Abstract
1. We have examined the ability of the Ca(2+)-binding proteins (CABP) calbindin D28k and paravalbumin to modulate increases in the intracellular free Ca2+ concentration ([Ca2+]i), produced by brief depolarizations, in rat dorsal root ganglion (DRG) neurones. 2. In order to obtain good voltage control, we replated DRG neurones prior to performing these experiments. Immunocytochemical staining of these cells revealed that approximately 10% stained for CABPs. 3. Using fluorescently labelled parvalbumin, we demonstrated that in the whole-cell voltage clamp mode the protein freely entered the cell soma with a mean half-life t0.5 of 6 min 22 s +/- 54 s. 4. Analysis of the effects of calbindin D28k (370 microM) and parvalbumin (1 mM) on Ca2+ currents in the whole-cell voltage clamp mode, revealed that neither protein changed the rate of inactivation of the Ca2+ current or its rate of run-down. 5. Introducing either calbindin D28k (370 microM) or parvalbumin (1 mM) into the cell soma did not significantly alter the basal [Ca2+]i when compared to control cells. 6. Compared to control cells, both CABPs significantly reduced the peak [Ca2+]i obtained for a Ca2+ influx of an equivalent charge density, whereas lysozyme (1 mM), a protein with low affinity for Ca2+, failed to do so. 7. Calbindin D28k caused an 8-fold decrease in the rate of rise in [Ca2+]i and altered the kinetics of decay of [Ca2+]i to a single slow component. Parvalbumin also slowed the rate of rise in [Ca2+]i. Parvalbumin selectively increased a fast component in the decay of the Ca2+ signal. 8. These data demonstrate that both calbindin D28k and paravalbumin effectively buffer Ca2+ in a cellular environment and may therefore regulate Ca(2+)-dependent aspects of neuronal function.
Full text
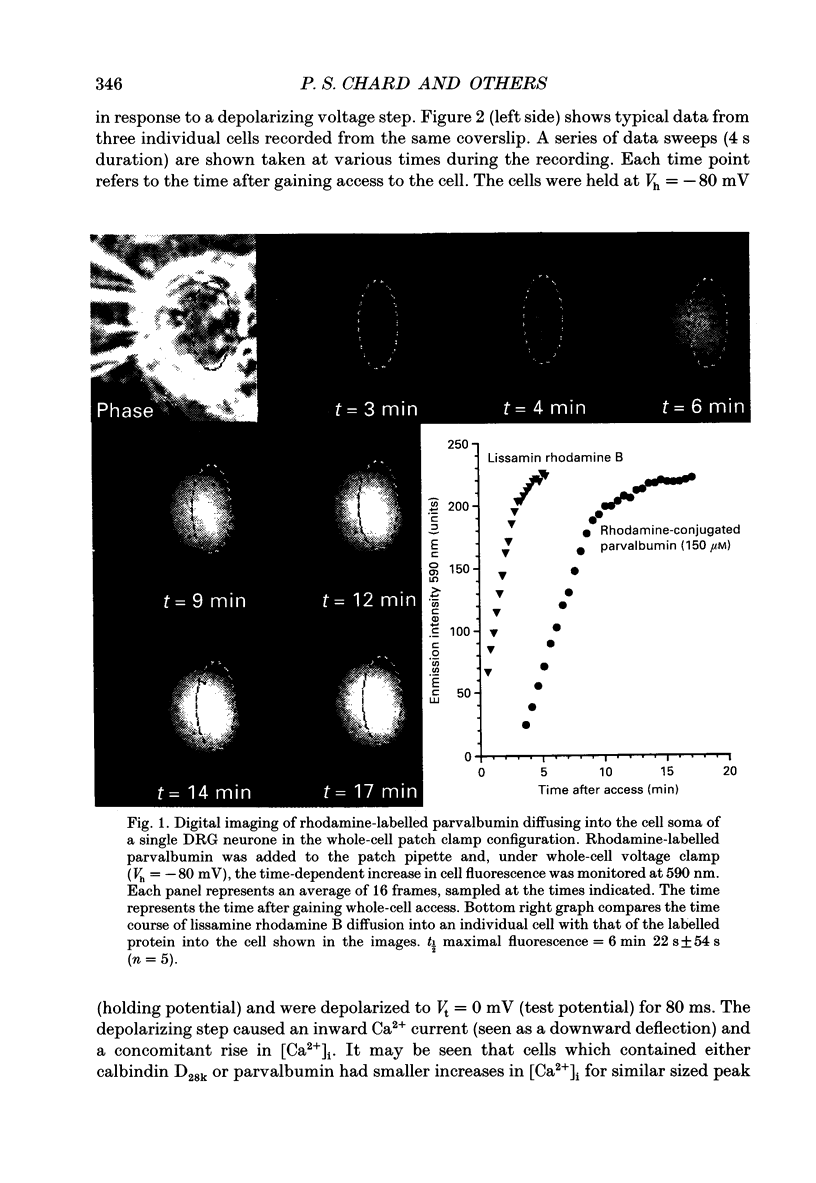
PDF




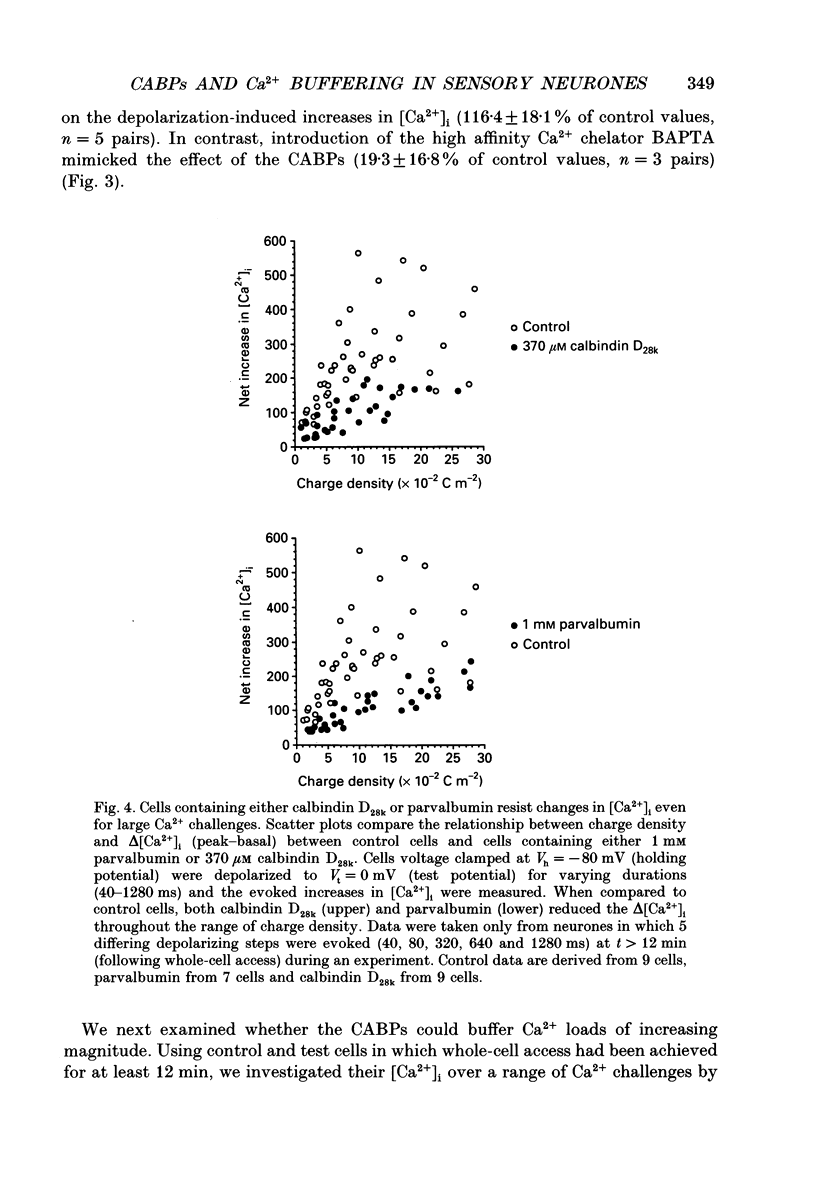
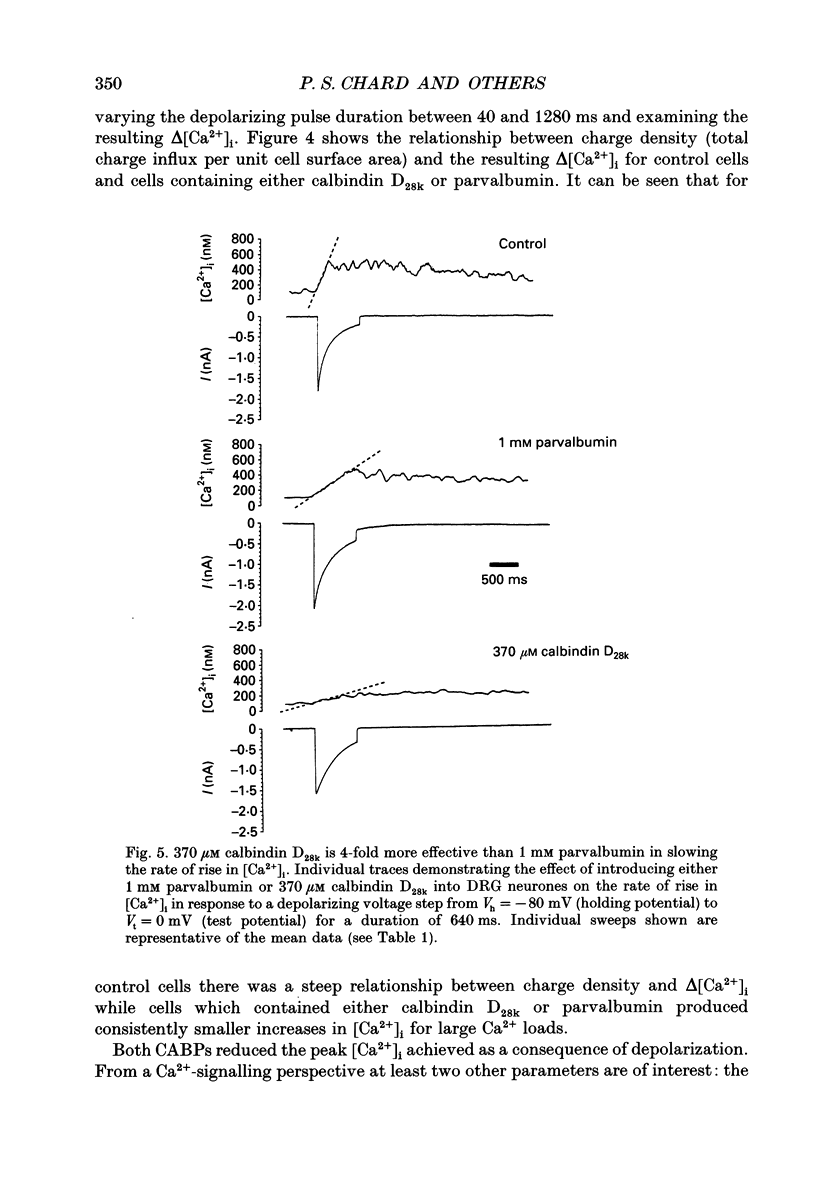
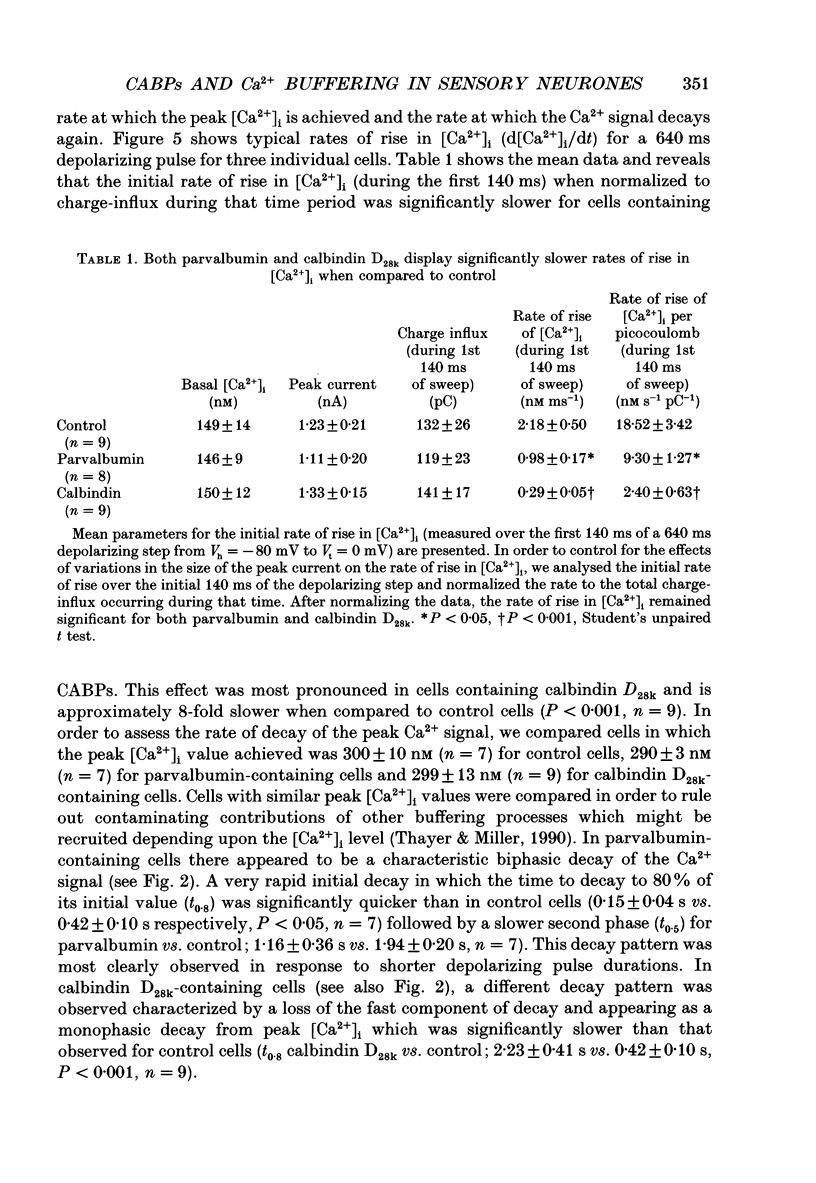






Images in this article
Selected References
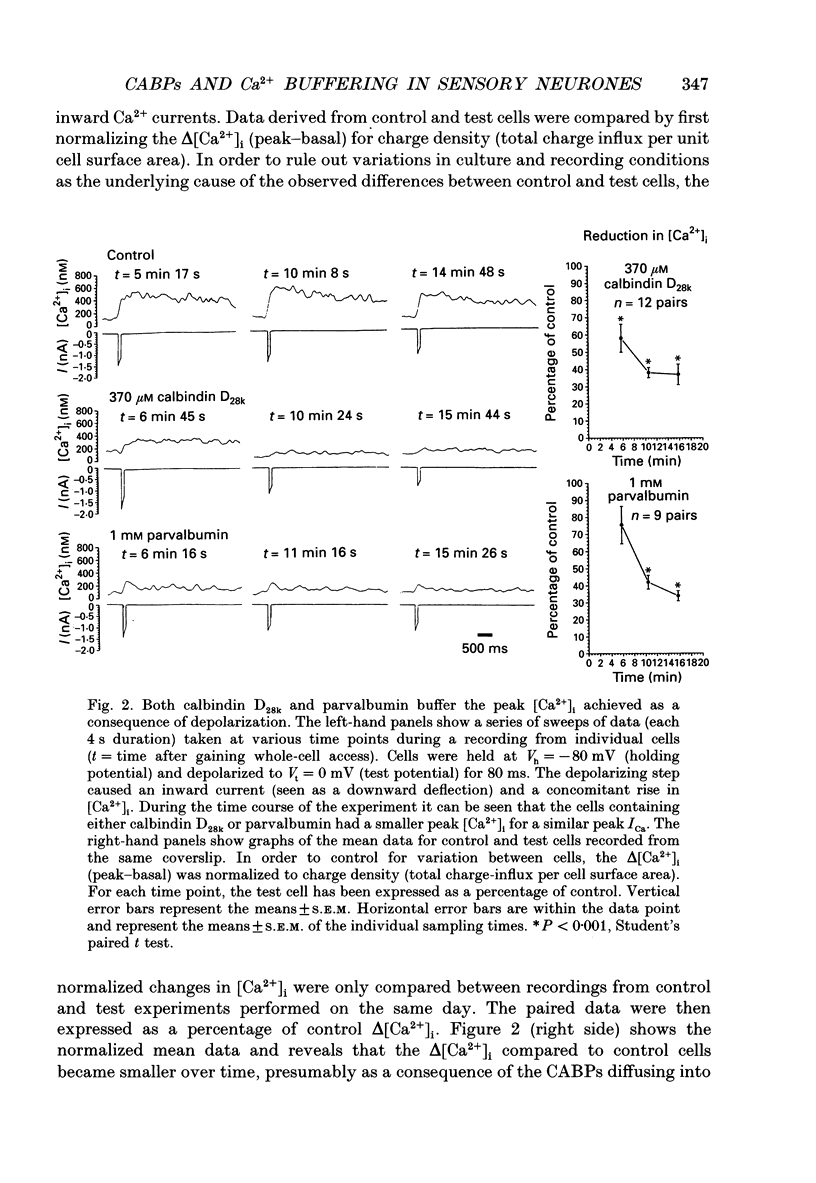
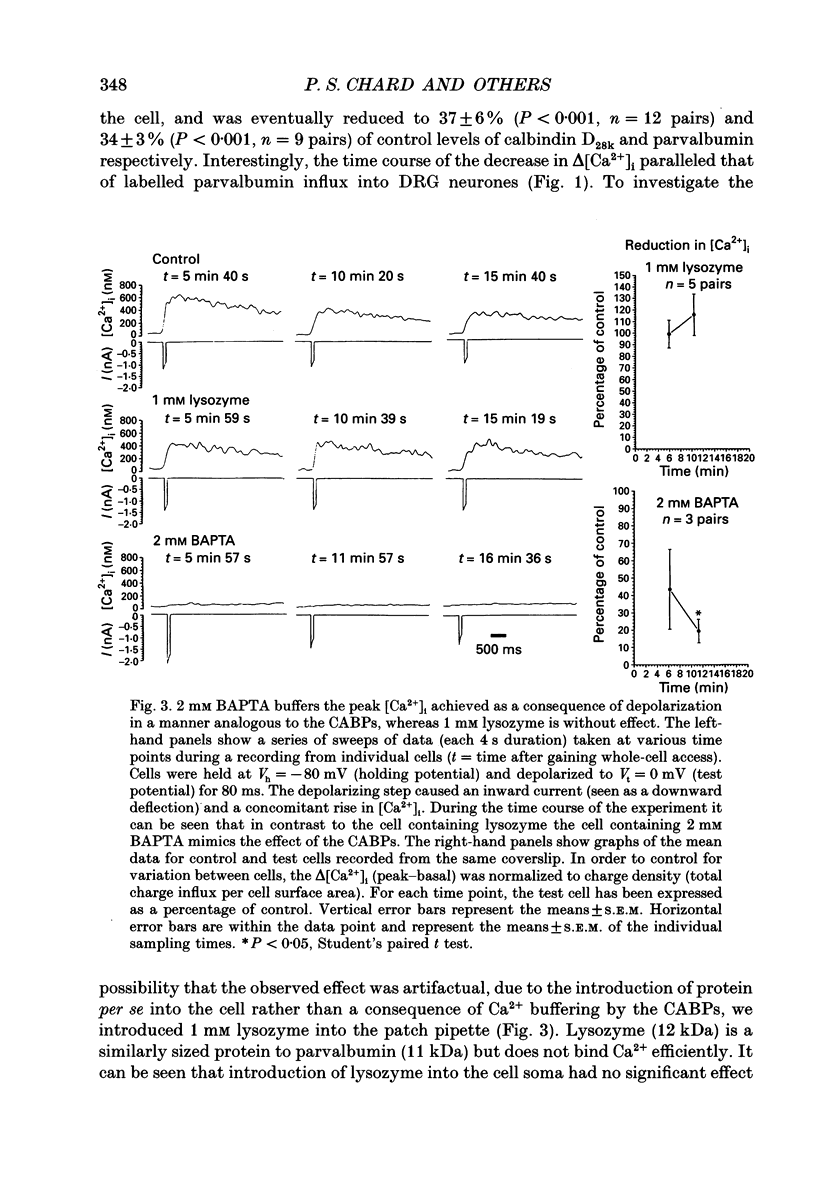
These references are in PubMed. This may not be the complete list of references from this article.
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Adler E. M., Charlton M. P. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann N Y Acad Sci. 1991;635:365–381. doi: 10.1111/j.1749-6632.1991.tb36505.x. [DOI] [PubMed] [Google Scholar]
- Baimbridge K. G., Celio M. R., Rogers J. H. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992 Aug;15(8):303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Schlaepfer W. W. Uptake and binding of calcium by axoplasm isolated from giant axons of Loligo and Myxicola. J Physiol. 1978 Mar;276:103–125. doi: 10.1113/jphysiol.1978.sp012222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Umbach J. A. Calcium buffering in axons and axoplasm of Loligo. J Physiol. 1987 Feb;383:369–394. doi: 10.1113/jphysiol.1987.sp016414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Evans M. L., McBain C. J. Ca2+ efflux mechanisms following depolarization evoked calcium transients in cultured rat sensory neurones. J Physiol. 1992 Sep;455:567–583. doi: 10.1113/jphysiol.1992.sp019316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredderman P. J., Wasserman R. H. Chemical composition, affinity for calcium, and some related properties of the vitamin D dependent calcium-binding protein. Biochemistry. 1974 Apr 9;13(8):1687–1694. doi: 10.1021/bi00705a021. [DOI] [PubMed] [Google Scholar]
- Calcium binding proteins in normal and transformed cells. Proceedings of the first European symposium. April 20-22, 1989, Brussels, Belgium. Adv Exp Med Biol. 1990;269:1–223. [PubMed] [Google Scholar]
- Carr P. A., Yamamoto T., Karmy G., Baimbridge K. G., Nagy J. I. Parvalbumin is highly colocalized with calbindin D28k and rarely with calcitonin gene-related peptide in dorsal root ganglia neurons of rat. Brain Res. 1989 Sep 11;497(1):163–170. doi: 10.1016/0006-8993(89)90983-9. [DOI] [PubMed] [Google Scholar]
- Celio M. R. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35(2):375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W. Excitotoxic cell death. J Neurobiol. 1992 Nov;23(9):1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Gillis J. M., Thomason D., Lefèvre J., Kretsinger R. H. Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil. 1982 Dec;3(4):377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heizmann C. W., Hunziker W. Intracellular calcium-binding proteins: more sites than insights. Trends Biochem Sci. 1991 Mar;16(3):98–103. doi: 10.1016/0968-0004(91)90041-s. [DOI] [PubMed] [Google Scholar]
- Hochner B., Parnas H., Parnas I. Effects of intra-axonal injection of Ca2+ buffers on evoked release and on facilitation in the crayfish neuromuscular junction. Neurosci Lett. 1991 Apr 29;125(2):215–218. doi: 10.1016/0304-3940(91)90032-o. [DOI] [PubMed] [Google Scholar]
- Hou T. T., Johnson J. D., Rall J. A. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J Physiol. 1991 Sep;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. II. Inactivation. J Gen Physiol. 1989 Jul;94(1):169–182. doi: 10.1085/jgp.94.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Katsumaru H., Kosaka T., Heizmann C. W., Hama K. Fast spiking cells in rat hippocampus (CA1 region) contain the calcium-binding protein parvalbumin. Brain Res. 1987 Jul 28;416(2):369–374. doi: 10.1016/0006-8993(87)90921-8. [DOI] [PubMed] [Google Scholar]
- Kellogg D. R., Mitchison T. J., Alberts B. M. Behaviour of microtubules and actin filaments in living Drosophila embryos. Development. 1988 Aug;103(4):675–686. doi: 10.1242/dev.103.4.675. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Kosaka K., Nakayama T., Hunziker W., Heizmann C. W. Axons and axon terminals of cerebellar Purkinje cells and basket cells have higher levels of parvalbumin immunoreactivity than somata and dendrites: quantitative analysis by immunogold labeling. Exp Brain Res. 1993;93(3):483–491. doi: 10.1007/BF00229363. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Nockolds C. E. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973 May 10;248(9):3313–3326. [PubMed] [Google Scholar]
- Köhr G., Mody I. Endogenous intracellular calcium buffering and the activation/inactivation of HVA calcium currents in rat dentate gyrus granule cells. J Gen Physiol. 1991 Nov;98(5):941–967. doi: 10.1085/jgp.98.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C., Ribak C. E. Calcium-binding proteins are concentrated in the CA2 field of the monkey hippocampus: a possible key to this region's resistance to epileptic damage. Exp Brain Res. 1991;85(1):129–136. doi: 10.1007/BF00229993. [DOI] [PubMed] [Google Scholar]
- Lledo P. M., Somasundaram B., Morton A. J., Emson P. C., Mason W. T. Stable transfection of calbindin-D28k into the GH3 cell line alters calcium currents and intracellular calcium homeostasis. Neuron. 1992 Nov;9(5):943–954. doi: 10.1016/0896-6273(92)90246-a. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Rychlik B., Chu C., Christakos S. Evidence for calcium-reducing and excito-protective roles for the calcium-binding protein calbindin-D28k in cultured hippocampal neurons. Neuron. 1991 Jan;6(1):41–51. doi: 10.1016/0896-6273(91)90120-o. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Pinter M. J. Time courses of calcium and calcium-bound buffers following calcium influx in a model cell. Biophys J. 1993 Jan;64(1):77–91. doi: 10.1016/S0006-3495(93)81342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberholtzer J. C., Buettger C., Summers M. C., Matschinsky F. M. The 28-kDa calbindin-D is a major calcium-binding protein in the basilar papilla of the chick. Proc Natl Acad Sci U S A. 1988 May;85(10):3387–3390. doi: 10.1073/pnas.85.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Résibois A., Rogers J. H. Calretinin in rat brain: an immunohistochemical study. Neuroscience. 1992;46(1):101–134. doi: 10.1016/0306-4522(92)90012-q. [DOI] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H. E., Schwartzkroin P. A. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science. 1989 Oct 13;246(4927):257–260. doi: 10.1126/science.2508225. [DOI] [PubMed] [Google Scholar]
- Scroggs R. S., Fox A. P. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol. 1992 Jan;445:639–658. doi: 10.1113/jphysiol.1992.sp018944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter R. S. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol. 1989 Feb 8;280(2):183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992 Mar;13(3):183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. A., Perney T. M., Miller R. J. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1988 Nov;8(11):4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. A., Sturek M., Miller R. J. Measurement of neuronal Ca2+ transients using simultaneous microfluorimetry and electrophysiology. Pflugers Arch. 1988 Jul;412(1-2):216–223. doi: 10.1007/BF00583753. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Vitamin d3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966 May 6;152(3723):791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]