Abstract
Introduction
In adults with moderate-to-severe atopic dermatitis (AD), rocatinlimab demonstrated significant and progressive improvement in clinical measures of disease severity compared with placebo. This post hoc analysis of a phase 2b study was undertaken to understand the disease burden and to assess the impact of rocatinlimab on patient-reported outcomes (PROs).
Methods
This analysis used baseline data from a multicenter, randomized, double-blind study of adults with moderate-to-severe AD, who completed a Worst Pruritus numerical rating scale (NRS), Sleep Disturbance NRS, and the Dermatology Life Quality Index (DLQI). A mixed model for repeated measures was used to estimate changes in PRO scores from baseline; scores were also compared with clinically meaningful change benchmarks.
Results
The analysis included 267 subjects, mean (SD) age 37.9 (14.7) years, 40.8% female; 55.1% grade 3 and 44.9% grade 4 Investigator Global Assessment for AD. Mean (SD) scores were: Worst Pruritus NRS 7.5 (1.9), Sleep Disturbance NRS 5.5 (2.9), DLQI total score 12.6 (7.1). Worst Pruritus and Sleep NRS scores had low positive correlations with SCORing AD (SCORAD) score (r = 0.44, r = 0.45 respectively) and negligible correlations with Eczema Area and Severity Index (EASI) score and area affected (r < 0.30). DLQI score varied by sex, study country, race, age, longer disease duration, disease severity (EASI and SCORAD), presence of asthma, and Worst Pruritus NRS, Sleep disturbance NRS, and DLQI scores. Rocatinlimab showed benefit on all three PROs, with significant improvements from baseline at the end of the double-blind period (week 18) and active treatment extension (week 36). Benefits were maintained over 20 weeks’ post-treatment follow-up. The benefit of rocatinlimab treatment on PROs is rapid and maintained for at least 20 weeks following treatment completion.
Conclusion
This analysis demonstrates the importance of characterizing the burden of moderate-to-severe AD from the patient’s perspective, alongside clinical disease measures, to develop a fuller picture of treatment benefit.
Trial Registration
ClinicalTrials.gov identifier, NCT03703102.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01303-z.
Keywords: Atopic dermatitis, Dermatology Life Quality Index, Health-related quality of life (HRQL), Pruritus, Rocatinlimab, Sleep disturbance
Key Summary Points
| Atopic dermatitis (AD) is a chronic, inflammatory skin disease with heterogeneous and persistent symptoms (particularly pruritus) that profoundly compromise health-related quality of life (HRQL). |
| Rocatinlimab has demonstrated significant and progressive improvement in multiple measures of clinical severity compared with placebo in adults with moderate-to-severe AD. |
| This analysis used baseline data from a phase 2 study to develop a deeper understanding of the burden of AD, and to generate a detailed and confirmatory assessment of the impact of rocatinlimab on patient-reported pruritus, sleep, and HRQL. |
| The benefit of rocatinlimab treatment on patient-reported pruritus, sleep, and HRQL is rapid and is maintained off-treatment for at least 20 weeks following treatment completion. |
| Assessment of treatment benefit should include the burden of moderate-to-severe AD alongside clinical disease measures in order to develop a full picture. |
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin disease with heterogeneous presentation [1–3]. It causes persistent symptoms, of which pruritus is the most common and burdensome across all severities of AD in adults [4], often leading to frequent scratching, skin pain, and skin infections [5–7]. Pruritus also compromises sleep [8–13], mental health [8–10, 14], and work productivity [11, 13, 15–17], thus profoundly impacting patients’ health-related quality of life (HRQL) [9, 10, 13, 18–22].
Rocatinlimab (KHK4083/AMG 451) is a T cell rebalancing therapy that inhibits and reduces pathogenic T cells by targeting the OX40 receptor [23–25]. In a phase 2b trial in adults with moderate-to-severe AD, rocatinlimab demonstrated significant and progressive improvement in multiple measures of clinical severity compared with placebo, while maintaining a tolerable safety profile [24, 26, 27]. Patient-reported outcomes (PROs) are an important complement to clinical outcomes in the evaluation of treatments for AD, to understand patients’ experience of the symptoms (e.g., pruritus, skin pain) and impacts (e.g., on sleep, mental health, and HRQL). In the phase 2b rocatinlimab trial, subjects completed three validated PRO measures: the Pruritus Numerical Rating Scale (NRS) [28–31], a Sleep Disturbance NRS [31–34], and the Dermatology Life Quality Index (DLQI) [30, 35]. For the Pruritus NRS, the proportion of subjects considered to have responded (i.e., ≥ 4 point improvement from baseline) [6] at week 16 was higher with rocatinlimab than with placebo, and the mean percentage change from baseline at week 16 was significantly higher in the rocatinlimab groups than in the placebo group. Subjects in the rocatinlimab groups reported a decrease in Sleep Disturbance NRS score, whereas subjects in the placebo group had no change. Improvements in DLQI score at week 16 were greater with rocatinlimab than with placebo [24]. The improvements in Worst Pruritus NRS score seen at week 16 continued in all rocatinlimab groups to week 36. Improvements in pruritus, sleep, and HRQL largely remained within the ranges observed while on treatment during the off-treatment follow-up through to week 56 [24].
The current post hoc analysis used baseline data from the phase 2b study to develop a deeper understanding of the burden of AD experienced by subjects, by assessing variation in HRQL by demographic and clinical variables, and relationships between PROs and clinical measures. In addition, changes in PRO scores from baseline to week 56 have been analyzed using alternative statistical techniques from the phase 2 study that control for covariates, in order to generate a detailed and confirmatory assessment of the impact of rocatinlimab on PROs. HRQL results have also been evaluated in the context of minimal clinically important difference (MCID) benchmarks.
Methods
Study Design
The phase 2b multicenter randomized double-blind parallel-group study was conducted at 65 sites in the USA, Canada, Japan, and Germany. Eligible subjects were adults (aged ≥ 18 years) with confirmed AD (American Academy of Dermatology Consensus or local diagnostic criteria) and moderate-to-severe disease activity defined by Eczema Area and Severity Index (EASI) score ≥ 16, validated Investigator’s Global Assessment for Atopic Dermatitis (vIGA-AD™) score of 3 (moderate) or 4 (severe), and affecting at least 10% of body surface area (BSA) at both screening and baseline. Eligible subjects also had a documented history (within 1 year) of inadequate response to topical medications, or topical medications were medically inadvisable. Full details of the study have been reported previously [24].
The study protocol was approved by an institutional review board or independent ethics committee and regulatory health authorities in accordance with local regulations before study commencement. The trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization’s consolidated Good Clinical Practice guideline. All subjects provided written informed consent.
Procedures
Subjects were randomized equally to receive 18 weeks’ subcutaneous treatment with rocatinlimab 150 mg every 4 weeks (Q4W), 600 mg Q4W, 300 mg every 2 weeks (Q2W), or 600 mg Q2W, or placebo. At week 18, subjects continued into an active treatment extension in a blinded fashion (weeks 18–36), during which those initially randomized to receive rocatinlimab continued on the same dose whereas subjects initially randomized to placebo switched to rocatinlimab 600 mg Q2W. The active treatment extension was followed by a 20-week off-treatment follow-up (weeks 36–56).
Assessments
PRO assessed in this analysis are Worst Pruritus NRS, Sleep Disturbance NRS, and DLQI domain and total scores. Subjects completed the PROs using an electronic device before any clinical assessments and then at screening, baseline, weeks 1 and 2, and every 2 weeks, though to week 36 (double-blind period and active treatment extension), and every 4 weeks from weeks 40 to 56 (follow-up) and at early termination, if applicable.
The Worst Pruritus NRS records the worst degree of itch in the previous 24 h on a scale from 0 (no itch) to 10 (worst itch imaginable) [6, 28, 29, 31]. The Sleep Disturbance NRS records the severity of sleep disturbance in the previous 24 h on a scale from 0 (no sleep loss) to 10 (cannot sleep at all) [31–34]. The DLQI measures the effect of skin symptoms on daily living over the previous week [30, 35]. It comprises 30 items across six domains. Four domains (Symptoms and feelings, Daily activities, Leisure, Personal relationships) are scored from 0 to 6; two domains (Work and school, Treatment) are scored from 0 to 3. Total scores range from 0 to 30. Higher scores indicate greater HRQL impairment for all domains and total score. The DLQI was scored according to instrument guidelines, including handling of missing data. A reduction of 4 points or more in the DLQI total score is considered clinically meaningful [35].
Demographic characteristics included in this post hoc analysis (sex, country, race) and medical history (body mass index [BMI], time since AD diagnosis, AD severity [vIGA-AD], EASI score, SCORing Atopic Dermatitis [SCORAD] score, % BSA affected, biological products used, and presence of asthma and allergic rhinitis) have been reported previously [24].
Analytical Methods
We assessed variation in HRQL by demographic and clinical variables, and evaluated relationships between PROs and clinical measures. Continuous and ordinal variables are reported as mean, standard deviation (SD), and range; categorical data are reported as number and percentage of subjects. For continuous and ordinal variables, the Spearman rank correlation coefficient was applied and coefficients interpreted according to Hinkle and colleagues [36]; for categorical variables, mean scores were compared using Mann–Whitney (two categories) and analysis of variance (ANOVA) F-test (more than two categories). P values < 0.05 were considered statistically significant. Mixed models for repeated measures (MMRM) were used to generate least square (LS) mean change from baseline in Worst Pruritus NRS, Sleep Disturbance NRS, and DLQI domain and total scores. The models included baseline score, treatment, time point, severity of AD (vIGA-AD) at baseline, region (Japan, rest of world), and previous use of biological products (yes, no) as main effects, with treatment–time and baseline score–time interactions. For each model the following are reported: Akaike’s information criterion (AIC) degrees of freedom, F value, and p value for each main effect. Handling of missing data has been reported previously [24].
Results
Demographics and Medical History
The analysis included 267 subjects. Demographic and clinical characteristics have been reported previously [24]. Mean (SD) age was 37.9 (14.7) years and 40.8% were female; 58.1% of subjects were in Japan, 20.2% in the USA, 12.4% in Germany, and 9.4% in Canada. Most participants were Asian (64.2%), followed by White (30.7%); fewest were Black or African American (4.9%). Mean (SD) BMI was 25.2 (6.0) kg/m2. Mean (SD) time since diagnosis of AD was 16.2 (14.9) years. The vIGA-AD was grade 3 in 55.1% of subjects and grade 4 in 44.9%. Mean (SD) EASI and SCORAD scores were 31.5 (12.7) and 68.3 (13.8), respectively, and the mean BSA affected was 56.7% (23.4). Thirteen percent of subjects had previously used biological products.
Burden of AD
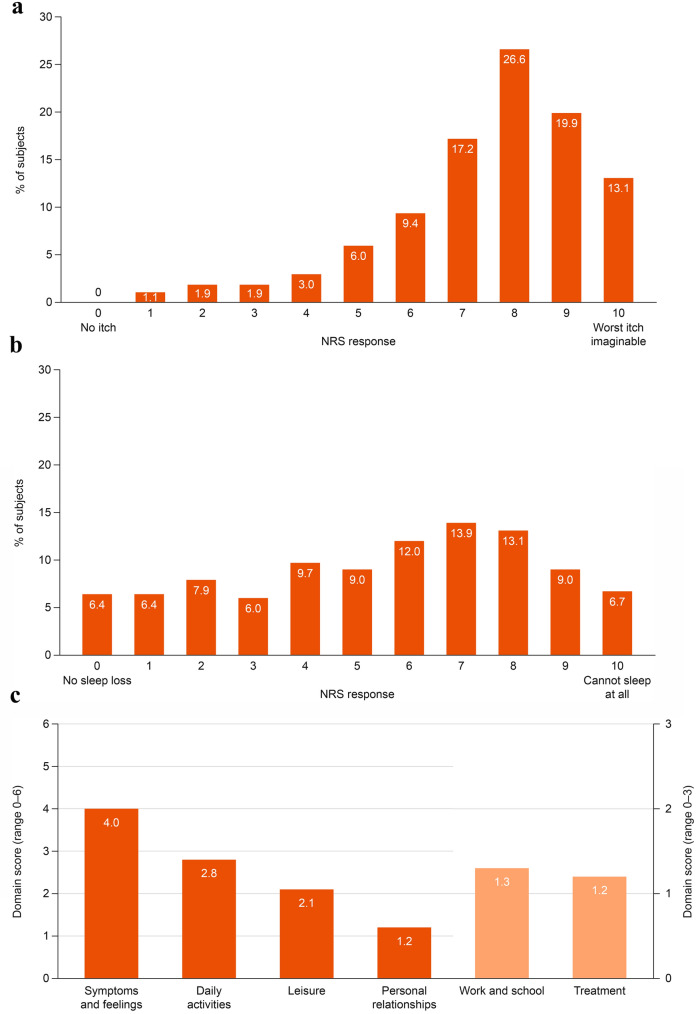
All subjects reported pruritus at study baseline. The mean (SD) score on the Worst Pruritus NRS was 7.5 (1.9); 75% scored 7 or higher (indicating severe pruritus [6]), with few reporting pruritus on the lower half of the scale (14% scored 1–5) (Fig. 1, Supplementary Table 1).
Fig. 1.
PRO scores at study baseline (n = 267). a Worst Pruritus NRS, b Sleep Disturbance NRS, c DLQI domain scores. Higher DLQI scores indicate greater HRQL impairment. DLQI Dermatology Life Quality Index, NRS numerical rating scale, PRO, patient-reported outcome
The mean (SD) score on the Sleep Disturbance NRS at baseline was 5.5 (2.9); 43% scored 7 or higher, 39% reported sleep disturbance on the lower half of the scale (scores 1–5), and few subjects (6.4%) reported no sleep loss (Fig. 1, Supplementary Table 1).
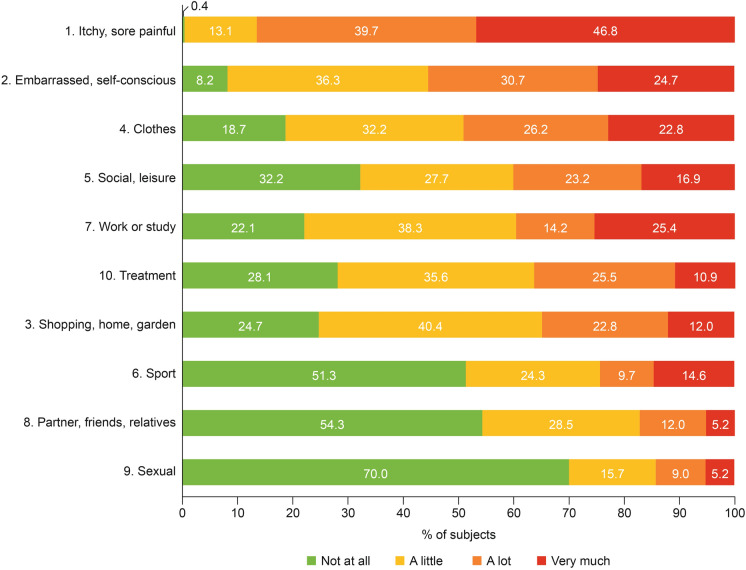
The mean (SD) DLQI total score was 12.6 (7.1) (Fig. 1, Supplementary Table 1).The greatest impact of treatment was on Symptoms and feelings, followed by Daily activities; the least impact was on Personal relationships, followed by Leisure. At the item level, subjects reported the greatest burden (i.e., responding “very much” or “a lot”) on the following items: “How itchy, sore, painful or stinging has your skin been?” (86.5%; Symptoms and feelings domain); “How embarrassed or self-conscious have you been because of your skin?” (55.4%; Symptoms and feelings domain); “How much has your skin influenced the clothes you wear?” (49.0%; Daily activities domain) (Fig. 2).
Fig. 2.
DLQI item responses at baseline. DLQI Dermatology Life Quality Index
Relationships Between Pruritus, Sleep, and Clinical Endpoints
The strongest correlations were between the Pruritus and Sleep Disturbance NRS scales (r = 0.61, moderate positive correlation) (Table 1). Both scales had a low positive correlation with SCORAD score (r = 0.44 for Worst Pruritus NRS; r = 0.45 for Sleep Disturbance NRS). Correlations between the NRS scales and EASI and BSA affected were negligible (i.e., r < 0.30).
Table 1.
Correlations between Worst Pruritus NRS and Sleep Disturbance NRS and clinical efficacy variables at baseline (n = 267)
| Clinical efficacy variable | Worst Pruritus NRS | Sleep Disturbance NRS | ||
|---|---|---|---|---|
| Coefficient (r) | Interpretationa | Coefficient (r) | Interpretationa | |
| Sleep disturbance NRS | 0.61 | Moderate positive | – | |
| EASI | 0.18 | Negligible | 0.18 | Negligible |
| % BSA affected | 0.13 | Negligible | 0.05 | Negligible |
| SCORAD | 0.44 | Low positive | 0.45 | Low positive |
BSA body surface area, EASI Eczema Area and Severity Index, NRS Numerical Rating Scale, SCORAD SCORing Atopic Dermatitis
aInterpretation of Spearman’s rank correlation coefficient (r): 0.90–1.00, very high positive correlation; 0.70–0.90, high positive; 0.50–0.70, moderate positive; 0.30–0.50, low positive; 0.00–0.30, negligible [36]
Variation in DLQI Scores
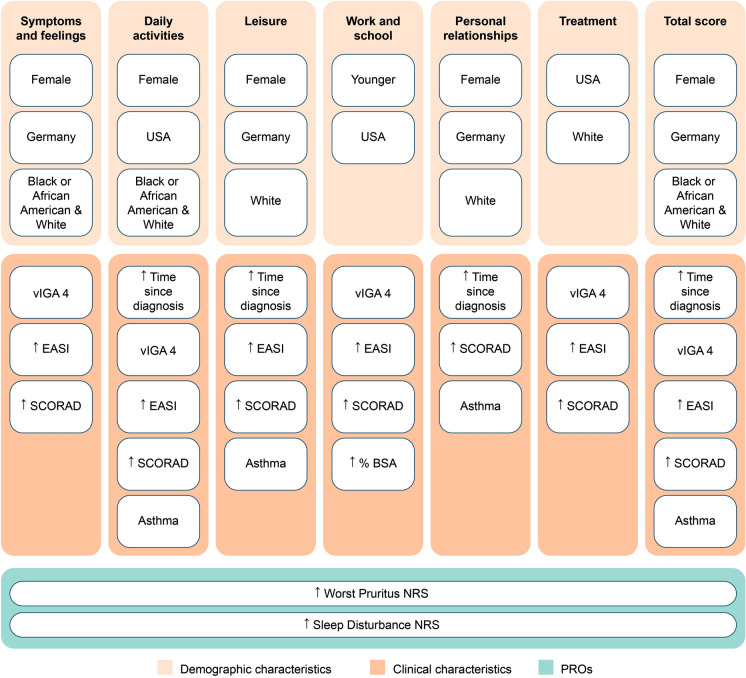
When variation in HRQL was assessed by demographic and clinical variables, female subjects had worse HRQL than male subjects in all domains except Work and school and for total score (Fig. 3, Supplementary Table 2). Subjects in Germany had worse HRQL than subjects in Canada, Japan, or the USA on three domains (Symptoms and feelings, Leisure, Personal relationships) and total score, and compared with those in the USA on two domains (Daily activities, Work and school). White subjects had worse HRQL than Black or African American and Asian subjects on all domains except Work and school and total score; Black or African American subjects had worse HRQL than White and Asian subjects on two domains (Symptoms and feelings, Daily activities) and total score. Younger age was related to worse HRQL only on the Work and school domain. BMI was not related to any HRQL domain or total score.
Fig. 3.
Indicators of worse DLQI domain and total scores at baseline (n = 267). Variation in HRQL by demographic and clinical variables was compared using Spearman’s rank correlation coefficient for continuous/ordinal variables, Mann–Whitney (two categories), and ANOVA F-test (more than two categories). P values < 0.05 were considered statistically significant. Categories: female/male; Canada/Germany/Japan/USA, Asian/Black or African American/Asian, vIGA 3/4. Higher DLQI scores indicate greater HRQL impairment. ↑, higher; BSA body surface area, DLQI Dermatology Life Quality Index, EASI Eczema Area and Severity Index, HRQL health-related quality of life, NRS Numerical Rating Scale, PRO patient-reported outcome, SCORAD SCORing Atopic Dermatitis, vIGA validated Investigator Global Assessment for Atopic Dermatitis
Across most domains and total score, worse HRQL was associated with longer disease duration (i.e., time since diagnosis), greater disease severity (vIGA-AD 4), worse EASI score, and worse SCORAD score (Fig. 3, Supplementary Table 3). The presence of asthma was associated with worse HRQL in the Daily activities and Leisure and Personal relationships domains, and in total score. HRQL was not related to prior use of biological products or presence of allergic rhinitis.
Higher Worst Pruritus NRS and Sleep disturbance NRS scores were significantly associated with worse HRQL across all DLQI domains and total score (Fig. 3, Supplementary Table 4).
PRO Change from Baseline
Worst Pruritus NRS
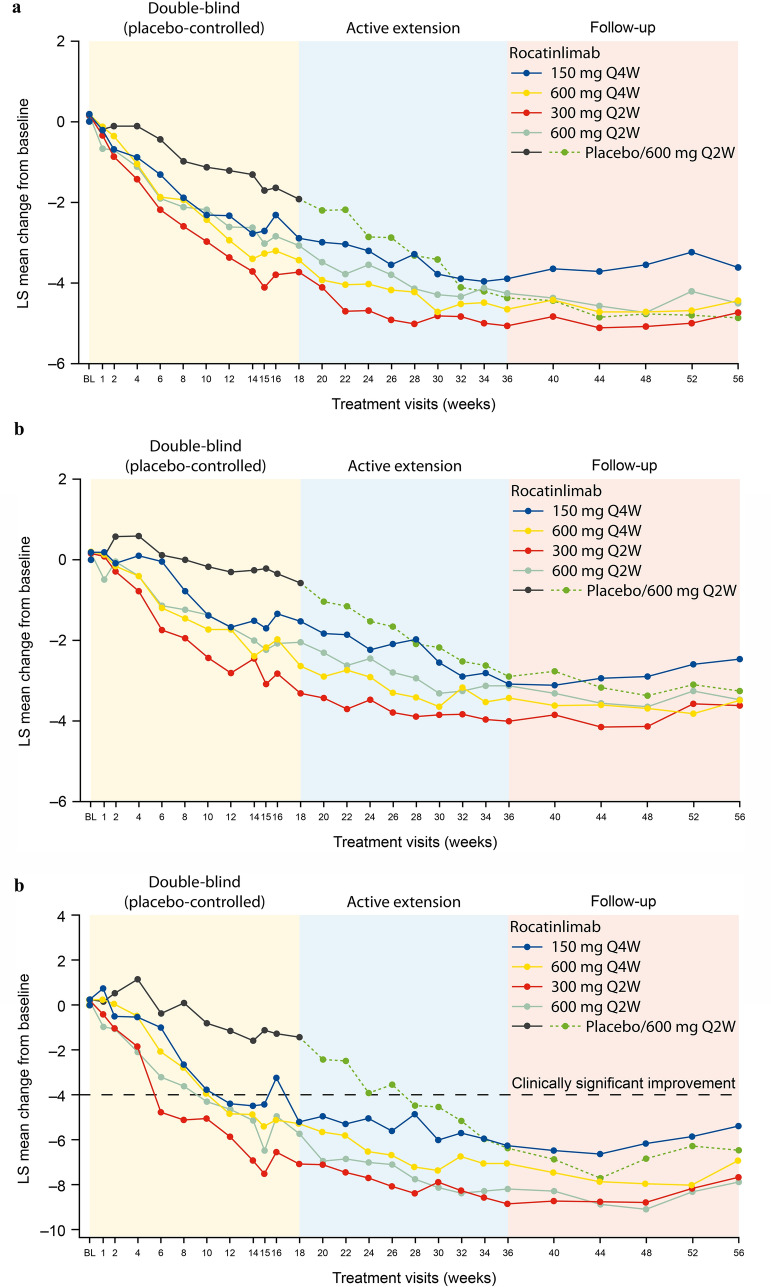
The MMRM showed significant improvements (p < 0.001) on the Worst Pruritus NRS in all five treatment groups at the end of the double-blind period (week 18), the active treatment extension (week 36), and follow-up (week 56) (Fig. 4a). The improvement by the end of the double-blind period was greatest in the 300 mg Q2W group and least in the placebo group. The greatest numerical improvement by the end of the active treatment extension was in the 300 mg Q2W group and the least improvement in the 150 mg Q4W group. In the placebo/600 mg Q2W group, improvement on the Worst Pruritus NRS at the end of the active treatment extension was similar to the improvement seen in the other four treatment groups. The improvements at the end of the active treatment extension were maintained through to week 56 (end of follow-up) in all treatment groups, with significant change from baseline. The greatest improvement at the end of the active treatment extension was in the placebo/600 mg Q2W group and the least improvement was in the 150 mg Q4W group.
Fig. 4.
MMRM PRO adjusted means score change from baseline (n = 267). a Worst Pruritus NRS. Worst Pruritus NRS measures the worst degree of itch in the previous 24 h; score range 0–10, with higher scores indicating greater worse itch. AIC 20272.1; main effects: baseline score (DF 1, F value 156.38, p < 0.001), treatment (DF 4, F value 4.74, p = 0.001), time point (DF 26, F value 2.03, p = 0.002), severity of AD (vIGA-AD) at baseline (DF 1, F value 1.99, p = 0.159), region (Japan, rest of world) (DF 1, F value 0.56, p = 0.456), and previous use of biological products (yes, no) (DF 1, F value 1.74, p = 0.189). Significant change from baseline in all treatment groups at weeks 18, 36, and 56 (p < 0.0001). b Sleep Disturbance NRS. Sleep Disturbance NRS measures the severity of sleep disturbance in the previous 24 h; score range 0–10, with higher scores indicating greater sleep disturbance. AIC 20377.4; main effects: baseline score (DF 1, F value 302.18, p < 0.001), treatment (DF 4, F value 6.00, p < 0.001), time point (DF 26, F value 6.27, p < 0.001), severity of AD (vIGA-AD) at baseline (DF 1, F value 0.05, p = 0.828), region (Japan, rest of world) (DF 1, F value 0.01, p = 0.939), and previous use of biological products (yes, no) (DF 1, F value 2.18, p = 0.141). Significant change from baseline in all treatment groups at weeks 18, 36, and 56 (p < 0.0001), except for the placebo group at week 18. c DLQI total score. DLQI total score ranges from 0 to 30; higher scores indicate greater impairment. AIC 23764.9; main effects: baseline score (DF 1, F value 154.41, p < 0.001), treatment (DF 4, F value 6.26, p < 0.001), time point (DF 26, F value 2.45, p < 0.001), severity of AD (vIGA-AD) at baseline (DF 1, F value 0.06, p = 0.801), region (Japan, rest of world) (DF 1, F value 1.16, p = 0.283), and previous use of biological products (yes, no) (DF 1, F value 1.11, p = 0.294). Significant change from baseline in all treatment groups at weeks 18, 36, and 56 (p < 0.0001), except for the placebo group at week 18. AD atopic dermatitis, AIC Akaike’s information criterion, BL baseline, DF degrees of freedom, DLQI Dermatology Life Quality Index, LS least squares, MMRM mixed models for repeated measures, NRS Numerical Rating Scale, PRO patient-reported outcome, Q2W every 2 weeks, Q4W every 4 weeks, vIGA-AD validated Investigator’s Global Assessment for Atopic Dermatitis
Sleep Disturbance NRS
Significant improvements (p < 0.001) were seen at the end of the double-blind period in the four active treatment groups but not in the placebo group (Fig. 4b). The greatest improvement was in the 300 mg Q2W group. Significant improvements were seen by the end of the active treatment extension in all treatment groups including the placebo group (who had switched to active treatment). The greatest improvement was in the 300 mg Q2W group and the least improvement in the placebo group. Significant change from baseline was maintained during the follow-up in all treatment groups. The greatest improvement from baseline to week 56 was in the 300 mg Q2W group and the least improvement in the 150 mg Q4W group.
DLQI Total Score
DLQI total score improved significantly (p < 0.001) from baseline at the end of the double-blind period in the four active treatment groups but not in the placebo group (Fig. 4c). The greatest improvement was in the 300 mg Q2W group. The 300 mg Q2W group also had the greatest improvement at the end of the active treatment extension and least improvement was seen in the 150 mg Q4W group. All treatment groups had significant improvements by the end of the active treatment extension, at which time improvement in the placebo group was similar to improvements in the four active treatment groups. The improvements seen at the end of the active treatment extension were broadly maintained during the follow-up in all treatment groups, with significant changes from baseline maintained in all treatment groups. The greatest improvement was maintained in the 600 mg Q2W group and the least improvement in the 150 mg Q4W group.
Clinically significant improvements in DLQI total score (i.e., exceeding the MCID of 4 points) were seen in all active treatment groups at the end of the double-blind period but not in the placebo group, and in all groups at the end of the active treatment extension and follow-up periods, including the placebo group (who had switched to active treatment). Clinically significant improvement was seen as early as week 6 in the 300 mg Q2W group and by week 12 in the other active treatment groups.
DLQI Domain Scores
Significant improvements (p < 0.05) at all three time points (weeks 18, 36, and 56) were seen in the four active treatment groups but not the placebo group in four domains: Daily activities, Leisure, Personal relationships, and Treatment (Supplementary Fig. 1). Significant improvement (p < 0.05) at all three time points was seen in all active treatment groups and the placebo group on two domains: Symptoms and feelings, Work and school. Greatest improvements were seen on the Symptoms and feelings and Work and school domains. Least improvements were seen on the Relationships and Leisure domains (taking into consideration different scale ranges).
Discussion
The phase 2b study demonstrated that rocatinlimab provided several benefits on PROs in subjects with moderate-to-severe AD: improvements from baseline to week 16 (% change) were seen in Worst Pruritus NRS, Sleep Disturbance NRS, and DLQI scores and in the proportion of subjects achieving a clinically meaningful improvement (≥ 4 point improvement) on the DLQI. Improvements through week 56 (end of follow-up) were also reported descriptively for these measures [24]. The current analysis assessed the burden reported by subjects on these PROs and the relationships between the PROs and clinical outcomes, using statistical techniques that control for covariates; the analysis also assessed the impact of rocatinlimab on PROs from baseline to week 56. Overall, we found that the clinical trial population described here had a substantial disease burden at baseline in pruritus, sleep disturbance, and HRQL and that only low or negligible correlations were seen between improvements in PROs and clinical measurements. Rocatinlimab showed consistent benefit on all three PROs, with significant improvements from baseline at the end of the double-blind period (week 18) and active treatment extension (week 36), and maintenance of benefit over the 20-week post-treatment follow-up.
The mean Worst Pruritus NRS score at baseline was 7.5 (median 8). Similar scores were reported for maximum itch intensity in a phase 3 study of dupilumab in subjects with moderate-to-severe AD (7.6 or 7.7) [37]. Subjects in the current study also reported a marked impact of AD on sleep. The mean Sleep Disturbance NRS score at baseline was 5.5 (median 6). A study of 218 adults with moderate-to-severe AD reported a mean score of 7.8 [34]. The Sleep Disturbance NRS is a relatively new instrument and has yet to be widely used in AD.
The mean DLQI scores at baseline in the current study are also consistent with a substantial disease burden: the mean total score of 12.6 (median 11.0) is slightly lower (indicating better HRQL) than the median scores of 13 and 14 reported in the phase 3 study of dupilumab [37]. A US cross-sectional study reported a mean DLQI total score of 9.2 in patients with moderate/severe AD [22] and was consistent with the current study in terms of items associated with the greatest burden (i.e., “How itchy, sore, painful or stinging has your skin been?”, “How embarrassed or self-conscious have you been because of your skin?”, “How much has your skin influenced the clothes you wear?”). Multiple studies have reported that HRQL burden increases with worsening disease severity [4, 9, 10, 13, 18–21, 38], worse itch [4, 18], and worse sleep [4, 18].
Importantly, only low or negligible correlations were seen between improvements in PROs (Worst Pruritus NRS, Sleep Disturbance NRS, DLQI) and clinical endpoints (EASI, BSA affected, SCORAD score). The strongest correlations were with the SCORAD score, which likely reflects content overlap as the SCORAD includes patient-reported symptoms of itch and sleep dysfunction. Other studies have also reported negligible or low correlation between itch-specific PROs and clinical endpoints but stronger correlations between itch-specific PROs and DLQI scores [39–42]. The limited correlation between clinical endpoints and PROs found in this study underlines the importance of including PROs in the evaluation of AD and its treatment, to ensure that the burden of disease is not underestimated. PROs are complementary to clinical endpoints, capturing information that is relevant and meaningful to patients but that might be overlooked in clinical assessments. PROs should also inform treatment decision-making alongside clinical disease measures [43].
The current study used MMRM to analyze change in PRO scores from baseline with rocatinlimab treatment, controlling for baseline score, treatment, time point, severity of AD, region, and previous use of biological products in the model. Rocatinlimab showed consistent benefit in worst pruritus, sleep disturbance, and HRQL, with significant improvements from baseline at the end of the double-blind period (week 18) and active treatment extension (week 36). Importantly, improvements in DLQI total score were clinically relevant, exceeding the MCID of 4 points in all active treatment groups at the end of the double-blind period and in all groups at the end of the active treatment extension. Clinically meaningful improvement in the DLQI total score was seen as early as week 6 with 300 mg Q2W and in all rocatinlimab treatment groups by week 12. Clinically meaningful improvements in the DLQI total score were maintained during the follow-up period in all the treatment groups. Thus, the benefits of rocatinlimab on HRQL are realized early.
Benefits observed in worst pruritus, sleep disturbance, and HRQL during rocatinlimab treatment were maintained over the 20-week off-treatment follow-up. Clinically meaningful improvements in the DLQI total score were also maintained during this off-treatment follow-up in all treatment groups. Thus, the benefits of rocatinlimab are maintained for at least 20 weeks after stopping treatment.
Improvements from baseline were reported in the Worst Pruritus NRS and on two DLQI domains (Symptoms and feelings, Work and school) for the placebo group during the double-blind phase (baseline to week 18). The study design required subjects to apply a topical emollient twice daily from 1 week before baseline until at least week 36, which may have contributed to these improvements. Of note, improvements in PROs in the placebo group at the end of the double-blind phase were less than in the active treatment groups.
All subjects reported pruritus at study baseline, with most reporting pruritus on the higher half of the scale. However, not all subjects reported burden in terms of sleep loss: 6.4% reported no sleep loss and 39% reported sleep disturbance on the lower half of the scale. Similarly, many subjects reported limited burden of AD on several DLQI items. Lower burden at baseline compromises the sensitivity to capture improvement following treatment intervention. This may have impacted the results reported here for the Sleep Disturbance NRS and the DLQI Leisure and Personal relationships domains. A future analysis could consider subsets of subjects reporting a specific level of burden at baseline (e.g., Sleep Disturbance NRS > 5) to better understand the impact of treatment for patients experiencing burden in these specific areas of their lives.
There are several study limitations that have been discussed in the context of the broader study [24] but are relevant to reiterate here. As this is a phase 2b study, the sample size is limited, and diversity is also limited—64% of the sample is Asian. While benefits of treatment are seen through the 20 weeks’ follow-up, a longer study is needed to determine the full durability of response. This study did not consider the combination of rocatinlimab with topical corticosteroids. This is a post hoc analysis and should therefore be considered exploratory and requiring validation with further research, and there in an ongoing phase 3 program for rocatinlimab which includes PRO assessments. There was no statistical correction for multiple comparisons in the evaluation of variation in DLQI scores and there was no evaluation of power for the MMRM. MCID benchmarks to allow group-level analysis of the Worst Pruritus NRS and Sleep Disturbance NRS have yet to be established in moderate-to-severe AD [24], so group-level changes in these two outcomes were not assessed in this analysis. The influence of sleep aids on the Sleep Disturbance NRS was not considered in the current analysis.
Conclusions
This analysis builds on the evidence demonstrating that, in addition to improving clinical symptoms, rocatinlimab improves AD symptoms that are relevant and meaningful to patients with moderate-to-severe AD, and their HRQL. The benefit of rocatinlimab treatment as evaluated by PROs is rapid and is maintained for at least 20 weeks following treatment completion. Given the low or negligible correlations between clinical measures and PROs, it is important to characterize the burden of moderate-to-severe AD from the patient’s self-reported perspective, alongside clinical disease measures, in order to develop a fuller picture of treatment benefit.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the subjects and healthcare professionals who participated in this study.
Medical Writing, Editorial, and Other Assistance
The authors also acknowledge Helen Barham PhD (The Text Doctor) for writing support and Jenna Zan (Zed, Oxford) for creating the figures.
Author Contributions
Melinda Gooderham, Emma Guttman-Yassky, Ken Igawa, Kenji Kabashima and Eric Simpson are study investigators, contributed to the concept and design of the analysis, provided review and interpretation of the statistical results and contributed to drafting the manuscript. Ehsanollah Esfandiari, Angela J Rylands, and Angela Williams contributed to the concept and design of the analysis, provided review and interpretation of the statistical results, and led the drafting of the manuscript. Annabel Nixon contributed to the concept and design of the analysis, contributed to the statistical analysis, and led the drafting of the manuscript. Jennifer E Dent designed and undertook the statistical analysis and contributed to drafting the manuscript.
Funding
This research was funded by, and the Raid service fee was paid by Kyowa Kirin Services Ltd.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of Interest
Melinda Gooderham has been an investigator, speaker, and/or advisor for: AbbVie, Acelyrin, Alumis, Amgen, Akros, Arcutis, Aristea, AnaptysBio, Apogee Therapeutics, Bausch Health, Bristol Myers Squibb, Boehringer Ingelheim, Cara Therapeutics, Dermira, Dermavant, Eli Lilly, Galderma, GSK, Incyte, Inmagene, JAMP Pharma, Janssen, LEO Pharma, L’Oreal, MedImmune, Meiji, MoonLake, Nektar, Nimbus, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, Tarsus, Takeda, UCB, Union, Ventyx, and Vyne. Emma Guttman-Yassky is an employee of Mount Sinai; this institution has received research grants from: LEO Pharma, Pfizer, Amgen, GSK, Incyte, Sanofi, Bristol Myers Squibb, ASLAN, Regeneron, AnaptysBio, Concert, Janssen, Q32 Bio, AbbVie, Eli Lilly, Arcutis, and Inmagene. Emma Guttman-Yassky has been a consultant for: AbbVie, Arcutis, Almirall, Amgen, AnaptysBio, Apogee Therapeutics, Apollo Therapeutics Limited, Artax Biopharma Inc., Astria, Bristol Myers Squibb, Boehringer Ingelheim, Calliditas Therapeutics, Cara Therapeutics, Celldex, Centrexion Therapeutics Corporation, Connect Biopharma, Coty, DBV Technologies, Eli Lilly, Enveda Biosciences, Escient Pharmaceuticals, Inc., Fairmount Funds Management LLC, FL2022-001, Inc., Galderma, Gate Bioscience, Google Ventures (GV), GSK Immunology, Incyte, Inmagene, Janssen Biotech, Jasper Therapeutics, Kymera Therapeutics, Kyowa Kirin, Leo Pharma, Matchpoint Therapeutics, Merck, Nektar Therapeutics, Novartis Pharmaceuticals, NUMAB Therapeutics AG, Nuvig, OrbiMed Advisors LLC, Otsuka, Pfizer, Pharmaxis Ltd, Pioneering Medicine VII, Inc., Proteologix US Inc., RAPT, RayThera, Inc., Regeneron Pharmaceuticals, RibonTherapeutics, Inc., Sagiment Biosciences, Sanofi, SATO, Schrödinger, Inc., Sitryx, Sun Pharma Advanced Research Company (SPARC), Takeda, Teva Branded Pharmaceutical Products R&D, Inc., TRex, UCB, and Ventyx Biosciences. Ken Igawa has received a lecture fee from: Sanofi, AbbVie, Lilly, Leo Pharma, Maruho, and Otsuka. Kenji Kabashima has received consulting fees, honoraria, grant support, and/or lecturing fees from: Amgen, Kyowa Kirin, Japan Tobacco, LEO Pharma, Maruho, Mitsubishi Tanabe, Ono Pharmaceutical, Procter & Gamble, Sanofi, Taiho, and Torii Pharmaceutical. Ehsanollah Esfandiari, Angela J Rylands and Angela Williams are employees of Kyowa Kirin International. Ehsanollah Esfandiari has stock options in Kyowa Kirin. Annabel Nixon and Jennifer E. Dent work for Chilli Consultancy, which received payment from Kyowa Kirin International for this work. Eric Simpson received personal fees from AbbVie, Amgen, Arcutis, Areteia Therapeutics, Bristol Myers Squibb, CorEvitas, Corvus, Dermira, Eli Lilly, Evelo Biosciences, FIDE, Forte Bio RX, Galderma, GlaxoSmithKline, Gilead Sciences, Impetus Healthcare, Incyte, Innovaderm Research, Janssen, Johnson & Johnson, Kyowa Kirin Pharmaceutical Development, LEO Pharma, Merck, NUMAB Therapeutics AG, Pfizer, Physicians World LLC, PRImE, Recludix Pharma, Regeneron, Roivant, Sanofi Genzyme, Sitryx Therapeutics, Trevi Therapeutics, and Valeant. Eric Simpson received research grants from or served as a principal investigator for: AbbVie, Acrotech, Amgen, Arcutis, ASLAN, Castle, CorEvitas, Dermavant, Dermira, Incyte, Lilly, Kymab, Kyowa Kirin, National Jewish Health, LEO Pharma, Pfizer, Regeneron, Sanofi, Target, and VeriSkin.
Ethical Approval
The trial was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization consolidated Good Clinical Practice guideline, and any applicable national and local laws and regulations. The protocol and all subsequent amendments were reviewed and approved by institutional review boards or independent ethics committees at each site. All subjects provided written informed consent.
References
- 1.Girolomoni G, de Bruin-Weller M, Aoki V, et al. Nomenclature and clinical phenotypes of atopic dermatitis. Ther Adv Chronic Dis. 2021;12:20406223211002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bylund S, Kobyletzki LB, Svalstedt M, Svensson A. Prevalence and incidence of atopic dermatitis: a systematic review. Acta Derm Venereol. 2020;100(12):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richard MA, Paul C, Nijsten T, et al. Prevalence of most common skin diseases in Europe: a population-based study. J Eur Acad Dermatol Venereol. 2022;36(7):1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–7. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg J, Gelfand JM, Margolis DJ, et al. Pain is a common and burdensome symptom of atopic dermatitis in United States adults. J Allergy Clin Immunol Pract. 2019;7(8):2699-706.e7. [DOI] [PubMed] [Google Scholar]
- 6.Vakharia PP, Chopra R, Sacotte R, et al. Burden of skin pain in atopic dermatitis. Ann A llergy Asthma Immunol. 2017;119(6):548-52.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thyssen JP, Halling-Sønderby AS, Wu JJ, Egeberg A. Pain severity and use of analgesic medication in adults with atopic dermatitis: a cross-sectional study. Br J Dermatol. 2019;20:5. 10.1111/bjd.18557. [DOI] [PubMed] [Google Scholar]
- 8.Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): Insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–8. [DOI] [PubMed] [Google Scholar]
- 9.Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–90. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Pérez J, Daudén-Tello E, Mora AM, Lara SN. Impact of atopic dermatitis on health-related quality of life in Spanish children and adults: the PSEDA study. Actas Dermosifiliogr. 2013;104(1):44–52. [DOI] [PubMed] [Google Scholar]
- 11.Torrelo A, Ortiz J, Alomar A, Ros S, Prieto M, Cuervo J. Atopic dermatitis: impact on quality of life and patients’ attitudes toward its management. Eur J Dermatol. 2012;22(1):97–105. [DOI] [PubMed] [Google Scholar]
- 12.Bobotsis R, Fleming P, Eshtiaghi P, Cresswell-Melville A, Drucker AM. A Canadian adult cross-sectional survey of the burden of moderate to severe atopic dermatitis. J Cutan Med Surg. 2018;22(4):445–6. [DOI] [PubMed] [Google Scholar]
- 13.Wei W, Ghorayeb E, Andria M, et al. A real-world study evaluating adeQUacy of existing systemic treatments for patients with moderate-to-severe atopic dermatitis (QUEST-AD): baseline treatment patterns and unmet needs assessment. Ann Allergy Asthma Immunol. 2019;123(4):381-8.e2. [DOI] [PubMed] [Google Scholar]
- 14.de Bruin-Weller M, Gadkari A, Auziere S, et al. The patient-reported disease burden in adults with atopic dermatitis: a cross-sectional study in Europe and Canada. J Eur Acad Dermatol Venereol. 2019. 10.1111/jdv.16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiteley J, Emir B, Seitzman R, Makinson G. The burden of atopic dermatitis in US adults: results from the 2013 National Health and Wellness Survey. Curr Med Res Opin. 2016;32(10):1645–51. [DOI] [PubMed] [Google Scholar]
- 16.Yano C, Saeki H, Ishiji T, et al. Impact of disease severity on work productivity and activity impairment in Japanese patients with atopic dermatitis. J Dermatol. 2013;40(9):736–9. [DOI] [PubMed] [Google Scholar]
- 17.Andersen L, Nyeland ME, Nyberg F. Increasing severity of atopic dermatitis is associated with a negative impact on work productivity among adults with atopic dermatitis in France, Germany, the UK and the USA. Br J Dermatol. 2019. 10.1111/bjd.18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverberg J, Gelfand JM, Margolis DJ, et al. Health utility scores of atopic dermatitis in US adults. J Allergy Clin Immunol Pract. 2019;7(4):1246-52.e1. [DOI] [PubMed] [Google Scholar]
- 19.Maksimović N, Janković S, Marinković J, Sekulović LK, Zivković Z, Spirić VT. Health-related quality of life in patients with atopic dermatitis. J Dermatol. 2012;39(1):42–7. [DOI] [PubMed] [Google Scholar]
- 20.Kim DH, Li K, Seo SJ, et al. Quality of life and disease severity are correlated in patients with atopic dermatitis. J Korean Med Sci. 2012;27(11):1327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz de Frutos FJ, Torrelo A, de Lucas R, et al. Patient perspectives on triggers, adherence to medical recommendations, and disease control in atopic dermatitis: the DATOP study. Actas Dermosifiliogr. 2014;105(5):487–96. [DOI] [PubMed]
- 22.Simpson EL, Guttman-Yassky E, Margolis DJ, et al. Association of inadequately controlled disease and disease severity with patient-reported disease burden in adults with atopic dermatitis. JAMA Dermatol. 2018;154(8):903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furue M, Furue M. OX40L-OX40 signaling in atopic dermatitis. J Clin Med. 2021;10(12):2578. [DOI] [PMC free article] [PubMed]
- 24.Guttman-Yassky E, Simpson EL, Reich K, et al. An anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: a multicentre, double-blind, placebo-controlled phase 2b study. Lancet. 2023;401(10372):204–14. [DOI] [PubMed] [Google Scholar]
- 25.Papp KA, Gooderham MJ, Girard G, Raman M, Strout V. Phase I randomized study of KHK4083, an anti-OX40 monoclonal antibody, in patients with mild to moderate plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31(8):1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le AM, Torres T. OX40-OX40L inhibition for the treatment of atopic dermatitis-focus on rocatinlimab and amlitelimab. Pharmaceutics. 2022;14(12):2753. [DOI] [PMC free article] [PubMed]
- 27.Nakagawa H, Iizuka H, Nemoto O, et al. Safety, tolerability and efficacy of repeated intravenous infusions of KHK4083, a fully human anti-OX40 monoclonal antibody, in Japanese patients with moderate to severe atopic dermatitis. J Dermatol Sci. 2020;99(2):82–9. [DOI] [PubMed] [Google Scholar]
- 28.Rams A, Baldasaro J, Bunod L, et al. Assessing itch severity: content validity and psychometric properties of a patient-reported pruritus numeric rating scale in atopic dermatitis. Adv Ther. 2024;41(4):1512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverberg JI, DeLozier A, Sun L, et al. Psychometric properties of the itch numeric rating scale, skin pain numeric rating scale, and atopic dermatitis sleep scale in adult patients with moderate-to-severe atopic dermatitis. Health Qual Life Outcomes. 2021;19(1):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel KR, Singam V, Vakharia PP, et al. Measurement properties of three assessments of burden used in atopic dermatitis in adults. Br J Dermatol. 2019;180(5):1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blauvelt A. Content evaluation of pruritus, skin pain, and sleep disturbance patient-reported outcome measures in adolescents and adults with moderate-to-severe atopic dermatitis: qualitative interviews. Br J Dermatol. 2024:ljae346. 10.1093/bjd/ljae346. [DOI] [PubMed]
- 32.Dias-Barbosa C, Matos R, Vernon M, Carney CE, Krystal A, Puelles J. Content validity of a sleep numerical rating scale and a sleep diary in adults and adolescents with moderate-to-severe atopic dermatitis. J Patient Rep Outcomes. 2020;4(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton L, DeLozier AM, Griffiths PC, et al. Exploring content and psychometric validity of newly developed assessment tools for itch and skin pain in atopic dermatitis. J Patient Rep Outcomes. 2019;3(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puelles J, Fofana F, Rodriguez D, et al. Psychometric validation and responder definition of the sleep disturbance numerical rating scale in moderate-to-severe atopic dermatitis. Br J Dermatol. 2022;186(2):285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230(1):27–33. [DOI] [PubMed] [Google Scholar]
- 36.Hinkle D, Wiersma W, Jurs S. Applied Statistics for the Behavioral Sciences. 5th ed: Houghton Mifflin; 2003.
- 37.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–48. [DOI] [PubMed] [Google Scholar]
- 38.Silverberg JI, Gelfand JM, Margolis DJ, et al. Severity strata for POEM, PO-SCORAD, and DLQI in US adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2018;121(4):464–8.e3. [DOI] [PubMed]
- 39.Holm EA, Wulf HC, Stegmann H, Jemec GB. Life quality assessment among patients with atopic eczema. Br J Dermatol. 2006;154(4):719–25. [DOI] [PubMed] [Google Scholar]
- 40.Shim WH, Park HJ, Kim HS, et al. Does the EASI score reflect itch severity? Ann Allergy Asthma Immunol. 2011;106(6):540–1. [DOI] [PubMed] [Google Scholar]
- 41.Yosipovitch G, Reaney M, Mastey V, et al. Peak pruritus numerical rating scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisshaar E, Bentz P, Apfelbacher C, et al. Itching in atopic dermatitis: patient- and physician-reported outcomes in the German Atopic Dermatitis Registry TREATgermany. Acta Derm Venereol. 2023;103:adv00854. [DOI] [PMC free article] [PubMed]
- 43.Feldman SR, Guerin A, Gauthier-Loiselle M, et al. Patient preferences for treatment attributes in moderate-to-severe atopic dermatitis: a discrete choice experiment. J Dermatol Treat. 2024;35(1):2345739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.