Abstract
Introduction
Psoriasis in high-impact areas, including the scalp, nails, palms, and soles, can disproportionately impair patient quality of life. Here, we evaluate the 2-year efficacy of bimekizumab treatment in patients with moderate to severe plaque psoriasis in post hoc analyses of five phase 3/3b trials.
Methods
High-impact area efficacy data were pooled through 2 years across five phase 3/3b trials: BE VIVID, BE READY, BE SURE, their ongoing open-label extension (OLE) BE BRIGHT, and BE RADIANT (including its double-blinded treatment period and the first year of its OLE). Complete clearance of psoriasis in high-impact areas is reported over 2 years using the scalp Investigator’s Global Assessment (IGA), palmoplantar IGA, and modified Nail Psoriasis Severity Index (mNAPSI). Patients included in these analyses had baseline moderate to severe scalp or palmoplantar involvement (scalp or palmoplantar IGA score ≥ 3) or mNAPSI score > 10.
Results
A total of 1107 patients were randomized to bimekizumab and entered the OLEs. Subsets of 821 patients had scalp IGA ≥ 3 at baseline, 377 had mNAPSI > 10, and 193 had palmoplantar IGA ≥ 3. Complete scalp clearance in patients with baseline scalp IGA ≥ 3 randomized to bimekizumab was achieved rapidly, with high responses sustained from first (86.4%) to second year (85.9%). Nail clearance responses in patients with baseline mNAPSI > 10 increased from 63.4% to 68.5% from first to second year. Palmoplantar clearance in patients with baseline palmoplantar IGA ≥ 3 was sustained from first (88.3%) to second year (89.8%). Similar trends were seen in the 374 patients who received bimekizumab 320 mg every 4 weeks (Q4W)/every 8 weeks (Q8W) initial/maintenance dosing.
Conclusion
In these analyses pooled across 2 years, bimekizumab showed sustained efficacy in psoriasis in high-impact areas.
Clinicaltrials.gov Trial Registration Numbers
NCT03370133, NCT03410992, NCT03412747, NCT03598790, NCT03536884.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01295-w.
Keywords: Bimekizumab, Clinical trial, Efficacy, High-impact areas, Nail, Palmoplantar, Palms, Soles, Psoriasis, Scalp
Plain Language Summary
Psoriasis in some body areas can have a bigger impact on the self-confidence and well-being of patients. These body areas, called high-impact areas, are often very visible or important for day-to-day activities. They include the scalp, fingernails, palms, and soles of the feet. People with psoriasis often find applying creams or ointments to these areas challenging. The treatment may also not be effective. Therefore, new medications that can clear psoriasis from these areas are needed by patients and physicians. Bimekizumab is a drug given by injection. We examined whether bimekizumab can clear psoriasis in high-impact areas over 2 years in five clinical trials. Psoriasis of the scalp, palms, and soles cleared quickly with bimekizumab. Most patients reported clear skin in these areas after 4 months, and skin remained clear for the rest of the 2-year period. After 2 years, 90% (18 in 20) of patients with psoriasis on their palms and soles saw it clear completely; 86% of patients (around 17 in 20) saw their scalp psoriasis completely cleared. Nail psoriasis took slightly longer to clear, because nails grow more slowly. Nevertheless, 63% of patients (around 13 in 20) had completely clear nails after 1 year and 69% of patients (around 14 in 20) had clear nails after 2 years. Bimekizumab can clear psoriasis in high-impact areas quickly, and this is maintained over the long-term. Bimekizumab can provide a lasting treatment option for areas of the body which are difficult to treat and have a big impact on patients’ lives.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01295-w.
Key Summary Points
| Why carry out this study? |
| Plaque psoriasis in high-impact areas, including the scalp, nails, palms, and soles, can disproportionately impair the health-related quality of life of patients. Therefore, the efficacy of biologic treatments in these areas is of considerable interest to patients and physicians. |
| This study evaluated the 2-year efficacy of bimekizumab treatment in patients with moderate to severe plaque psoriasis in post hoc analyses of five phase 3/3b trials. |
| What was learned from the study? |
| Bimekizumab-treated patients showed high efficacy responses in these high-impact areas, which were sustained over 2 years. |
| Bimekizumab can provide a lasting treatment option for patients with psoriasis affecting high-impact areas. |
Digital Features
This article is published with digital features, including a video to facilitate understanding of the article. To view digital features for this article, go to 10.6084/m9.figshare.27210411
Bimekizumab efficacy in high-impact areas: pooled 2-year analysis in scalp, nail, and palmoplantar psoriasis from phase 3/3b randomized controlled trials (MP4 532263 KB)
Introduction
Psoriatic lesions in highly visible and functional areas, such as the scalp, nails, palms, and soles, can cause patients disproportionately high levels of physical impairment and emotional distress [1–4]. In patients with psoriasis, involvement of the scalp, nail, and palmoplantar areas (palms and soles) is among the strongest predictors of reduced health-related quality of life [1]. Physical manifestations result in substantial internalized stigma, feelings of shame and embarrassment, and difficulties with daily life, and place a significant burden on the families of patients with psoriasis [5–7]. Therefore, clearance of psoriasis in these high-impact areas is of considerable interest to both patients and physicians [8, 9].
Scalp, nail, and palmoplantar psoriasis can be difficult to treat topically, with topical administration in these regions often limiting patient satisfaction, leading clinicians to offer systemic therapy instead [2, 9–11]. While biologics are the recommended treatment for psoriasis in high-impact areas, the limited published data in these regions can impact the decision-making ability of physicians [2, 12]; specifically, there is a lack of long-term results in large study populations [2, 12–14]. This poses challenges to the understanding of psoriasis in these areas, potentially leading to undertreatment and poorer clinical outcomes [2, 12].
Bimekizumab is a monoclonal immunoglobulin G1 antibody that selectively inhibits interleukin (IL)-17F in addition to IL-17A [15]. Dual inhibition with bimekizumab in patients with moderate to severe plaque psoriasis has resulted in superior efficacy compared with placebo, adalimumab (anti-tumor necrosis factor [TNF]), ustekinumab (anti-IL-12/23), and secukinumab (anti-IL-17A) [16–19]. Bimekizumab has demonstrated long-term efficacy across phase 3/3b trials over 2 years, as indicated by complete and near-complete skin clearance [20, 21], and is well tolerated [22].
Here, we evaluate scalp, nail, and palmoplantar outcome data through 2 years of bimekizumab treatment among patients with plaque psoriasis involvement of these areas at baseline, across five phase 3/3b trials in the bimekizumab in plaque psoriasis clinical program.
Methods
Patients
Efficacy data were pooled from the BE VIVID (NCT03370133), BE READY (NCT03410992), and BE SURE (NCT03412747) phase 3 trials, the first year of their ongoing open-label extension (OLE), BE BRIGHT (NCT03598790), and 2 years of the BE RADIANT (NCT03536884) phase 3b trial, incorporating the first year of its ongoing OLE [16–19, 23]. The last patient’s 2-year study visit (OLE Week 48) occurred on 8 December 2020 in BE BRIGHT and 6 May 2020 in BE RADIANT.
Full inclusion and exclusion criteria have been reported previously [16–19, 22]. Eligible patients were adults with moderate to severe plaque psoriasis, defined as a baseline Psoriasis Area and Severity Index (PASI) score ≥ 12, body surface area (BSA) affected by psoriasis ≥ 10%, and an Investigator’s Global Assessment (IGA) score ≥ 3 on a 5-point scale. Included patients were eligible for systemic psoriasis therapy and/or phototherapy. Patients were excluded if they had experienced primary failure (no response within 12 weeks) to ≥ 1 IL-17 biologic response modifier (such as brodalumab, ixekizumab, and secukinumab), or to ≥ 2 non-IL-17 biologic response modifiers (such as adalimumab and ustekinumab). Patients included in these analyses were randomized to receive bimekizumab 320 mg subcutaneously every 4 weeks (Q4W) to Week 16, received bimekizumab either Q4W or every 8 weeks (Q8W) to the end of the first year (BE RADIANT Week 48; BE VIVID Week 52; BE READY/BE SURE Week 56), and entered the OLEs.
Regional psoriasis presentation was analyzed in these studies using three measures: the modified Nail Psoriasis Severity Index (mNAPSI), providing a total fingernail score on a 0–130 scale (0–13 per fingernail), and scalp and palmoplantar IGA, 5-point scales ranging 0–4 (3, moderate; 4, severe). Subsets of patients included in these analyses had moderate to severe scalp or palmoplantar involvement (i.e., scalp or palmoplantar IGA score ≥ 3) or an mNAPSI score > 10 at baseline.
Study Designs
Study designs for BE VIVID, a 52-week trial comparing bimekizumab versus placebo and ustekinumab; BE READY, a 56-week trial comparing bimekizumab versus placebo; BE SURE, a 56-week trial comparing bimekizumab versus adalimumab; and the 48-week double-blinded treatment period of BE RADIANT, comparing bimekizumab versus secukinumab, have been reported previously [16–19].
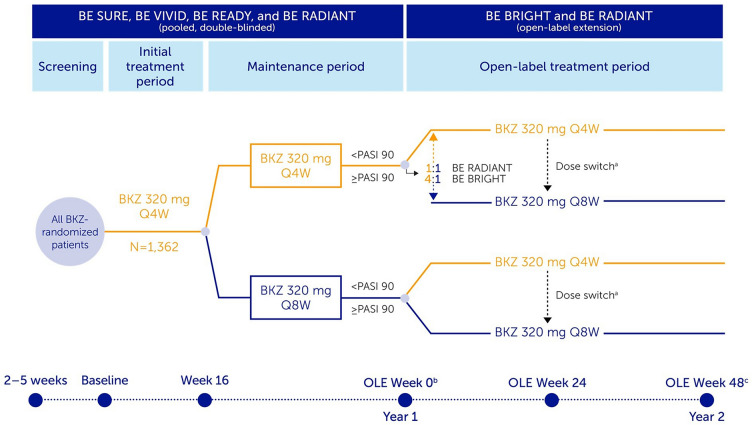
Patients eligible for enrollment into the BE BRIGHT OLE had completed Week 52 of BE VIVID, or Week 56 of the BE READY or BE SURE feeder trials. BE BRIGHT is an ongoing, open-label, multicenter trial in which patients’ bimekizumab dose was dependent on treatment, dose, and PASI response at the end of the feeder trials (Week 52/56; Fig. 1). Those eligible for enrollment into the BE RADIANT OLE had completed Week 48 of the BE RADIANT trial. BE RADIANT is an ongoing, phase 3b, multicenter trial; the OLE began at Week 48. Upon OLE entry, all patients received open-label bimekizumab 320 mg Q4W or Q8W. BE RADIANT OLE dose frequency was dependent on PASI response at Week 48 and double-blinded period treatment (Fig. 1).
Fig. 1.
Study design. At baseline, 1362 patients with plaque psoriasis were randomized to bimekizumab (BKZ) across the included trials; 1107 of these patients received continuous BKZ and entered the open-label extensions (OLEs). In this analysis, BKZ every 4 weeks (Q4W) and every 8 weeks (Q8W) treatment arms shown are pooled for the BKZ total group. At OLE Week 0, patients receiving BKZ Q4W who achieved ≥ 90% improvement from baseline in Psoriasis Area and Severity Index (PASI 90) were re-randomized 4:1 in BE BRIGHT and 1:1 in BE RADIANT to open-label BKZ Q4W or Q8W; patients receiving BKZ Q8W who achieved PASI 90 at OLE Week 0 remained on Q8W dosing. Patients who did not achieve PASI 90 received open-label BKZ Q4W dosing. In BE READY, patients who did not achieve PASI 90 at Week 16, and patients who do not achieve ≥ 75% improvement from baseline in PASI (PASI 75) at any visit during the maintenance period, could enter a 12-week open-label BKZ 320 mg Q4W escape arm; if they achieved ≥ 50% improvement from baseline in PASI (50) at the end of the 12-week escape arm, they could then enter the BE BRIGHT OLE. Study designs for included trials have been published previously [16–19]. aDose switch: BE BRIGHT OLE Week 24, for patients achieving PASI 90 at investigator discretion; BE RADIANT OLE Week 16 or next scheduled clinic visit, dose switch added via protocol amendment; bAs a result of a lack of common visits across the studies, OLE Week 0 (the end of Year 1) corresponds to Week 48 for BE SURE, BE READY, and BE RADIANT, and Week 52 for BE VIVID;cOLE Week 48 (the end of Year 2) corresponds to BE RADIANT Week 96, BE VIVID/BE BRIGHT Week 100, and BE READY/BE BRIGHT and BE SURE/BE BRIGHT Week 104. BKZ bimekizumab, OLE open-label extension, PASI 90 ≥ 90% improvement from baseline in Psoriasis Area and Severity Index, Q4W every 4 weeks, Q8W every 8 weeks
Across both studies, patients and study sites remained blinded to double-blinded period treatments and dose adjustments could occur in the OLE periods. Following implementation of a protocol amendment in BE RADIANT, all patients receiving bimekizumab 320 mg Q4W were switched to bimekizumab Q8W at OLE Week 16, or the next scheduled clinic visit. At Week 24 of the BE BRIGHT OLE, for patients receiving bimekizumab 320 mg Q4W and achieving ≥ 90% improvement from baseline in PASI (PASI 90), the investigator was permitted to change the patient’s dosing interval from Q4W to Q8W.
Here, we report results of an interim analysis which included data collected over 2 years, from baseline of the BE VIVID, BE READY, BE SURE, and BE RADIANT trials, up to OLE Week 48 in BE BRIGHT and the BE RADIANT OLE.
Studies were conducted in accordance with the principles of the Declaration of Helsinki and approved by an independent review board and independent ethics committee. All participants provided informed written consent documented in accordance with local regulations.
Outcomes
The proportions of patients who entered the OLEs and achieved complete regional psoriasis clearance (scalp IGA 0, mNAPSI 0, palmoplantar IGA 0) are reported through 2 years (OLE Week 48). Photographs taken of psoriatic nails at baseline and Week 48, to show clearance over time, are also presented. Additionally, for the same overall group of patients, we report the mean percentage change from feeder study baseline in scalp IGA, mNAPSI, and palmoplantar IGA through 2 years (OLE Week 48).
Statistical Analysis
For all results, descriptive statistics were used to provide an overview of the results. No statistical hypothesis testing was performed on these post hoc analyses.
Pooled data are reported for all patients randomized to bimekizumab at baseline who received at least one bimekizumab dose in BE BRIGHT or in the BE RADIANT OLE period (bimekizumab total group). Data were also analyzed for the subset of patients who received bimekizumab 320 mg Q4W from baseline to Week 16, followed by Q8W for the maintenance period, and continued to receive Q8W upon OLE entry (bimekizumab Q4W/Q8W).
For scalp, nail, and palmoplantar outcomes over 2 years, missing data were primarily accounted for using modified non-responder imputation (mNRI); NRI and observed case (OC) data are also reported, with NRI analyzed continuously from baseline. For mNRI, patients with missing data following treatment discontinuation due to lack of efficacy or treatment-related adverse events were considered non-responders; multiple imputation methodology was used for all other missing data. Patients who entered the BE READY escape arm (Fig. 1) were considered as non-responders from the date of escape until the end of BE READY, after which they were considered in the same way as all other non-escape patients during the BE BRIGHT OLE. For change from baseline data, data are reported using last observation carried forward (LOCF) to account for missing data, and as OC.
Results
Patient Disposition and Baseline Characteristics
Of the 1362 patients who were randomized to receive bimekizumab at baseline across the included trials, 1107 continued to receive bimekizumab at Week 16 and entered the BE BRIGHT and BE RADIANT OLEs (bimekizumab total group). Among these 1107 patients, a subset of 821 had scalp IGA ≥ 3 at baseline (74.2%), 377 had mNAPSI > 10 (34.1%), and 193 had palmoplantar IGA ≥ 3 (17.4%). The bimekizumab Q4W/Q8W dosing regimen was followed by a subset of 374 patients; among these, 277 had scalp IGA ≥ 3 at baseline (74.1%), 129 had mNAPSI > 10 (34.5%), and 52 had palmoplantar IGA ≥ 3 (13.9%).
Baseline characteristics were representative of patients with plaque psoriasis eligible for biologic therapy and were generally comparable between bimekizumab groups and across the high-impact area subgroups assessed, with the exception that > 80% of patients with a baseline mNAPSI > 10 were male, whereas < 70% of patients with scalp IGA ≥ 3 were male (Table 1) [22].
Table 1.
Baseline patient characteristics
| Scalp IGA ≥ 3 (N = 821) |
mNAPSI > 10 (N = 377) |
Palmoplantar IGA ≥ 3 (N = 193) |
||||
|---|---|---|---|---|---|---|
| BKZ total (N = 821) |
BKZ Q4W/Q8W (n = 277) |
BKZ total (N = 377) |
BKZ Q4W/Q8W (n = 129) |
BKZ total (N = 193) |
BKZ Q4W/Q8W (n = 52) |
|
| Age (years), mean ± SD | 44.8 ± 13.7 | 44.0 ± 13.9 | 44.8 ± 13.1 | 44.5 ± 13.2 | 45.0 ± 12.9 | 43.8 ± 11.5 |
| Male, n (%) | 569 (69.3) | 192 (69.3) | 316 (83.8) | 107 (82.9) | 144 (74.6) | 41 (78.8) |
| White, n (%) | 715 (87.1) | 259 (93.5) | 328 (87.0) | 123 (95.3) | 162 (83.9) | 49 (94.2) |
| Weight (kg), mean ± SD | 89.8 ± 21.4 | 88.8 ± 21.0 | 92.2 ± 20.7 | 92.1 ± 20.6 | 85.9 ± 18.7 | 87.0 ± 17.4 |
| Duration of psoriasis (years), mean ± SD | 18.1 ± 12.6 | 18.6 ± 12.4 | 18.9 ± 12.4 | 18.8 ± 12.2 | 17.7 ± 12.1 | 18.8 ± 9.8 |
| PsA status, n (%) | ||||||
| PASE ≥ 47 | 131 (16.0) | 36 (13.0) | 80 (21.2) | 25 (19.4) | 38 (19.7) | 6 (11.5) |
| Reported history of PsA | 194 (23.6) | 53 (19.1) | 107 (28.4) | 31 (24.0) | 54 (28.0) | 8 (15.4) |
| Either PASE ≥ 47 or reported history of PsA | 194 (23.6) | 53 (19.1) | 107 (28.4) | 31 (24.0) | 54 (28.0) | 8 (15.4) |
| PASI, mean ± SD | 21.4 ± 8.0 | 20.9 ± 7.7 | 22.4 ± 8.5 | 21.6 ± 8.0 | 23.9 ± 9.0 | 26.9 ± 10.6 |
| BSA (%), mean ± SD | 26.6 ± 16.0 | 24.5 ± 13.5 | 28.9 ± 17.6 | 25.7 ± 13.8 | 30.5 ± 17.4 | 31.6 ± 15.6 |
| IGA score, n (%) | ||||||
| 3: moderate | 527 (64.2) | 189 (68.2) | 212 (56.2) | 75 (58.1) | 109 (56.5) | 24 (46.2) |
| 4: severe | 294 (35.8) | 88 (31.8) | 163 (43.2) | 53 (41.1) | 83 (43.0) | 27 (51.9) |
|
DLQI total score, mean ± SD |
10.8 ± 6.5 | 10.7 ± 6.6 | 10.7 ± 6.6 | 11.1 ± 6.0 | 11.3 ± 7.1 | 11.8 ± 7.0 |
|
Scalp IGA score, mean ± SD |
3.2 ± 0.4 | 3.2 ± 0.4 | 2.8 ± 1.0 | 2.8 ± 0.9 | 3.0 ± 0.8 | 3.1 ± 0.7 |
|
mNAPSI score, mean ± SD |
11.6 ± 17.8 | 11.1 ± 16.2 | 31.0 ± 20.5 | 28.2 ± 16.9 | 21.9 ± 28.0 | 22.9 ± 23.7 |
| Pattern of nail involvement, n (%) | ||||||
| Nail matrix | 410 (86.0) | 135 (85.4) | 359 (95.2) | 124 (96.1) | 124 (93.2) | 35 (94.6) |
| Nail bed | 405 (84.9) | 140 (88.6) | 357 (94.7) | 121 (93.8) | 117 (88.0) | 35 (94.6) |
| Palmoplantar IGA score, mean ± SD | 0.9 ± 1.3 | 0.8 ± 1.2 | 1.3 ± 1.4 | 1.1 ± 1.4 | 3.2 ± 0.4 | 3.2 ± 0.4 |
| Any prior systemic therapy, n (%) | 635 (77.3) | 209 (75.5) | 297 (78.8) | 100 (77.5) | 163 (84.5) | 45 (86.5) |
| Any prior biologic therapy, n (%) | 306 (37.3) | 95 (34.3) | 139 (36.9) | 41 (31.8) | 70 (36.3) | 18 (34.6) |
| Anti-TNF | 123 (15.0) | 33 (11.9) | 63 (16.7) | 14 (10.9) | 34 (17.6) | 4 (7.7) |
| Anti-IL-17 | 160 (19.5) | 51 (18.4) | 83 (22.0) | 27 (20.9) | 39 (20.2) | 15 (28.8) |
| Anti-IL-12/23 | 54 (6.6) | 19 (6.9) | 19 (5.0) | 6 (4.7) | 7 (3.6) | 1 (1.9) |
| Anti-IL-23 | 47 (5.7) | 18 (6.5) | 12 (3.2) | 6 (4.7) | 8 (4.1) | 1 (1.9) |
Data are presented for patients with plaque psoriasis initially randomized to bimekizumab (BKZ) who later entered the open-label extension (OLE). In this analysis, BKZ 320 mg every 4 weeks (Q4W) and every 8 weeks (Q8W) treatment arms are pooled for the BKZ total group
BKZ bimekizumab, BSA body surface area, DLQI Dermatology Life Quality Index, IGA Investigator’s Global Assessment, IL interleukin, mNAPSI modified Nail Psoriasis Severity Index, OLE open-label extension, PASE Psoriatic Arthritis Screening and Evaluation, PASI Psoriasis Area and Severity Index, PsA psoriatic arthritis, Q4W every 4 weeks, Q8W every 8 weeks, SD standard deviation, TNF tumor necrosis factor
Among the 1107 patients in the bimekizumab total group who entered the OLEs, the mean PASI score at baseline was 20.9, with mean scalp IGA, mNAPSI, and palmoplantar IGA baseline scores of 2.8, 11.8, and 0.9, respectively. Within the subset of 821 patients with scalp IGA ≥ 3 at baseline, the mean scalp IGA score was 3.2; within the 377 with mNAPSI > 10 at baseline, the mean mNAPSI score was 31.0; and within the 193 with palmoplantar IGA ≥ 3 at baseline, the mean palmoplantar IGA was 3.2 (Table 1). Mean PASI scores in these subsets at baseline were 21.4, 22.4, and 23.9, respectively. Similar values were observed for the patient subsets receiving bimekizumab Q4W/Q8W.
Proportions Achieving Complete Clearance
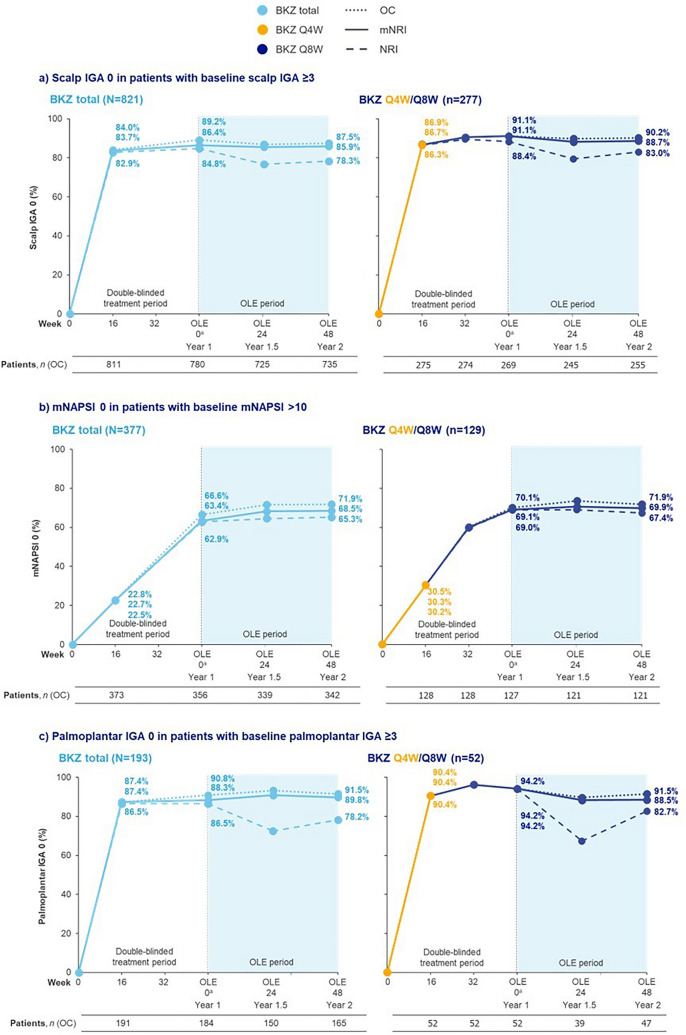
Among patients with scalp IGA ≥ 3 at baseline, complete scalp clearance was achieved rapidly, with 83.7% of patients who received bimekizumab achieving complete scalp clearance at Week 16. These high levels of response were stable through to the end of the second year, sustained from 86.4% and 91.1% of patients at Year 1 (OLE Week 0) to 85.9% and 88.7% of patients at Year 2 (OLE Week 48) in the bimekizumab total group and bimekizumab Q4W/Q8W subgroup, respectively (Fig. 2a).
Fig. 2.
Complete clearance of high-impact areas over 2 years. Data are presented for patients with plaque psoriasis initially randomized to bimekizumab (BKZ) who later entered the open-label extension (OLE). For modified non-responder imputation (mNRI), patients with missing data following treatment discontinuation due to lack of efficacy or treatment-related adverse events were considered non-responders; multiple imputation methodology was used for all other missing data. BKZ total consists of patients randomized to receive BKZ 320 mg every 4 weeks (Q4W) to Week 16, received either BKZ Q4W or every 8 weeks (Q8W) to the end of the first year (Week 48/52/56), and entered the OLE. BKZ Q4W/Q8W consists of patients randomized to BKZ 320 mg Q4W to Week 16, who received BKZ Q8W throughout the maintenance period and on OLE entry; there are no BE VIVID patients in this treatment arm. Only visits common to all the studies included in a treatment arm are presented. aAs a result of a lack of common visits across the studies, OLE Week 0 corresponds to Week 48 for BE SURE, BE READY, and BE RADIANT, and Week 52 for BE VIVID. BKZ bimekizumab, IGA Investigator’s Global Assessment, mNAPSI modified Nail Psoriasis Severity Index, mNRI modified non-responder imputation, NRI non-responder imputation, OC observed case, OLE open-label extension, Q4W every 4 weeks, Q8W every 8 weeks
For patients with baseline mNAPSI > 10, rates of complete nail clearance increased to 63.4% at the end of the first year of treatment for patients in the bimekizumab total group, and 69.1% for the bimekizumab Q4W/Q8W subgroup. In the second year, the complete nail clearance rate in the bimekizumab total group was stable, at 68.5% at Year 2 (Fig. 2b), and similarly at 69.9% for the bimekizumab Q4W/Q8W subgroup.
Comparable trends to scalp IGA were observed in the proportions of patients achieving complete palmoplantar clearance among patients with palmoplantar IGA ≥ 3 at baseline. A rapid response was observed, with a large proportion of patients achieving palmoplantar clearance at Week 16, at 87.4% for the bimekizumab total group. High rates of complete palmoplantar clearance were maintained through to the end of the second year, sustained from 88.3% at Year 1 to 89.8% at Year 2 in patients in the bimekizumab total group (Fig. 2c). Similarly, for the patient subset treated with bimekizumab Q4W/Q8W, results remained high and largely stable over 2 years at 94.2% at Year 1 to 88.5% at Year 2.
NRI data are presented for complete scalp, nail, and palmoplantar clearance in Table S2.
BE RADIANT Bimekizumab Treatment Examples
Representative photographs showing complete clearance of nail psoriasis at Week 48 are provided in Fig S1.
Change from Baseline
For included patients with regional psoriasis involvement at baseline, after 1 year of treatment in the bimekizumab total group, the mean percentage change in score from baseline for scalp IGA, mNAPSI, and palmoplantar IGA was − 93.4%, − 90.0%, and − 95.2%, respectively (Fig. 3); these scores were sustained after 2 years at − 93.4%, − 90.7%, and − 96.3% for the respective outcomes (Fig. 3).
Fig. 3.
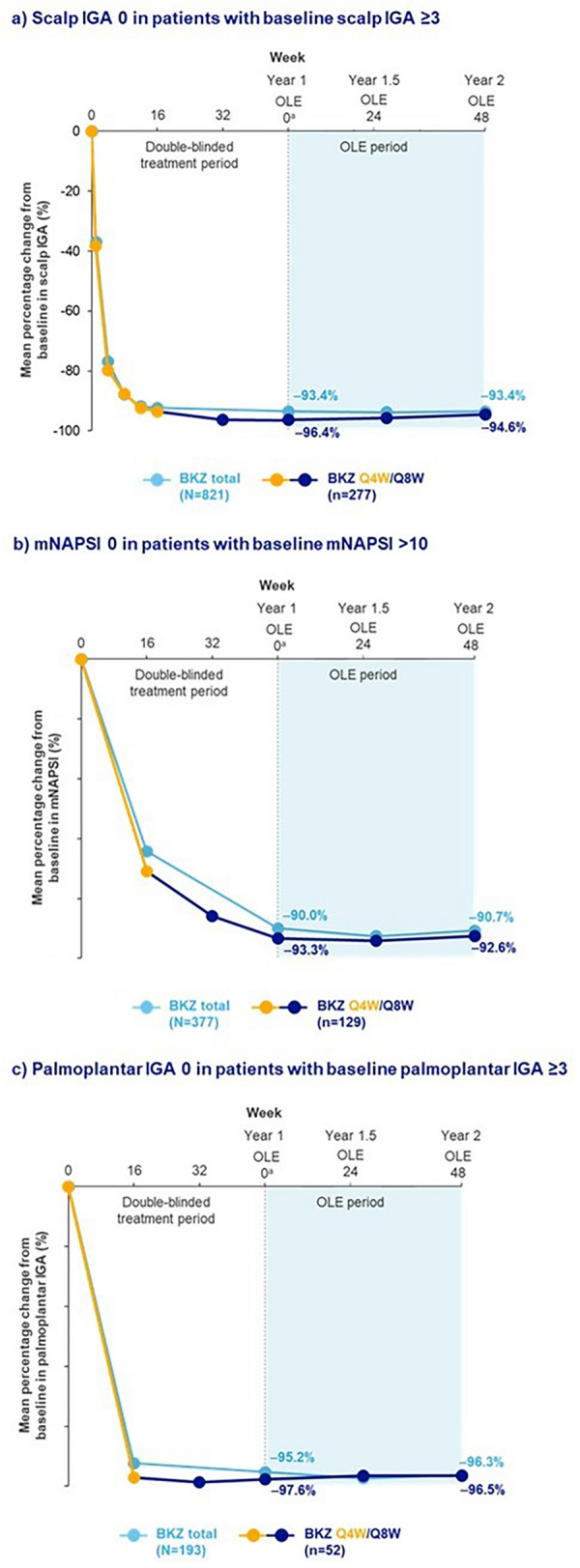
Change from baseline in high-impact areas over 2 years. Data are presented for patients with plaque psoriasis initially randomized to bimekizumab (BKZ) who later entered the open-label extension (OLE). Last observation carried forward (LOCF) was used for missing data. For BKZ every 4 weeks (Q4W)/every 8 weeks (Q8W), the switch from yellow line to blue line represents the switch from BKZ 320 mg Q4W to BKZ 320 mg Q8W at Week 16. BKZ total consists of patients randomized to receive BKZ 320 mg Q4W to Week 16, and who received either BKZ Q4W or Q8W to the end of the first year (Week 48/52/56), and entered the OLE. BKZ Q4W/Q8W consists of patients randomized to BKZ 320 mg Q4W to Week 16, who received BKZ Q8W throughout the maintenance period and on OLE entry; there are no BE VIVID patients in this treatment arm. Only visits common to all the studies included in a treatment arm are presented. aAs a result of a lack of common visits across the studies, OLE Week 0 corresponds to Week 48 for BE SURE, BE READY, and BE RADIANT, and Week 52 for BE VIVID. BKZ bimekizumab, IGA Investigator’s Global Assessment, LOCF last observation carried forward, mNAPSI modified Nail Psoriasis Severity Index, OLE open-label extension, Q4W every 4 weeks, Q8W every 8 weeks
In the patient subset treated with bimekizumab Q4W/Q8W, after 1 year of treatment, the mean change in score from baseline for scalp IGA, mNAPSI, and palmoplantar IGA was − 96.4%, − 93.3%, and − 97.6%, respectively (Fig. 3). After 2 years, these scores were sustained at − 94.6%, − 92.6%, and − 96.5% for the respective outcome (Fig. 3). OC data are presented in Table S1.
Discussion
These analyses report pooled 2-year bimekizumab efficacy data for patients who had plaque psoriasis in high-impact areas at baseline.
Complete and sustained clearance of scalp and palmoplantar psoriasis was achieved in a high percentage of bimekizumab-treated patients over 2 years. Scalp clearance and palmoplantar clearance were achieved rapidly, and clearance rates remained generally stable from Week 16 through to the end of the 2-year observation period. Rates of complete nail clearance increased through the first year of bimekizumab treatment, reflective of the longer timescale required for nail growth and repair, and were largely sustained through the second year across patients receiving bimekizumab. In parallel, high mean reductions from baseline in scalp, nail, and palmoplantar psoriasis were achieved after 1 year of bimekizumab treatment and these reductions were durable over 2 years, including for the patient subset receiving bimekizumab Q4W/Q8W.
To date, there has been a lack of biologic agents with specific indications for nail and palmoplantar psoriasis, despite biologics being the recommended treatment for high-impact areas. Study data directly comparing the efficacy of biologics are also sparse. This highlights the importance of the few trials evaluating biologic efficacy in these regions [2, 12, 24, 25]. The IL-17A inhibitor, secukinumab, was assessed in palmoplantar psoriasis in the GESTURE phase 3b trial, with 53% and 59% of patients achieving palmoplantar IGA 0/1 after 2.5 years of treatment with 150 mg and 300 mg secukinumab, respectively [26]. Secukinumab was also assessed in nail psoriasis in the TRANSFIGURE phase 3b trial, demonstrating efficacy after 2.5 years of treatment, with a mean percentage change in NAPSI score of − 73.3% [27]. The higher response rates observed after 2 years of bimekizumab treatment in the trials reported here (bimekizumab total group: 89.8% palmoplantar clearance, − 90.7% mean change from baseline in mNAPSI), while not directly comparable, support a hypothesis that there is an additional benefit from inhibiting IL-17F in addition to IL-17A in palmoplantar and nail psoriasis [20]. Adalimumab, a TNF inhibitor, has also been evaluated in nail psoriasis, with 54.5% of patients achieving at least 75% reduction from baseline in mNAPSI after 52 weeks in a phase 3 trial [28]. Furthermore, while few network meta-analyses comparing the efficacy of biologics in nail, palmoplantar, and scalp psoriasis have been performed, one previously published network meta-analysis ranked bimekizumab among the top three/four most efficacious biologics for complete resolution of nail psoriasis at 24–28 weeks/48–52 weeks, though longer-term comparisons are limited [29].
Nail psoriasis is observed in 80% of patients with psoriatic arthritis (PsA), and strong evidence suggests that it is a predictor of joint disease [30]. The high efficacy of bimekizumab in nail regions (shown here), and positive results of recent bimekizumab in PsA phase 3 trials [31, 32], may highlight the connection between the nail matrix and enthesitis of nearby joints, supporting the presence of nail psoriasis as an at-risk phenotype that bimekizumab can target [30, 33].
Over 2 years, bimekizumab has demonstrated a favorable safety profile with no safety signals observed for all included patients, as reported previously [21, 22].
The main strengths of this analysis are length of observation (2 years; 100–104 weeks for BE BRIGHT and 96 weeks for BE RADIANT) and large sample size obtained from pooling patients from across five phase 3/3b trials, enabling a thorough examination of bimekizumab efficacy in high-impact areas. Furthermore, this study provides long-term high-impact area efficacy data for the bimekizumab Q8W dosing regimen, the recommended maintenance dose for the vast majority of patients [34].
Limitations of this analysis involve the inclusion of open-label trial designs, which could have increased risk of bias. The small number of patients (52) treated with bimekizumab Q4W/Q8W in the palmoplantar IGA ≥ 3 group may limit conclusions that can be drawn regarding this patient subgroup. Moreover, there may be limitations to how applicable findings are to real-world populations due to minimal racial diversity in the patient population, and stringent patient eligibility criteria, which excluded certain groups, including patients with comorbidities. Finally, this study examined bimekizumab’s efficacy in patients with moderate to severe plaque psoriasis overall, who also had scalp, nail, and palmoplantar high-impact area involvement; further study in patients with inverse psoriasis (i.e., patients with psoriasis of the body folds with or without involvement of classical body regions) [35] may be valuable. Additionally, many patients experience psoriasis affecting the genitals, which can result in significant impacts on quality of life [36, 37]; while no genital psoriasis data were available from these clinical trials, in a recent real-world study in Italy, 98.4% of patients with moderate to severe plaque psoriasis with genital involvement achieved a Static Physician Global Assessment of Genitalia score of 0 (clear) after 16 weeks of bimekizumab treatment [38]. Further future studies exploring bimekizumab’s efficacy in patients with genital psoriasis would also be of interest.
Conclusion
Bimekizumab provided long-term maintenance of efficacy outcomes over 2 years in high-impact areas in patients with moderate to severe plaque psoriasis. In addition to positive scalp and palmoplantar responses, complete nail clearance was also observed and was generally sustained through the second year.
To view a video summary of this manuscript, please view the online version or follow the digital features link under the abstract.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients, the investigators, and their teams who took part in this study. This research was funded by UCB and supported by the National Institute for Health and Care Research (NIHR) Manchester Biomedical Research Centre (NIHR203308).
Medical Writing and Editorial Assistance
The authors also acknowledge Poppy Wilson, MBiol, Costello Medical, London, UK for medical writing and editorial assistance based on the authors’ input and direction, funded by UCB.
Author Contributions
Substantial contributions to study conception and design: Joseph F. Merola, Alice B. Gottlieb, Andreas Pinter, Boni Elewski, Melinda Gooderham, Richard B. Warren, Stefano Piaserico, Krista Wixted, Nancy Cross, Nicola Tilt, Susanne Wiegratz, Ulrich Mrowietz; substantial contributions to analysis and interpretation of the data: Joseph F. Merola, Alice B. Gottlieb, Andreas Pinter, Boni Elewski, Melinda Gooderham, Richard B. Warren, Stefano Piaserico, Krista Wixted, Nancy Cross, Nicola Tilt, Susanne Wiegratz, Ulrich Mrowietz; drafting the article or reviewing it critically for important intellectual content: Joseph F. Merola, Alice B. Gottlieb, Andreas Pinter, Boni Elewski, Melinda Gooderham, Richard B. Warren, Stefano Piaserico, Krista Wixted, Nancy Cross, Nicola Tilt, Susanne Wiegratz, Ulrich Mrowietz; final approval of the version of the article to be published: Joseph F. Merola, Alice B. Gottlieb, Andreas Pinter, Boni Elewski, Melinda Gooderham, Richard B. Warren, Stefano Piaserico, Krista Wixted, Nancy Cross, Nicola Tilt, Susanne Wiegratz, Ulrich Mrowietz.
Funding
These studies were sponsored by UCB. This article was based on the original studies BE VIVID (NCT03370133), BE READY (NCT03410992), BE SURE (NCT03412747), BE BRIGHT (NCT03598790) and BE RADIANT (NCT03536884), sponsored by UCB. Support for third-party writing assistance for this article, provided by Poppy Wilson, MBiol, Costello Medical, London, UK, was funded by UCB in accordance with Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022). The journal’s Rapid Service Fee was funded by UCB.
Data Availability
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient-level data and redacted trial documents, which may include: analysis-ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password protected portal.
Declarations
Conflicts of Interest
All details of authors’ affiliation or involvement in an organization or entity with a financial or nonfinancial interest in the subject matter or materials discussed in this manuscript are disclosed. Joseph F. Merola: A consultant and/or investigator for AbbVie, Amgen, Biogen, Bristol Myers Squibb, Dermavant, Eli Lilly, Janssen, LEO Pharma, Pfizer, Novartis, Regeneron, Sanofi, Sun Pharma, and UCB. Alice B. Gottlieb: Received honoraria as an advisory board member and consultant for Amgen, AnaptypsBio, Avotres Therapeutics, Bristol Myers Squibb, Boehringer Ingelheim, Dice Therapeutics, Eli Lilly, Janssen, Novartis, Sanofi, UCB, and Xbiotech; received research/educational grants from AnaptypsBio, Bristol Myers Squibb, Moonlake Immunotherapeutics, Novartis, and UCB; all funds paid to Mount Sinai School of Medicine. Andreas Pinter: Investigator and/or speaker and/or advisor for AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, GSK, Hexal, Janssen, LEO Pharma, MC2, Medac, Merck Serono, Mitsubishi Pharma, MSD, MoonLake Immunotherapeutics, Novartis, Pfizer, Regeneron, Roche, Sandoz, Schering-Plough, Tigercat Pharma, and UCB. Boni Elewski: Research support as funding to Case Western Reserve University from AbbVie, AnaptysBio, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Incyte, LEO Pharma, Menlo, Merck, Novartis, Pfizer, Regeneron, Sun Pharma, Valeant, and Vanda; Consultant (honoraria) from Arcutis, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, LEO Pharma, Menlo, Novartis, Pfizer, Sun Pharma, UCB, Valeant, and Verrica. Melinda Gooderham: Investigator, speaker, consultant, or advisory board member for AbbVie, Akros, Amgen, AnaptysBio, Arcutis, Aslan, Aristea, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly, Galderma, GSK, Incyte, Janssen, Kyowa Kirin, MedImmune, Meiji, Merck, Moonlake Immunotherapeutics, Nimbus, Novartis, Pfizer, Regeneron, Reistone, Sanofi Genzyme, Sun Pharma, and UCB. Richard B. Warren: Consulting fees from AbbVie, Almirall, Amgen, Arena, Astellas, Avillion, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, GSK, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi, and UCB; research grants to institution from AbbVie, Almirall, Janssen, LEO Pharma, Novartis, and UCB; honoraria from Astellas, DICE Therapeutics, GSK, and Union Therapeutics. Stefano Piaserico: Served as consultant and/or speaker for AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Sandoz, and UCB. Krista Wixted, Nancy Cross, Nicola Tilt, Susanne Wiegratz: Employees and shareholders of UCB. Ulrich Mrowietz: Advisor and/or clinical study investigator for, and/or received honoraria and/or grants from AbbVie, Aditxt, Almirall, Amgen, Aristea, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dr. Reddy’s, Eli Lilly, Formycon, Immunic, Janssen-Cilag, LEO Pharma, Merck, MetrioPharm, Novartis, Phi-Stone, Sanofi-Aventis, Merck Sharp & Dohme, UCB, and Union Therapeutics.
Ethical Approval
Reviewed and approved by the relevant IRBs. Clinicaltrials.gov trial registration: NCT03370133, NCT03410992, NCT03412747, NCT03598790, NCT03536884. Studies were conducted in accordance with the principles of the Declaration of Helsinki and approved by an independent review board and independent ethics committee. All participants provided informed written consent documented in accordance with local regulations. Written consent for the publication of recognizable patient photographs or other identifiable material was obtained by the authors and attested to at the time of article submission to the journal stating that all patients gave consent with the understanding that this information may be publicly available.
Footnotes
Prior Presentation: (1) Merola JF, Conrad C, Hampton P, et al. Bimekizumab 3-year efficacy in high-impact areas in moderate to severe plaque psoriasis: Pooled results from five phase 3/3b trials. Poster presented at the European Academy of Dermatology and Venereology meeting; Berlin, Germany; 11–14 October 2023; poster P2547. (2) Lauffer F, Pinter A, Kokolakis G, et al. Bimekizumab efficacy in high-impact areas for patients with moderate to severe plaque psoriasis: Change from baseline through two years pooled from five phase 3 and 3b trials. Poster presented at the Annual Meeting of the German Dermatological Society; Berlin, Germany; 26–29 April 2023; poster P066.
References
- 1.Augustin M, Sommer R, Kirsten N, et al. Topology of psoriasis in routine care: results from high-resolution analysis of 2009 patients. Br J Dermatol. 2019;181(2):358–65. [DOI] [PubMed] [Google Scholar]
- 2.Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3): e12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mrowietz U, Augustin M. Using the upgrade criteria of the European psoriasis consensus is best practice care according to the people-centred healthcare concept of the World Health Organization. Br J Dermatol. 2022;187(6):1007–8. [DOI] [PubMed] [Google Scholar]
- 5.Eghlileb AM, Davies EE, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156(6):1245–50. [DOI] [PubMed] [Google Scholar]
- 6.Pariser D, Schenkel B, Carter C, Farahi K, Brown TM, Ellis CN. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Korte J, Sprangers MA, Mombers FM, Bos JD. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc. 2004;9(2):140–7. [DOI] [PubMed] [Google Scholar]
- 8.Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol. 2008;26(5):448–59. [DOI] [PubMed] [Google Scholar]
- 9.Dopytalska K, Sobolewski P, Błaszczak A, Szymańska E, Walecka I. Psoriasis in special localizations. Reumatologia/Rheumatology. 2018;56(6):392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilton R. Management of psoriasis affecting high-impact sites. Dermatol Nurs. 2016;15:4. [Google Scholar]
- 11.Samarasekera E, Sawyer L, Parnham J, Smith CH. Assessment and management of psoriasis: summary of NICE guidance. BMJ. 2012;345:e6712. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Regaña M, Aldunce Soto MJ, Belinchón Romero I, et al. Evidence-based guidelines of the spanish psoriasis group on the use of biologic therapy in patients with psoriasis in difficult-to-treat sites (nails, scalp, palms, and soles). Actas Dermosifiliogr. 2014;105(10):923–34. [DOI] [PubMed] [Google Scholar]
- 13.Foley P, Gordon K, Griffiths CEM, et al. Efficacy of guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol. 2018;154(6):676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31(3):477–82. [DOI] [PubMed] [Google Scholar]
- 15.Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475–86. [DOI] [PubMed] [Google Scholar]
- 17.Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142–52. [DOI] [PubMed] [Google Scholar]
- 18.Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385(2):130–41. [DOI] [PubMed] [Google Scholar]
- 19.Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–98. [DOI] [PubMed] [Google Scholar]
- 20.Thaçi D, Vender R, de Rie MA, et al. Safety and efficacy of bimekizumab through 2 years in patients with moderate-to-severe plaque psoriasis: longer-term results from the BE SURE randomized controlled trial and the open-label extension from the BE BRIGHT trial. Br J Dermatol. 2022;188(1):22–31. [DOI] [PubMed] [Google Scholar]
- 21.Strober B, Tada Y, Mrowietz U, et al. Bimekizumab maintenance of response through 3 years in patients with moderate-to-severe plaque psoriasis: results from the BE BRIGHT open-label extension trial. Br J Dermatol. 2023;188(6):749–59. [DOI] [PubMed] [Google Scholar]
- 22.Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158(7):735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A Study to Evaluate the Efficacy and Safety of Bimekizumab in Adult Subjects With Moderate to Severe Chronic Plaque Psoriasis (BE BRIGHT). Available at: https://clinicaltrials.gov/study/NCT03598790. Accessed Nov 2024.
- 24.Galluzzo M, Talamonti M, Cioni A, et al. Efficacy of tildrakizumab for the treatment of difficult-to-treat areas: scalp, nail, palmoplantar and genital psoriasis. J Clin Med. 2022;11(9):2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–72. [DOI] [PubMed] [Google Scholar]
- 26.Gottlieb AB, Kubanov A, van Doorn M, et al. Sustained efficacy of secukinumab in patients with moderate-to-severe palmoplantar psoriasis: 2.5-year results from GESTURE, a randomized, double-blind placebo-controlled trial. Br J Dermatol. 2020;182(4):889–99. [DOI] [PubMed] [Google Scholar]
- 27.Reich K, Sullivan J, Arenberger P, et al. Secukinumab shows high and sustained efficacy in nail psoriasis: 2.5-year results from the randomized placebo-controlled TRANSFIGURE study. Br J Dermatol. 2021;184(3):425–36. [DOI] [PubMed] [Google Scholar]
- 28.Elewski BE, Baker CS, Crowley JJ, et al. Adalimumab for nail psoriasis: efficacy and safety over 52 weeks from a phase-3, randomized, placebo-controlled trial. J Eur Acad Dermatol Venereol. 2019;33(11):2168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egeberg A, Kristensen LE, Puig L, et al. Network meta-analyses comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis at 24–28 and 48–52 weeks. J Dermatolog Treat. 2023;34(1):2263108. [DOI] [PubMed] [Google Scholar]
- 30.Sobolewski P, Walecka I, Dopytalska K. Nail involvement in psoriatic arthritis. Reumatologia. 2017;55(3):131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritchlin CT, Coates LC, McInnes IB, et al. Bimekizumab treatment in biologic DMARD-naïve patients with active psoriatic arthritis: 52-week efficacy and safety results from the phase III, randomised, placebo-controlled, active reference BE OPTIMAL study. Ann Rheum Dis. 2023;82(11):1404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet. 2023;401(10370):38–48. [DOI] [PubMed] [Google Scholar]
- 33.Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol. 2019;15(3):153–66. [DOI] [PubMed] [Google Scholar]
- 34.European Medicines Agency. Bimzelx Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/bimzelx-epar-product-information_en.pdf. Accessed Nov 2024.
- 35.Micali G, Verzì AE, Giuffrida G, Panebianco E, Musumeci ML, Lacarrubba F. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019;12:953–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu M, Fischer G. Adult genital psoriasis: an updated review for clinicians. Australas J Dermatol. 2024;65(3):e1–12. [DOI] [PubMed] [Google Scholar]
- 37.Meeuwis KAP, Potts Bleakman A, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29(8):754–60. [DOI] [PubMed] [Google Scholar]
- 38.Orsini D, Malagoli P, Balato A, et al. Bimekizumab for the treatment of plaque psoriasis with involvement of genitalia: a 16-week multicenter real-world experience—IL PSO (Italian landscape psoriasis). Dermatol Pract Concept. 2024;14(2):e2024052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient-level data and redacted trial documents, which may include: analysis-ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password protected portal.