Abstract
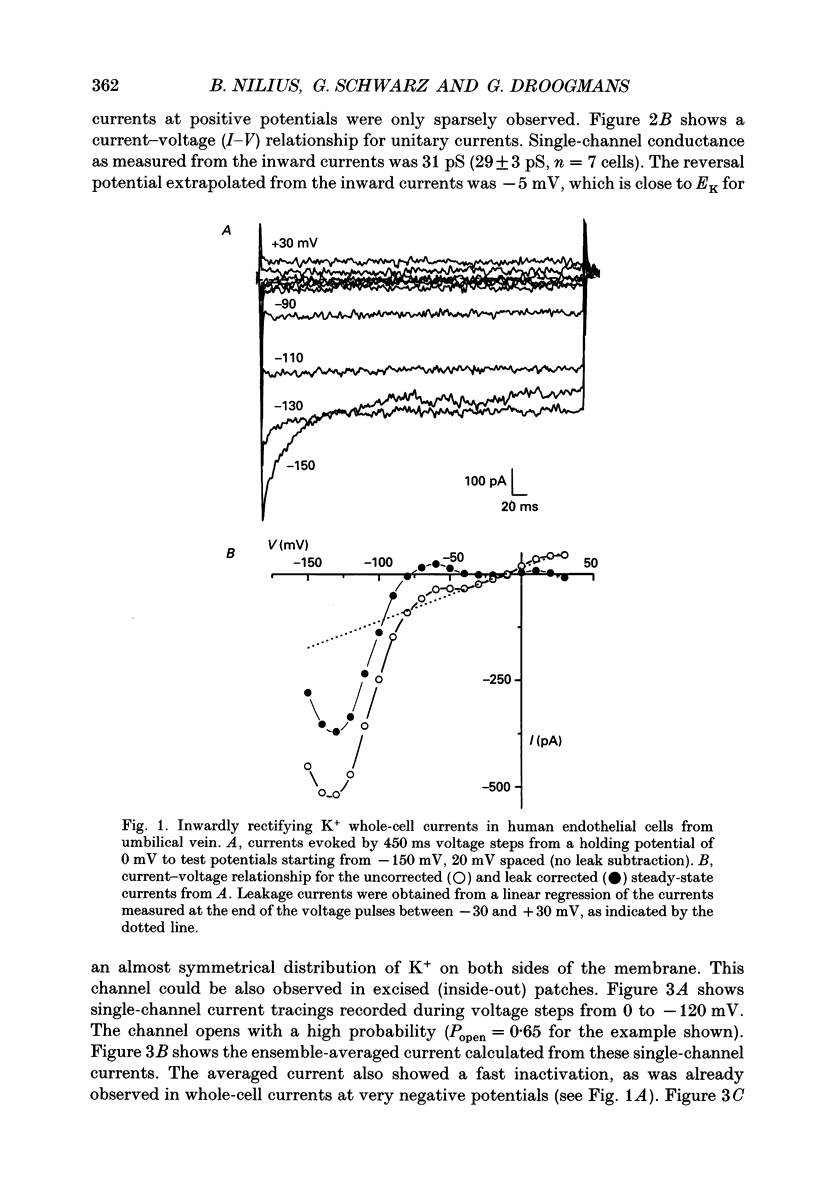
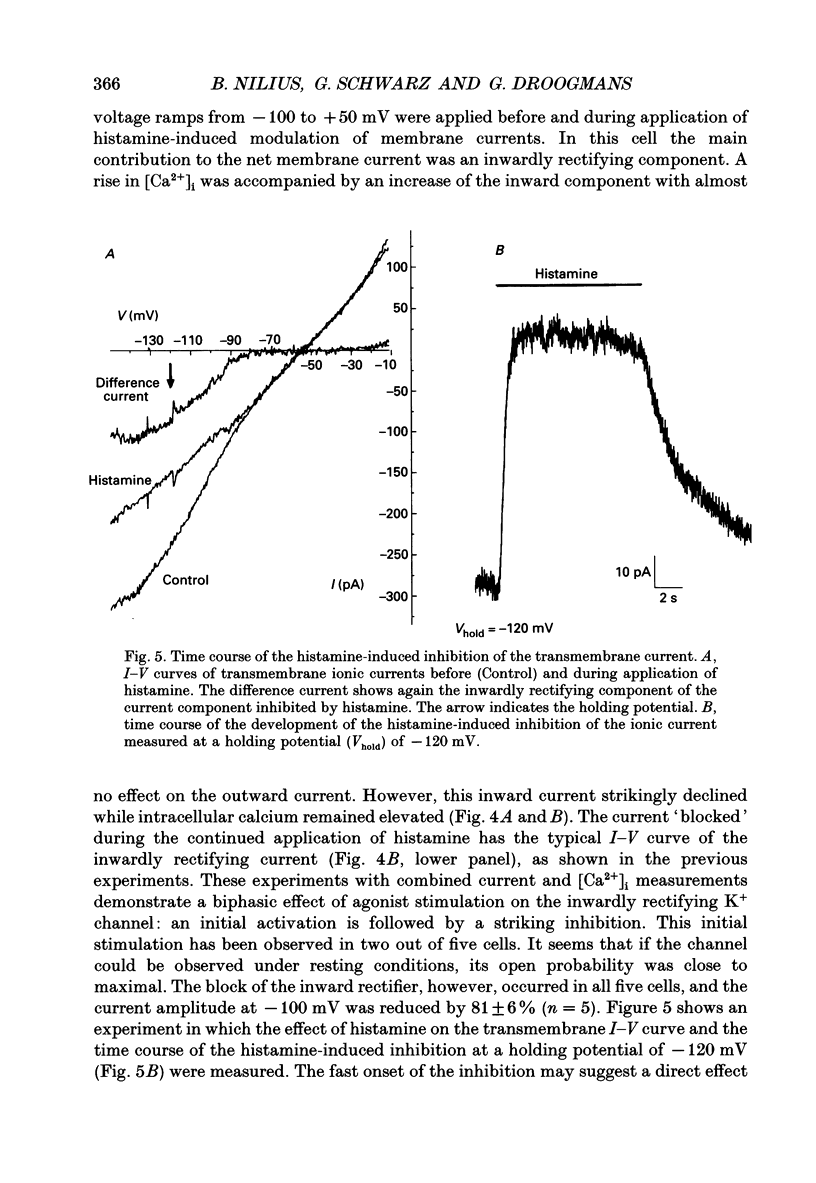
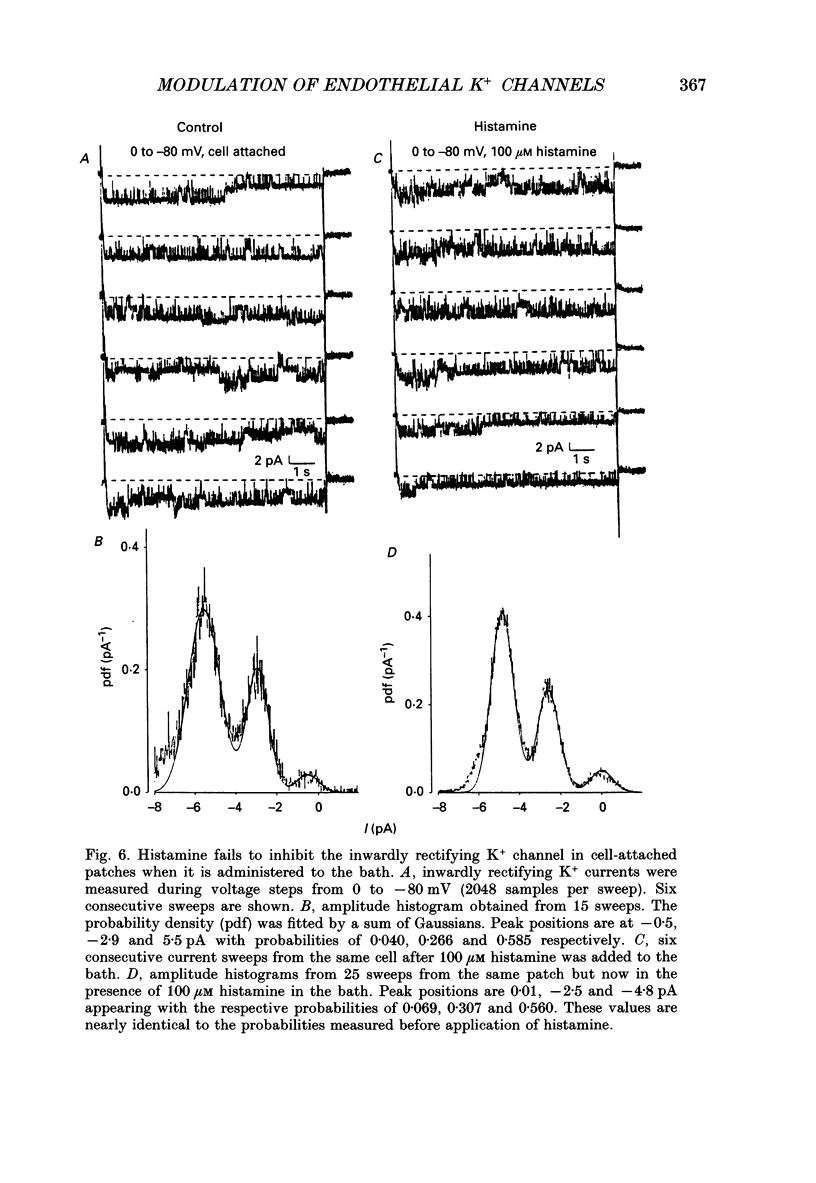
1. Whole-cell and single-channel currents were recorded together with intracellular Ca2+ in voltage clamped, single endothelial cells isolated from human umbilical vein. 2. The major current component under resting conditions in the whole-cell configuration was a strongly inwardly rectifying potassium current. 3. This current is due to activation of a K+ channel with an inward conductance of 29 +/- 3 pS (n = 7) with symmetrical 140 mM K+ on both sides of the membrane. This channel could be measured both in the cell-attached and in the inside-out configuration. At potentials below -110 mV both whole-cell and averaged single-channel currents showed a fast inactivation. 4. During stimulation of endothelial cells with histamine, whole-cell K+ currents initially increased but then substantially declined, despite the sustained increase in intracellular Ca2+ concentration ([Ca2+]i). 5. The blockade of the inwardly rectifying K+ channel by histamine could not be observed in cell-attached patches if histamine was added to the bath. 6. It is concluded that endothelial cells possess K+ channels that are directly inhibited by agonists, such as histamine. Blocking these channels may depolarize the cell membrane and thereby reduce the driving force for Ca2+ influx.
Full text
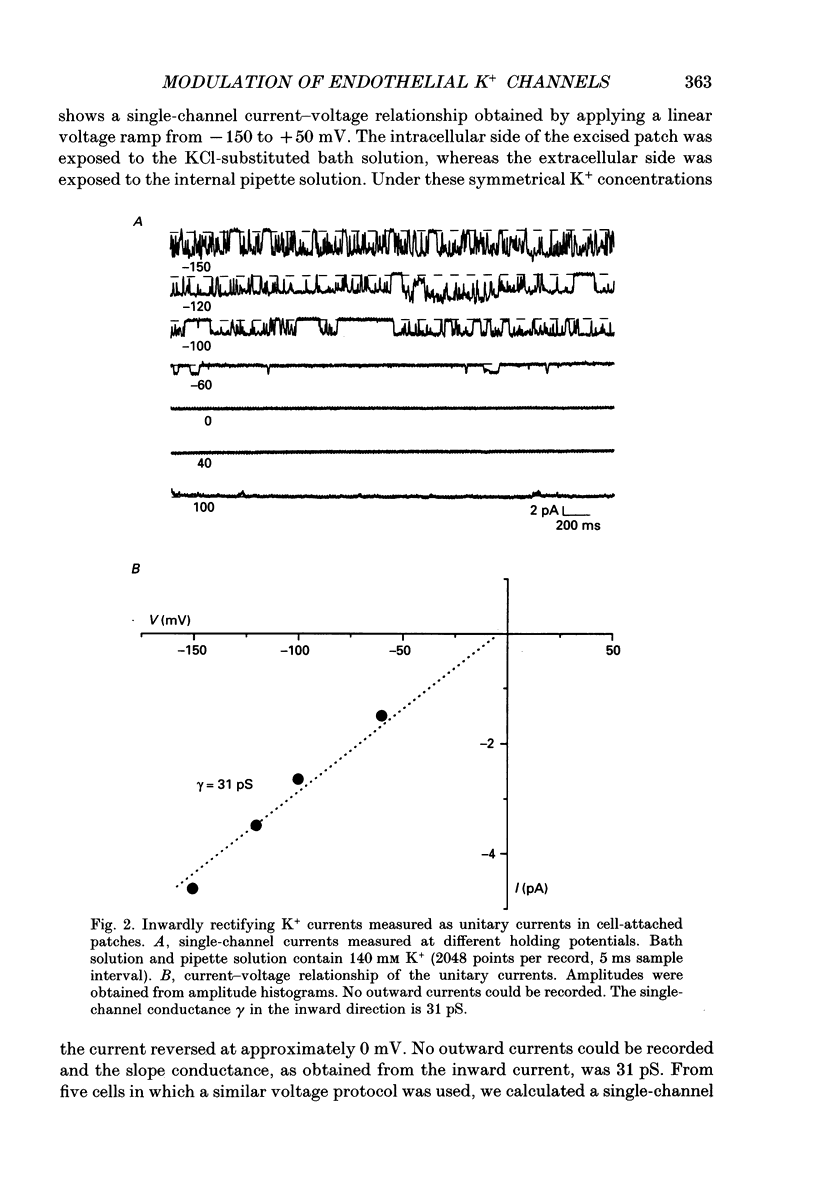
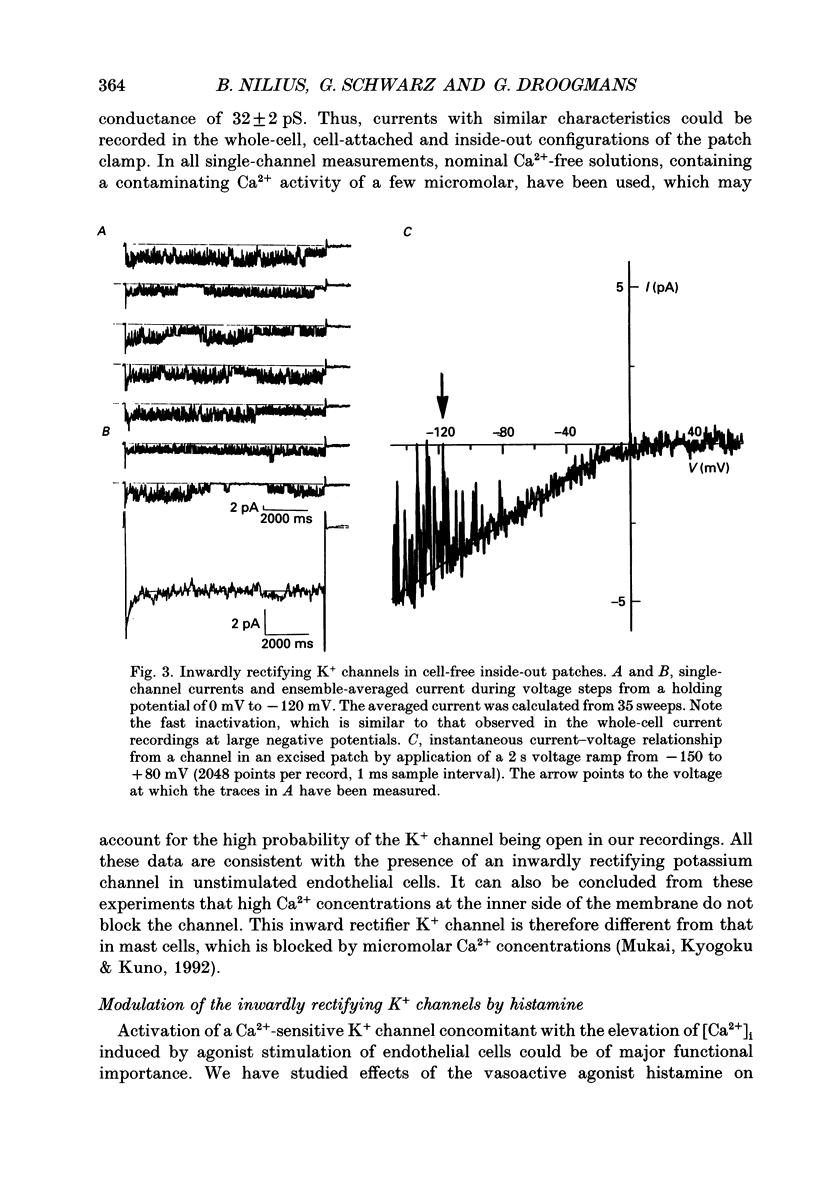
PDF


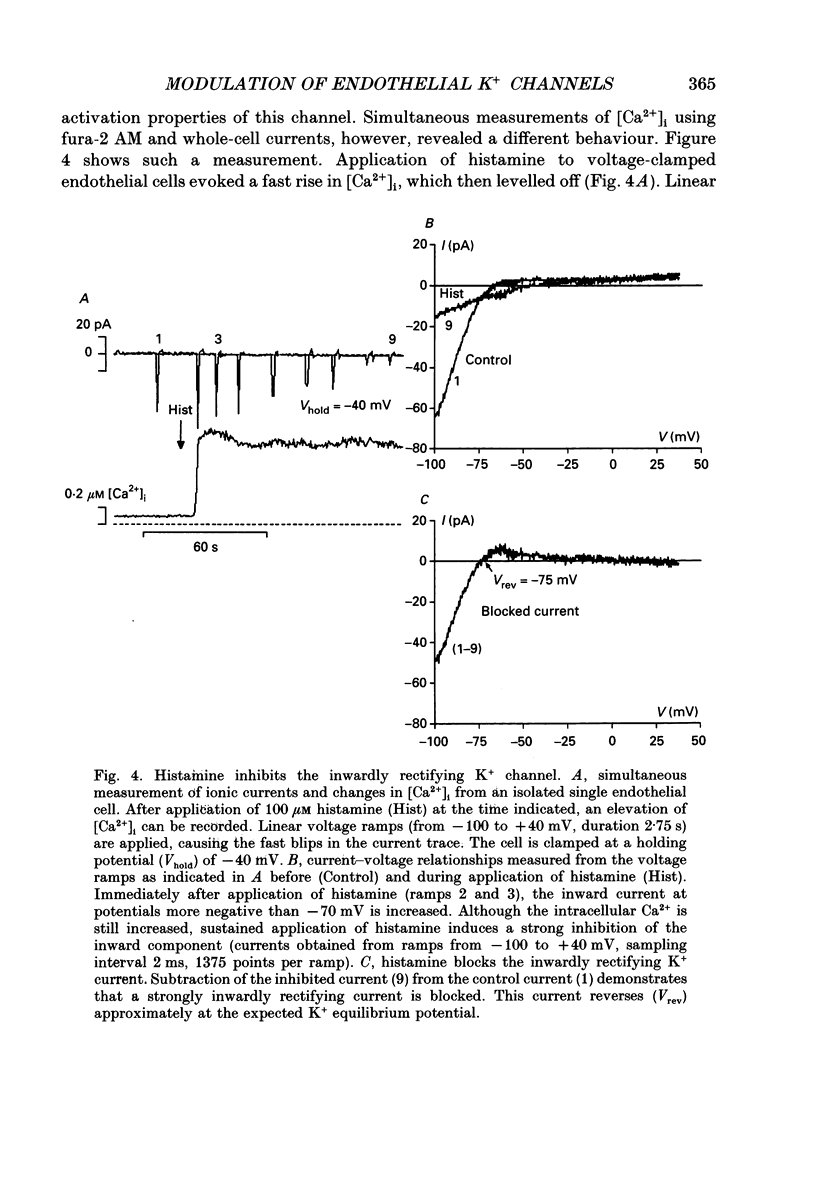




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M., Cramer E. B., Gallin E. K. Comparison of apical and basal surfaces of confluent endothelial cells: patch-clamp and viral studies. Am J Physiol. 1992 Sep;263(3 Pt 1):C573–C583. doi: 10.1152/ajpcell.1992.263.3.C573. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Possani L. D., Kunze D. L. Bradykinin-induced potassium current in cultured bovine aortic endothelial cells. J Membr Biol. 1990 Jul;116(3):227–238. doi: 10.1007/BF01868462. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Ritchie A. K., Eskin S. G., Navarro L. T., Kunze D. L. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res. 1987 Nov;61(5):632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- Fichtner H., Fröbe U., Busse R., Kohlhardt M. Single nonselective cation channels and Ca2+-activated K+ channels in aortic endothelial cells. J Membr Biol. 1987;98(2):125–133. doi: 10.1007/BF01872125. [DOI] [PubMed] [Google Scholar]
- Flavahan N. A., Vanhoutte P. M. G-proteins and endothelial responses. Blood Vessels. 1990;27(2-5):218–229. doi: 10.1159/000158813. [DOI] [PubMed] [Google Scholar]
- Groschner K., Graier W. F., Kukovetz W. R. Activation of a small-conductance Ca(2+)-dependent K+ channel contributes to bradykinin-induced stimulation of nitric oxide synthesis in pig aortic endothelial cells. Biochim Biophys Acta. 1992 Oct 27;1137(2):162–170. doi: 10.1016/0167-4889(92)90198-k. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoyer J., Popp R., Meyer J., Galla H. J., Gögelein H. Angiotensin II, vasopressin and GTP[gamma-S] inhibit inward-rectifying K+ channels in porcine cerebral capillary endothelial cells. J Membr Biol. 1991 Jul;123(1):55–62. doi: 10.1007/BF01993963. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Penner R. Angiotensin II induces oscillations of intracellular calcium and blocks anomalous inward rectifying potassium current in mouse renal juxtaglomerular cells. Proc Natl Acad Sci U S A. 1989 May;86(9):3423–3427. doi: 10.1073/pnas.86.9.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Effects of external and internal K+ ions on magnesium block of inwardly rectifying K+ channels in guinea-pig heart cells. J Physiol. 1991 Apr;435:83–99. doi: 10.1113/jphysiol.1991.sp018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki T., Ohhashi T. Endothelium and mechanical responses of isolated monkey pulmonary veins to histamine. Am J Physiol. 1990 Oct;259(4 Pt 2):H1032–H1037. doi: 10.1152/ajpheart.1990.259.4.H1032. [DOI] [PubMed] [Google Scholar]
- Mehrke G., Pohl U., Daut J. Effects of vasoactive agonists on the membrane potential of cultured bovine aortic and guinea-pig coronary endothelium. J Physiol. 1991 Aug;439:277–299. doi: 10.1113/jphysiol.1991.sp018667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai M., Kyogoku I., Kuno M. Calcium-dependent inactivation of inwardly rectifying K+ channel in a tumor mast cell line. Am J Physiol. 1992 Jan;262(1 Pt 1):C84–C90. doi: 10.1152/ajpcell.1992.262.1.C84. [DOI] [PubMed] [Google Scholar]
- Nilius B. Permeation properties of a non-selective cation channel in human vascular endothelial cells. Pflugers Arch. 1990 Jul;416(5):609–611. doi: 10.1007/BF00382697. [DOI] [PubMed] [Google Scholar]
- Nilius B., Riemann D. Ion channels in human endothelial cells. Gen Physiol Biophys. 1990 Apr;9(2):89–111. [PubMed] [Google Scholar]
- Revest P. A., Abbott N. J. Membrane ion channels of endothelial cells. Trends Pharmacol Sci. 1992 Nov;13(11):404–407. doi: 10.1016/0165-6147(92)90124-o. [DOI] [PubMed] [Google Scholar]
- Rusko J., Tanzi F., van Breemen C., Adams D. J. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol. 1992 Sep;455:601–621. doi: 10.1113/jphysiol.1992.sp019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T. Acetylcholine induces Ca-dependent K currents in rabbit endothelial cells. Jpn J Pharmacol. 1990 Jun;53(2):235–246. doi: 10.1254/jjp.53.235. [DOI] [PubMed] [Google Scholar]
- Sauve R., Parent L., Simoneau C., Roy G. External ATP triggers a biphasic activation process of a calcium-dependent K+ channel in cultured bovine aortic endothelial cells. Pflugers Arch. 1988 Oct;412(5):469–481. doi: 10.1007/BF00582535. [DOI] [PubMed] [Google Scholar]
- Schwarz G., Callewaert G., Droogmans G., Nilius B. Shear stress-induced calcium transients in endothelial cells from human umbilical cord veins. J Physiol. 1992 Dec;458:527–538. doi: 10.1113/jphysiol.1992.sp019432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M. R., DeCoursey T. E. Intrinsic gating of inward rectifier in bovine pulmonary artery endothelial cells in the presence or absence of internal Mg2+. J Gen Physiol. 1990 Jul;96(1):109–133. doi: 10.1085/jgp.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca L., Schilling W. P., Kunze D. L. G-protein-mediated regulation of a Ca(2+)-dependent K+ channel in cultured vascular endothelial cells. Pflugers Arch. 1992 Oct;422(1):66–74. doi: 10.1007/BF00381515. [DOI] [PubMed] [Google Scholar]


