assembly of SWI/SNF family chromatin remodeling complexes. Cell. 2018;175(5):1272–88.e20. 10.1016/j.cell.2018.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen K, Yuan J, Sia Y, Chen Z. Mechanism of action of the SWI/SNF family complexes. Nucleus. 2023;14(1):2165604. 10.1080/19491034.2023.2165604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoadley KA, Yau C, Hinoue T, Wolf DM, Lazar AJ, Drill E, et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell. 2018;173(2):291–304.e6. 10.1016/j.cell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang Y, Jin YH, Li HL, Xin H, Chen JD, Li XY, et al. PBRM1 deficiency oncogenic addiction is associated with activated AKT-mTOR signalling and aerobic glycolysis in clear cell renal cell carcinoma cells. J Cell Mol Med. 2022;26(14):3837–49. 10.1111/jcmm.17418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stachowiak M, Szymanski M, Ornoch A, Jancewicz I, Rusetska N, Chrzan A, et al. SWI/SNF chromatin remodeling complex and glucose metabolism are deregulated in advanced bladder cancer. IUBMB Life. 2020;72(6):1175–88. 10.1002/iub.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi L, Zhao C, Pu H, Zhang Q. FBP1 expression is associated with basal-like breast carcinoma. Oncol Lett. 2017;13(5):3046–56. 10.3892/ol.2017.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23(3):316–31. 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xing T, Li L, Chen Y, Ju G, Li G, Zhu X, et al. Targeting the TCA cycle through cuproptosis confers synthetic lethality on ARID1A-deficient hepatocellular carcinoma. Cell Rep Med. 2023;4(11):101264. 10.1016/j.xcrm.2023.101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lavoie H, Gagnon J, Therrien M. ERK signalling: a master regulator of cell behaviour, life and fate. Nat Rev Mol Cell Biol. 2020;21(10):607–32. 10.1038/s41580-020-0255-7. [DOI] [PubMed] [Google Scholar]
- 118.Animireddy S, Kavadipula P, Kotapalli V, Gowrishankar S, Rao S, Bashyam MD. Aberrant cytoplasmic localization of ARID1B activates ERK signaling and promotes oncogenesis. J Cell Sci. 2021;134(4). 10.1242/jcs.251637. [DOI] [PubMed]
- 119.Inoue C, Zhao C, Tsuduki Y, Udono M, Wang L, Nomura M, et al. SMARCD1 regulates senescence-associated lipid accumulation in hepatocytes. NPJ Aging Mech Dis. 2017;3:11. 10.1038/s41514-017-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferguson LP, Gatchalian J, McDermott ML, Nakamura M, Chambers K, Rajbhandari N, et al. Smarcd3 is an epigenetic modulator of the metabolic landscape in pancreatic ductal adenocarcinoma. Nat Commun. 2023;14(1):292. 10.1038/s41467-023-35796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hou P, Kapoor A, Zhang Q, Li J, Wu CJ, Li J, et al. Tumor microenvironment remodeling enables bypass of oncogenic KRAS dependency in pancreatic cancer. Cancer Discov. 2020;10(7):1058–77. 10.1158/2159-8290.Cd-19-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiazhu F. The study on role and mechanism of ARID1A in hepatocellular carcinoma [Doctoral]. 2014. [Google Scholar]
- 123.Qu YL, Deng CH, Luo Q, Shang XY, Wu JX, Shi Y, et al. Arid1a regulates insulin sensitivity and lipid metabolism. EBioMedicine. 2019;42:481–93. 10.1016/j.ebiom.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu S, Fukumoto T, Lin J, Nacarelli T, Wang Y, Ong D, et al. Targeting glutamine dependence through GLS1 inhibition suppresses ARID1A-inactivated clear cell ovarian carcinoma. Nature Cancer. 2021;2(2):189–200. 10.1038/s43018-020-00160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu M, Zhang S, Zhang WX, et al. Progress in the regulation of energy metabolic homeostasis by the SWI/SNF complex subunit Baf60a. Chin J Biotech. 2021;37(02):500–12. 10.13345/j.cjb.200312. [DOI] [PubMed] [Google Scholar]
- 126.Curcio C, Brugiapaglia S, Bulfamante S, Follia L, Cappello P, Novelli F. The glycolytic pathway as a target for novel onco-immunology therapies in pancreatic cancer. Molecules. 2021;26(6). 10.3390/molecules26061642. [DOI] [PMC free article] [PubMed]
- 127.Cappello P, Rolla S, Chiarle R, Principe M, Cavallo F, Perconti G, et al. Vaccination with ENO1 DNA prolongs survival of genetically engineered mice with pancreatic cancer. Gastroenterology. 2013;144(5):1098–106. 10.1053/j.gastro.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 128.Allen MD, Freund SMV, Bycroft M, Zinzalla G. SWI/SNF subunit BAF155 N-terminus structure informs the impact of cancer-associated mutations and reveals a potential drug binding site. Commun Biol. 2021;4(1):528. 10.1038/s42003-021-02050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.El-Far AH, Al Jaouni SK, Li X, Fu J. Cancer metabolism control by natural products: pyruvate kinase M2 targeting therapeutics. Phytother Res. 2022;36(8):3181–201. 10.1002/ptr.7534. [DOI] [PubMed] [Google Scholar]
- 130.Yang Y, Gao Y, Xiong Y, Gong Y, Lu J, Zhang Y, et al. Research progress of warburg effect in hepatocellular carcinoma. Front Biosci (Landmark Ed). 2024;29(5):178. 10.31083/j.fbl2905178. [DOI] [PubMed] [Google Scholar]
- 131.Park W, Han JH, Wei S, Yang ES, Cheon SY, Bae SJ et al. Natural product-based glycolysis inhibitors as a therapeutic strategy for epidermal growth factor receptor-tyrosine kinase inhibitor-resistant non-small cell lung cancer. Int J Mol Sci. 2024;25(2). 10.3390/ijms25020807. [DOI] [PMC free article] [PubMed]
- 132.Obaid QA, Khudair KK, Al-Shammari AM. Glucose deprivation using 2-deoxyglucose and acarbose induce metabolic oxidative stress and apoptosis in female mice bearing breast cancer. Biochimie. 2022;195:59–66. 10.1016/j.biochi.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 133.Tyagi K, Mandal S, Roy A. Recent advancements in therapeutic targeting of the Warburg effect in refractory ovarian cancer: a promise towards disease remission. Biochim Biophys Acta Rev Cancer. 2021;1876(1):188563. 10.1016/j.bbcan.2021.188563. [DOI] [PubMed] [Google Scholar]
- 134.Škorja Milić N, Dolinar K, Miš K, Matkovič U, Bizjak M, Pavlin M et al. Suppression of pyruvate dehydrogenase kinase by dichloroacetate in cancer and skeletal muscle cells is isoform specific and partially independent of HIF-1α. Int J Mol Sci. 2021;22(16). 10.3390/ijms22168610. [DOI] [PMC free article] [PubMed]
- 135.Weiss JM. The promise and peril of targeting cell metabolism for cancer therapy. Cancer Immunol Immunother. 2020;69(2):255–61. 10.1007/s00262-019-02432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fatma H, Siddique HR. Cancer cell plasticity, stem cell factors, and therapy resistance: how are they linked? Cancer Metastasis Rev. 2024;43(1):423–40. 10.1007/s10555-023-10144-9. [DOI] [PubMed] [Google Scholar]
- 137.Huang Y, Sun G, Sun X, Li F, Zhao L, Zhong R et al. The potential of lonidamine in combination with chemotherapy and physical therapy in cancer treatment. Cancers (Basel). 2020;12(11). 10.3390/cancers12113332. [DOI] [PMC free article] [PubMed]
- 138.Jiang SH, Dong FY, Da LT, Yang XM, Wang XX, Weng JY, et al. Ikarugamycin inhibits pancreatic cancer cell glycolysis by targeting hexokinase 2. Faseb J. 2020;34(3):3943–55. 10.1096/fj.201901237R. [DOI] [PubMed] [Google Scholar]
- 139.Zhou Q, Li J, Pang J, Fan F, Li S, Liu H. Gefitinib inhibits glycolysis and induces programmed cell death in non-small cell lung cancer cells. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(6):884–92. 10.12122/j.issn.1673-4254.2020.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Song G, Fang J, Shang C, Li Y, Zhu Y, Xiu Z, et al. Ad-apoptin inhibits glycolysis, migration and invasion in lung cancer cells targeting AMPK/mTOR signaling pathway. Exp Cell Res. 2021;409(2):112926. 10.1016/j.yexcr.2021.112926. [DOI] [PubMed] [Google Scholar]
- 141.Jia KG, Feng G, Tong YS, Tao GZ, Xu L. miR-206 regulates non-small-cell lung cancer cell aerobic glycolysis by targeting hexokinase 2. J Biochem. 2020;167(4):365–70. 10.1093/jb/mvz099. [DOI] [PubMed] [Google Scholar]
- 142.Li L, Liu H, Du L, Xi P, Wang Q, Li Y, et al. miR-449a suppresses LDHA-mediated glycolysis to enhance the sensitivity of non-small cell lung cancer cells to ionizing radiation. Oncol Res. 2018;26(4):547–56. 10.3727/096504017x15016337254605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arora S, Singh P, Tabassum G, Dohare R, Syed MA. miR-16-5p regulates aerobic glycolysis and tumorigenesis of NSCLC cells via LDH-A/lactate/NF-κB signaling. Life Sci. 2022;304:120722. 10.1016/j.lfs.2022.120722. [DOI] [PubMed] [Google Scholar]
- 144.Lu J, Wang L, Chen W, Wang Y, Zhen S, Chen H, et al. miR-603 targeted hexokinase-2 to inhibit the malignancy of ovarian cancer cells. Arch Biochem Biophys. 2019;661:1–9. 10.1016/j.abb.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 145.Teng Y, Zhang Y, Qu K, Yang X, Fu J, Chen W, et al. MicroRNA-29B (mir-29b) regulates the Warburg effect in ovarian cancer by targeting AKT2 and AKT3. Oncotarget. 2015;6(38):40799–814. 10.18632/oncotarget.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dai Y, Liu Y, Li J, Jin M, Yang H, Huang G. Shikonin inhibited glycolysis and sensitized cisplatin treatment in non-small cell lung cancer cells via the exosomal pyruvate kinase M2 pathway. Bioengineered. 2022;13(5):13906–18. 10.1080/21655979.2022.2086378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Heydarzadeh S, Moshtaghie AA, Daneshpour M, Hedayati M. The effect of Apigenin on glycometabolism and cell death in an anaplastic thyroid cancer cell line. Toxicol Appl Pharmacol. 2023;475:116626. 10.1016/j.taap.2023.116626. [DOI] [PubMed] [Google Scholar]
- 148.Xu D, Jin J, Yu H, Zhao Z, Ma D, Zhang C, et al. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J Exp Clin Cancer Res. 2017;36(1):44. 10.1186/s13046-017-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hong Z, Lu Y, Liu B, Ran C, Lei X, Wang M, et al. Glycolysis, a new mechanism of oleuropein against liver tumor. Phytomedicine. 2023;114:154770. 10.1016/j.phymed.2023.154770. [DOI] [PubMed] [Google Scholar]
- 150.Wang K, Li Q, Fan Y, Fang P, Zhou H, Huang J. OBHS drives abnormal glycometabolis reprogramming via GLUT1 in breast cancer. Int J Mol Sci. 2023;24(8). 10.3390/ijms24087136. [DOI] [PMC free article] [PubMed]
- 151.Yen K, Travins J, Wang F, David MD, Artin E, Straley K, et al. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 2017;7(5):478–93. 10.1158/2159-8290.Cd-16-1034. [DOI] [PubMed] [Google Scholar]
- 152.Medeiros BC, Fathi AT, DiNardo CD, Pollyea DA, Chan SM, Swords R. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia. 2017;31(2):272–81. 10.1038/leu.2016.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pardee TS, Lee K, Luddy J, Maturo C, Rodriguez R, Isom S, et al. A phase I study of the first-in-class antimitochondrial metabolism agent, CPI-613, in patients with advanced hematologic malignancies. Clin Cancer Res. 2014;20(20):5255–64. 10.1158/1078-0432.Ccr-14-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lycan TW, Pardee TS, Petty WJ, Bonomi M, Alistar A, Lamar ZS, et al. A phase II clinical trial of CPI-613 in patients with relapsed or refractory small cell lung carcinoma. PLoS One. 2016;11(10):e0164244. 10.1371/journal.pone.0164244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.De A, Kuppusamy G. Metformin in breast cancer: preclinical and clinical evidence. Curr Probl Cancer. 2020;44(1):100488. 10.1016/j.currproblcancer.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 156.Kamarudin MNA, Sarker MMR, Zhou JR, Parhar I. Metformin in colorectal cancer: molecular mechanism, preclinical and clinical aspects. J Exp Clin Cancer Res. 2019;38(1):491. 10.1186/s13046-019-1495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Molina JR, Sun Y, Protopopova M, Gera S, Bandi M, Bristow C, et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med. 2018;24(7):1036–46. 10.1038/s41591-018-0052-4. [DOI] [PubMed] [Google Scholar]
- 158.Gupta N, Srivastava SK. Atovaquone: an antiprotozoal drug suppresses primary and resistant breast tumor growth by inhibiting HER2/β-catenin signaling. Mol Cancer Ther. 2019;18(10):1708–20. 10.1158/1535-7163.Mct-18-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res. 2018;24(11):2482–90. 10.1158/1078-0432.Ccr-17-3070. [DOI] [PubMed] [Google Scholar]
- 160.Tian S, Chen H, Tan W. Targeting mitochondrial respiration as a therapeutic strategy for cervical cancer. Biochem Biophys Res Commun. 2018;499(4):1019–24. 10.1016/j.bbrc.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 161.Shang R, Liao Y, Zheng X. Inhibition of Wnt signaling by atovaquone inhibits gastric cancer and enhances chemotherapy effectiveness through activation of casein kinase 1α. Nutr Cancer. 2024;76(5):452–62. 10.1080/01635581.2024.2328377. [DOI] [PubMed] [Google Scholar]
- 162.Baskaran R, Lee J, Yang SG. Clinical development of photodynamic agents and therapeutic applications. Biomater Res. 2018;22:25. 10.1186/s40824-018-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kalyanaraman B, Cheng G, Hardy M, You M. OXPHOS-targeting drugs in oncology: new perspectives. Expert Opin Ther Targets. 2023;27(10):939–52. 10.1080/14728222.2023.2261631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sawyer BT, Qamar L, Yamamoto TM, McMellen A, Watson ZL, Richer JK, et al. Targeting fatty acid oxidation to promote anoikis and inhibit ovarian cancer progression. Mol Cancer Res. 2020;18(7):1088–98. 10.1158/1541-7786.Mcr-19-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Divakaruni AS, Hsieh WY, Minarrieta L, Duong TN, Kim KKO, Desousa BR, et al. Etomoxir inhibits macrophage polarization by disrupting CoA homeostasis. Cell Metab. 2018;28(3):490–503.e7. 10.1016/j.cmet.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Raud B, Roy DG, Divakaruni AS, Tarasenko TN, Franke R, Ma EH, et al. Etomoxir actions on regulatory and memory T cells are independent of Cpt1a-mediated fatty acid oxidation. Cell Metab. 2018;28(3):504–15.e7. 10.1016/j.cmet.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ma Y, Wang W, Devarakonda T, Zhou H, Wang XY, Salloum FN, et al. Functional analysis of molecular and pharmacological modulators of mitochondrial fatty acid oxidation. Sci Rep. 2020;10(1):1450. 10.1038/s41598-020-58334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu X, Meng Y, Li L, Xu P, Wang J, Li Z, et al. Overview of the development of glutaminase inhibitors: achievements and future directions. J Med Chem. 2019;62(3):1096–115. 10.1021/acs.jmedchem.8b00961. [DOI] [PubMed] [Google Scholar]
- 169.Jin L, Li D, Alesi GN, Fan J, Kang HB, Lu Z, et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell. 2015;27(2):257–70. 10.1016/j.ccell.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ma Y, Wang L, Jia R. The role of mitochondrial dynamics in human cancers. Am J Cancer Res. 2020;10(5):1278–93. [PMC free article] [PubMed] [Google Scholar]
- 171.Genovese I, Carinci M, Modesti L, Aguiari G, Pinton P, Giorgi C. Mitochondria: insights into crucial features to overcome cancer chemoresistance. Int J Mol Sci. 2021;22(9). 10.3390/ijms22094770. [DOI] [PMC free article] [PubMed]
- 172.Dai W, Wang G, Chwa J, Oh ME, Abeywardana T, Yang Y, et al. Mitochondrial division inhibitor (mdivi-1) decreases oxidative metabolism in cancer. Br J Cancer. 2020;122(9):1288–97. 10.1038/s41416-020-0778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Qin Y, Yu Y, Yang C, Wang Z, Yang Y, Wang C, et al. Atractylenolide I inhibits NLRP3 inflammasome activation in colitis-associated colorectal cancer via suppressing Drp1-mediated mitochondrial fission. Front Pharmacol. 2021;12:674340. 10.3389/fphar.2021.674340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rossi T, Iorio E, Chirico M, Pisanu ME, Amodio N, Cantafio MEG et al. BET inhibitors (BETi) influence oxidative phosphorylation metabolism by affecting mitochondrial dynamics leading to alterations in apoptotic pathways in triple-negative breast cancer (TNBC) cells. Cell Prolif. 2024:e13730. 10.1111/cpr.13730. [DOI] [PMC free article] [PubMed]
- 175.Mubthasima PP, Singh SA, Kannan A. Sesamol-mediated targeting of EPHA2 sensitises cervical cancer for cisplatin treatment by regulating mitochondrial dynamics, autophagy, and mitophagy. Mol Biol Rep. 2024;51(1):949. 10.1007/s11033-024-09875-x. [DOI] [PubMed] [Google Scholar]
- 176.Baumgartner V, Schaer D, Moch H, Salemi S, Eberli D. Mitochondrial Elongation and ROS-mediated apoptosis in prostate cancer cells under therapy with apalutamide and complex I inhibitor. Int J Mol Sci. 2024;25(13). 10.3390/ijms25136939. [DOI] [PMC free article] [PubMed]
- 177.Wu Z, Xiao C, Li F, Huang W, You F, Li X. Mitochondrial fusion-fission dynamics and its involvement in colorectal cancer. Mol Oncol. 2024;18(5):1058–75. 10.1002/1878-0261.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Du D, Liu C, Qin M, Zhang X, Xi T, Yuan S, et al. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm Sin B. 2022;12(2):558–80. 10.1016/j.apsb.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Torcasio R, Gallo Cantafio ME, Ikeda RK, Ganino L, Viglietto G, Amodio N. Lipid metabolic vulnerabilities of multiple myeloma. Clin Exp Med. 2023;23(7):3373–90. 10.1007/s10238-023-01174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xiao Y, Hu B, Guo Y, Zhang D, Zhao Y, Chen Y, et al. Targeting glutamine metabolism as an attractive therapeutic strategy for acute myeloid leukemia. Curr Treat Options Oncol. 2023;24(8):1021–35. 10.1007/s11864-023-01104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gremke N, Polo P, Dort A, Schneikert J, Elmshäuser S, Brehm C, et al. mTOR-mediated cancer drug resistance suppresses autophagy and generates a druggable metabolic vulnerability. Nat Commun. 2020;11(1):4684. 10.1038/s41467-020-18504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shima T, Taniguchi K, Inomata Y, Arima J, Lee SW. Glycolysis in gastrointestinal stromal tumor: a brief overview. Neoplasia. 2024;55:101022. 10.1016/j.neo.2024.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chaudhry S, Thomas SN, Simmons GE Jr. Targeting lipid metabolism in the treatment of ovarian cancer. Oncotarget. 2022;13:768–83. 10.18632/oncotarget.28241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yu Y, Nie Q, Wang Z, Di Y, Chen X, Ren K. Targeting acetyl-CoA carboxylase 1 for cancer therapy. Front Pharmacol. 2023;14:1129010. 10.3389/fphar.2023.1129010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135(7):463–71. 10.1182/blood.2019002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Okazaki A, Gameiro PA, Christodoulou D, Laviollette L, Schneider M, Chaves F, et al. Glutaminase and poly(ADP-ribose) polymerase inhibitors suppress pyrimidine synthesis and VHL-deficient renal cancers. J Clin Invest. 2017;127(5):1631–45. 10.1172/jci87800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Momcilovic M, Bailey ST, Lee JT, Fishbein MC, Magyar C, Braas D, et al. Targeted inhibition of EGFR and glutaminase induces metabolic crisis in EGFR mutant lung cancer. Cell Rep. 2017;18(3):601–10. 10.1016/j.celrep.2016.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Reckzeh ES, Karageorgis G, Schwalfenberg M, Ceballos J, Nowacki J, Stroet MCM, et al. Inhibition of glucose transporters and glutaminase synergistically impairs tumor cell growth. Cell Chem Biol. 2019;26(9):1214–28.e25. 10.1016/j.chembiol.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 189.Pashaei-Asl F, Pashaei-Asl R, Khodadadi K, Akbarzadeh A, Ebrahimie E, Pashaiasl M. Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artif Cells Nanomed Biotechnol. 2018;46(7):1483–7. 10.1080/21691401.2017.1374281. [DOI] [PubMed] [Google Scholar]
- 190.Hatipoglu A, Menon D, Levy T, Frias MA, Foster DA. Inhibiting glutamine utilization creates a synthetic lethality for suppression of ATP citrate lyase in KRas-driven cancer cells. PLoS One. 2022;17(10):e0276579. 10.1371/journal.pone.0276579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhu X, Fu Z, Chen SY, Ong D, Aceto G, Ho R, et al. Alanine supplementation exploits glutamine dependency induced by SMARCA4/2-loss. Nat Commun. 2023;14(1):2894. 10.1038/s41467-023-38594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lucantoni F, Dussmann H, Prehn JHM. Metabolic targeting of breast cancer cells with the 2-deoxy-d-glucose and the mitochondrial bioenergetics inhibitor MDIVI-1. Front Cell Dev Biol. 2018;6:113. 10.3389/fcell.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Raninga PV, Lee A, Sinha D, Dong LF, Datta KK, Lu X, et al. Marizomib suppresses triple-negative breast cancer via proteasome and oxidative phosphorylation inhibition. Theranostics. 2020;10(12):5259–75. 10.7150/thno.42705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Radwan AM, Abosharaf HA, Sharaky M, Abdelmonem R, Effat H. Functional combination of resveratrol and tamoxifen to overcome tamoxifen-resistance in breast cancer cells. Arch Pharm (Weinheim). 2024:e2400261. 10.1002/ardp.202400261. [DOI] [PubMed]
- 195.Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366(6468):1013–21. 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Siska PJ, Rathmell JC. T cell metabolic fitness in antitumor immunity. Trends Immunol. 2015;36(4):257–64. 10.1016/j.it.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–63. 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhu L, Zhao Q, Yang T, Ding W, Zhao Y. Cellular metabolism and macrophage functional polarization. Int Rev Immunol. 2015;34(1):82–100. 10.3109/08830185.2014.969421. [DOI] [PubMed] [Google Scholar]
- 199.Ippolito L, Morandi A, Giannoni E, Chiarugi P. Lactate: a metabolic driver in the tumour landscape. Trends Biochem Sci. 2019;44(2):153–66. 10.1016/j.tibs.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 200.Soll D, Chu CF, Sun S, Lutz V, Arunkumar M, Gachechiladze M, et al. Sodium chloride in the tumor microenvironment enhances T cell metabolic fitness and cytotoxicity. Nat Immunol. 2024. 10.1038/s41590-024-01918-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wei F, Wang D, Wei J, Tang N, Tang L, Xiong F, et al. Metabolic crosstalk in the tumor microenvironment regulates antitumor immunosuppression and immunotherapy resisitance. Cell Mol Life Sci. 2021;78(1):173–93. 10.1007/s00018-020-03581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li H, Bullock K, Gurjao C, Braun D, Shukla SA, Bossé D, et al. Metabolomic adaptations and correlates of survival to immune checkpoint blockade. Nat Commun. 2019;10(1):4346. 10.1038/s41467-019-12361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mullard A. IDO takes a blow. Nat Rev Drug Discov. 2018;17(5):307. 10.1038/nrd.2018.67. [DOI] [PubMed] [Google Scholar]
- 204.Johnson TS, MacDonald TJ, Pacholczyk R, Aguilera D, Al-Basheer A, Bajaj M, et al. Indoximod-based chemo-immunotherapy for pediatric brain tumors: a first-in-children phase I trial. Neuro Oncol. 2024;26(2):348–61. 10.1093/neuonc/noad174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kesarwani P, Kant S, Zhao Y, Prabhu A, Buelow KL, Miller CR, et al. Quinolinate promotes macrophage-induced immune tolerance in glioblastoma through the NMDAR/PPARγ signaling axis. Nat Commun. 2023;14(1):1459. 10.1038/s41467-023-37170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531(7596):651–5. 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bordon Y. T cell flexibility points to a metabolic checkpoint for cancer therapy. Nat Rev Immunol. 2020;20(1):2–3. 10.1038/s41577-019-0256-y. [DOI] [PubMed] [Google Scholar]
- 208.Zheng M, Xu H, Huang Y, Sun J, Zhang H, Lv Z, et al. Hypoxia-activated glutamine antagonist prodrug combined with combretastatin A4 nanoparticles for tumor-selective metabolic blockade. J Control Release. 2024;365:480–90. 10.1016/j.jconrel.2023.11.054. [DOI] [PubMed] [Google Scholar]
- 209.Lu C, Yang D, Klement JD, Colson YL, Oberlies NH, Pearce CJ et al. G6PD functions as a metabolic checkpoint to regulate granzyme B expression in tumor-specific cytotoxic T lymphocytes. J Immunother Cancer. 2022;10(1). 10.1136/jitc-2021-003543. [DOI] [PMC free article] [PubMed]
- 210.Daneshmandi S, Cassel T, Higashi RM, Fan TW, Seth P. 6-Phosphogluconate dehydrogenase (6PGD), a key checkpoint in reprogramming of regulatory T cells metabolism and function. Elife. 2021;10. 10.7554/eLife.67476. [DOI] [PMC free article] [PubMed]
- 211.He T, Xiao L, Qiao Y, Klingbeil O, Young E, Wu XS, et al. Targeting the mSWI/SNF complex in POU2F-POU2AF transcription factor-driven malignancies. Cancer Cell. 2024;42(8):1336–51.e9. 10.1016/j.ccell.2024.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Han X, Luo R, Wang L, Zhang L, Wang T, Zhao Y, et al. Potential predictive value of serum targeted metabolites and concurrently mutated genes for EGFR-TKI therapeutic efficacy in lung adenocarcinoma patients with EGFR sensitizing mutations. Am J Cancer Res. 2020;10(12):4266–86. [PMC free article] [PubMed] [Google Scholar]
- 213.Kubik J, Humeniuk E, Adamczuk G, Madej-Czerwonka B, Korga-Plewko A. Targeting energy metabolism in cancer treatment. Int J Mol Sci. 2022;23(10). 10.3390/ijms23105572. [DOI] [PMC free article] [PubMed]
- 214.Chen Q, Yang Z, Liu H, Man J, Oladejo AO, Ibrahim S et al. novel drug delivery systems: an important direction for drug innovation research and development. Pharmaceutics. 2024;16(5). 10.3390/pharmaceutics16050674. [DOI] [PMC free article] [PubMed]
- 215.Xie Z, Zhou Z, Yang S, Zhang S, Shao B. Epigenetic regulation and therapeutic targets in the tumor microenvironment. Mol Biomed. 2023;4(1):17. 10.1186/s43556-023-00126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Smith LK, Parmenter T, Kleinschmidt M, Kusnadi EP, Kang J, Martin CA, et al. Adaptive translational reprogramming of metabolism limits the response to targeted therapy in BRAF(V600) melanoma. Nat Commun. 2022;13(1):1100. 10.1038/s41467-022-28705-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bacci M, Lorito N, Smiriglia A, Morandi A. Fat and furious: lipid metabolism in antitumoral therapy response and resistance. Trends Cancer. 2021;7(3):198–213. 10.1016/j.trecan.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 218.Wang Z, Wang Y, Li Z, Xue W, Hu S, Kong X. Lipid metabolism as a target for cancer drug resistance: progress and prospects. Front Pharmacol. 2023;14:1274335. 10.3389/fphar.2023.1274335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhou W, Wahl DR. Purine metabolism promotes radioresistance and is a therapeutic target in glioblastoma. Mol Cell Oncol. 2020;7(6):1834902. 10.1080/23723556.2020.1834902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Giarrizzo M, LaComb JF, Patel HR, Reddy RG, Haley JD, Graves LM, et al. TR-107, an agonist of caseinolytic peptidase proteolytic subunit, disrupts mitochondrial metabolism and inhibits the growth of human colorectal cancer cells. Mol Cancer Ther. 2024. 10.1158/1535-7163.Mct-24-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Erb HHH, Polishchuk N, Stasyk O, Kahya U, Weigel MM, Dubrovska A. Glutamine metabolism and prostate cancer. Cancers (Basel). 2024;16(16). 10.3390/cancers16162871. [DOI] [PMC free article] [PubMed]
- 222.Liu Y, Ge X, Pang J, Zhang Y, Zhang H, Wu H, et al. Restricting glutamine uptake enhances NSCLC sensitivity to third-generation EGFR-TKI Almonertinib. Front Pharmacol. 2021;12:671328. 10.3389/fphar.2021.671328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Feng WW, Wilkins O, Bang S, Ung M, Li J, An J, et al. CD36-mediated metabolic rewiring of breast cancer cells promotes resistance to HER2-targeted therapies. Cell Rep. 2019;29(11):3405–20.e5. 10.1016/j.celrep.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ma G, Li C, Zhang Z, Liang Y, Liang Z, Chen Y, et al. Targeted glucose or glutamine metabolic therapy combined with PD-1/PD-L1 checkpoint blockade immunotherapy for the treatment of tumors - mechanisms and strategies. Front Oncol. 2021;11:697894. 10.3389/fonc.2021.697894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liao L, Xu H, Zhao Y, Zheng X. Metabolic interventions combined with CTLA-4 and PD-1/PD-L1 blockade for the treatment of tumors: mechanisms and strategies. Front Med. 2023;17(5):805–22. 10.1007/s11684-023-1025-7. [DOI] [PubMed] [Google Scholar]
- 226.Lin C, Chen H, Han R, Li L, Lu C, Hao S, et al. Hexokinases II-mediated glycolysis governs susceptibility to crizotinib in ALK-positive non-small cell lung cancer. Thorac Cancer. 2021;12(23):3184–93. 10.1111/1759-7714.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pucci G, Minafra L, Bravatà V, Calvaruso M, Turturici G, Cammarata FP et al. Glut-3 gene knockdown as a potential strategy to overcome glioblastoma radioresistance. Int J Mol Sci. 2024;25(4). 10.3390/ijms25042079. [DOI] [PMC free article] [PubMed]
- 228.Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48(7):2053–108. 10.1039/c8cs00618k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bockamp E, Rosigkeit S, Siegl D, Schuppan D. Nano-enhanced cancer immunotherapy: immunology encounters nanotechnology. Cells. 2020;9(9). 10.3390/cells9092102. [DOI] [PMC free article] [PubMed]
- 230.Lin P, Lu Y, Zheng J, Lin Y, Zhao X, Cui L. Strategic disruption of cancer’s powerhouse: precise nanomedicine targeting of mitochondrial metabolism. J Nanobiotechnology. 2024;22(1):318. 10.1186/s12951-024-02585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Shan X, Lv S, Cheng H, Zhou L, Gao Y, Xing C et al. Evaluation of 3-O-β-D-galactosylated resveratrol-loaded polydopamine nanoparticles for hepatocellular carcinoma treatment. Eur J Pharm Biopharm. 2024:114454. 10.1016/j.ejpb.2024.114454. [DOI] [PubMed]
- 232.Hyroššová P, Milošević M, Škoda J, Vachtenheim J Jr, Rohlena J, Rohlenová K. Effects of metabolic cancer therapy on tumor microenvironment. Front Oncol. 2022;12:1046630. 10.3389/fonc.2022.1046630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tang S, Wang Q, Sun K, Song Y, Liu R, Tan X, et al. metabolic heterogeneity and potential immunotherapeutic responses revealed by single-cell transcriptomics of breast cancer. Apoptosis. 2024. 10.1007/s10495-024-01952-7. [DOI] [PubMed] [Google Scholar]
- 234.Lin X, Yang P, Wang M, Huang X, Wang B, Chen C, et al. Dissecting gastric cancer heterogeneity and exploring therapeutic strategies using bulk and single-cell transcriptomic analysis and experimental validation of tumor microenvironment and metabolic interplay. Front Pharmacol. 2024;15:1355269. 10.3389/fphar.2024.1355269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jin Y, Wu Y, Reuben A, Zhu L, Gay CM, Wu Q, et al. Single-cell and spatial proteo-transcriptomic profiling reveals immune infiltration heterogeneity associated with neuroendocrine features in small cell lung cancer. Cell Discov. 2024;10(1):93. 10.1038/s41421-024-00703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wu J, Chen Y, Zou H, Xu K, Hou J, Wang M, et al. 6-Phosphogluconate dehydrogenase promotes glycolysis and fatty acid synthesis by inhibiting the AMPK pathway in lung adenocarcinoma cells. Cancer Lett. 2024;601: 217177. 10.1016/j.canlet.2024.217177. [DOI] [PubMed] [Google Scholar]
- 237.Hakimi AA, Reznik E, Lee CH, Creighton CJ, Brannon AR, Luna A, et al. An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell. 2016;29(1):104–16. 10.1016/j.ccell.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Huang L, Wang L, Hu X, Chen S, Tao Y, Su H, et al. Machine learning of serum metabolic patterns encodes early-stage lung adenocarcinoma. Nat Commun. 2020;11(1):3556. 10.1038/s41467-020-17347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zou S, Li N, Zhang T, Geng Q. Research progress on tumor metabolic biomarkers in liquid biopsy of lung cancer. Zhongguo Fei Ai Za Zhi. 2024;27(2):126–32. 10.3779/j.issn.1009-3419.2023.106.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Pomyen Y, Budhu A, Chaisaingmongkol J, Forgues M, Dang H, Ruchirawat M, et al. Tumor metabolism and associated serum metabolites define prognostic subtypes of Asian hepatocellular carcinoma. Sci Rep. 2021;11(1):12097. 10.1038/s41598-021-91560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kaira K, Kuji I, Kagamu H. Value of (18)F-FDG-PET to predict PD-L1 expression and outcomes of PD-1 inhibition therapy in human cancers. Cancer Imaging. 2021;21(1):11. 10.1186/s40644-021-00381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rich LJ, Bagga P, Wilson NE, Schnall MD, Detre JA, Haris M, et al. (1)H magnetic resonance spectroscopy of (2)H-to-(1)H exchange quantifies the dynamics of cellular metabolism in vivo. Nat Biomed Eng. 2020;4(3):335–42. 10.1038/s41551-019-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wan J, Guo Y, Chen H, Sun P, Zhang X, Ye T, et al. Application and development of Deuterium Metabolic Imaging in tumor glucose metabolism: visualization of different metabolic pathways. Front Oncol. 2023;13:1285209. 10.3389/fonc.2023.1285209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bitencourt AGV, Bhowmik A, Marcal Filho EFL, Lo Gullo R, Mazaheri Y, Kapetas P, et al. Deuterium MR spectroscopy: potential applications in oncology research. BJR Open. 2024;6(1):tzae019. 10.1093/bjro/tzae019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kothandapani A, Gopalakrishnan K, Kahali B, Reisman D, Patrick SM. Downregulation of SWI/SNF chromatin remodeling factor subunits modulates cisplatin cytotoxicity. Exp Cell Res. 2012;318(16):1973–86. 10.1016/j.yexcr.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Xu X, Zheng Z, Jia L, Suo S, Liu B, Shao T, et al. Overexpression of SMARCA2 or CAMK2D is associated with cisplatin resistance in human epithelial ovarian cancer. Oncol Lett. 2018;16(3):3796–804. 10.3892/ol.2018.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kido K, Nojima S, Motooka D, Nomura Y, Kohara M, Sato K, et al. Ovarian high-grade serous carcinoma cells with low SMARCA4 expression and high SMARCA2 expression contribute to platinum resistance. J Pathol. 2023;260(1):56–70. 10.1002/path.6064. [DOI] [PubMed] [Google Scholar]
- 248.Ogiwara H, Takahashi K, Sasaki M, Kuroda T, Yoshida H, Watanabe R, et al. Targeting the Vulnerability of Glutathione Metabolism in ARID1A-Deficient Cancers. Cancer Cell. 2019;35(2):177–90 e8. 10.1016/j.ccell.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 249.Davidson J, Shen Z, Gong X, Pollack JR. SWI/SNF aberrations sensitize pancreatic cancer cells to DNA crosslinking agents. Oncotarget. 2018;9(11):9608–17. 10.18632/oncotarget.20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li W, Chen Q, Gao W, Zeng H. ARID1A promotes chemosensitivity to gemcitabine in pancreatic cancer through epigenetic silencing of RRM2. Pharmazie. 2022;77(7):224–9. 10.1691/ph.2022.1881. [DOI] [PubMed] [Google Scholar]
- 251.Kim J, Jang G, Sim SH, Park IH, Kim K, Park C. SMARCA4 depletion induces cisplatin resistance by activating YAP1-mediated epithelial-to-mesenchymal transition in triple-negative breast cancer. Cancers (Basel). 2021;13(21). 10.3390/cancers13215474. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.