Abstract
Sensitization to Staphylococcus aureus (S. aureus) enterotoxins (SEs) A (SEA) and B (SEB) is associated with the pathogenesis of several chronic airway diseases, including asthma and chronic rhinosinusitis, but its role in chronic obstructive pulmonary disease (COPD) remains unclear. This cohort study aimed to investigate the impact of sensitization to SEs on total IgE levels, and capsaicin cough reflex sensitivity (C-CS) in COPD. This study prospectively enrolled 68 patients with COPD from the outpatient department at Nagoya City University Hospital and Shizuoka General Hospital, Japan, from June 2018 to January 2020 for posthoc analysis. Patient characteristics and biomarkers, including total IgE, and C-CS, were collected. We additionally measured and serum SEA-IgE and SEB-IgE levels for this analysis. Total IgE and SEs-IgE levels in individuals with COPD were compared to those of 20 healthy individuals. The correlation between C-CS and IgE levels was evaluated using Pearson’s correlation coefficient. Multivariate analyses were used to adjust the impact on SE sensitization in total IgE levels and C-CS. The prevalence of SEs sensitization was higher in patients with COPD (23.5%, N = 16) than in healthy individuals (0%, N = 0) at a cut-off value of 0.35 UA/mL or more (p = 0.018). There was a negative correlation between total serum SEB-IgE levels and declined C-CS values. Multivariate analysis showed that SEs sensitization was associated with increased total IgE levels, and declined C-CS values. This study indicated that SEs sensitization was associated with increased IgE levels and decreased C-CS in COPD. This study was approved by the ethics committee of Nagoya City University (60-18-0012) and registered in the UMIN Clinical Trials Registry (Registry ID UMIN000032497).
Keywords: Capsaicin cough reflex sensitivity, Chronic obstructive pulmonary disease, Staphylococcus aureus, Staphylococcus aureus enterotoxins, IgE
Subject terms: Biomarkers, Medical research, Pathogenesis
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide1. COPD is a complex disease influenced by a multitude of factors, including environmental and genetic components2. Additionally, COPD exacerbations are associated with various pathogens and allergens2.
Allergens may be associated with the development of a Type-2 inflammatory environment in COPD. Sensitization to S. aureus enterotoxins (SEs), including SEA and SEB, is linked to the development of chronic upper and lower airway inflammation3. S. aureus colonization on the skin and nasopharyngeal membranes is commonly observed in approximately 25–30% of the healthy population4. S. aureus carriage and SEs sensitization are associated with allergic multimorbidity5. SEs can act as superantigens, triggering T-cell activation without conventional antigen presentation, thereby orchestrating airway inflammation6. Notably, previous research has suggested a role for SEs sensitization in the pathophysiology of chronic rhinosinusitis (CRS) with nasal polyps and asthma7–10. However, a recent study reported that SEs sensitization did not induce elevated eosinophil or fractional nitric oxide (FeNO) levels in patients with COPD11. Thus, potential impact of SEs sensitization on the pathophysiology of COPD remains unclear at present.
Acute exacerbation (AE) of COPD and pneumonia are the leading causes of death in patients with COPD. We have recently shown that declined capsaicin cough reflex sensitivity (C-CS) is associated with hospitalization in patients with COPD12. Meanwhile, we did not evaluate what factors were associated with declined C-CS in the original study. In asthma, lower levels of serum total IgE were associated with heightened C-CS in patients with asthma13. Since SE sensitization could facilitate IgE production, it could trigger the decline in C-CS in patients with COPD. However, no studies have investigated the association between SE sensitization and C-CS in either asthma or COPD.
Considering that SEs facilitate IgE production in COPD acting as superantigens, SE sensitization could contribute to declined C-CS. Furthermore, SE sensitization may be a risk factor for AE. Therefore, we performed a posthoc study to evaluate the impact of SE sensitization on the clinical outcomes of COPD.
Methods
Patients
We recruited patients with COPD from the outpatient departments at Nagoya City University Hospital and Shizuoka General Hospital, from June 2018 to January 2020 to assess the impact of C-CS on hospitalization due to community-acquired pneumonia (CAP) and AE in these patients12. COPD and comorbid asthma were diagnosed based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018 and the Japanese Respiratory Society Guidelines for the Management of Asthma-COPD overlap 201814, respectively. Inclusion criteria were as follows12: (1) a postbronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio of < 0.70; (2) persistent respiratory symptoms such as dyspnea, cough, sputum production, or wheezing; and (3) substantial exposure to noxious stimuli such as tobacco smoke or other environmental particles. Furthermore, we excluded patients if (1) they denied participation in the study; (2) had chronic respiratory diseases other than asthma; (3) had a postbronchodilator FEV1/FVC ratio of ≥ 0.70; (4) resided in a nursing care home, were bedridden at home; (5) had a history of aspiration pneumonia; (6) underwent tubal feeding; or (7) had a respiratory infection within four weeks of enrollment or hospitalization due to AE-COPD or CAP within 12 weeks prior to enrollment. Out of the 80 patients initially considered, 69 were eligible for enrollment, and 68 completed the study follow-up (Fig. S1). This study was approved by the ethics committee of Nagoya City University (60-18-0012) and registered in the UMIN Clinical Trials Registry (Registry ID UMIN000032497). All methods were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants. This study was conducted in accordance with the STROBE guidelines.
In our previous study, biomarkers, such as fractional exhaled nitric oxide (FeNO) and serum levels of total IgE, were measured in 28 healthy individuals between October 2015 and December 201715. In addition, serum SEs-IgE levels were measured in 20 healthy individuals using stored samples10. All healthy individuals had no history of upper or lower respiratory disease. They provided consent for secondary use of anonymized data. (approval number of the Ethics Committee of Nagoya City University: 1165).
Measurements
Biomarkers, including blood eosinophil counts, FeNO, serum total IgE, and C-CS, and pulmonary and cardiac functions were measured at enrollment. The capsaicin cough challenge involved testing ten doubling concentrations of capsaicin (ranging from 0.61 to 312.5 µM)16. Higher values of C2 and C5 represented a decline in C-CS15. The detailed method of the capsaicin cough challenge test is described in the online supplementary manuscript.
Disease control, symptoms, and cough-specific quality of life (QoL) were evaluated at the time of enrollment using the COPD Assessment Test, a modified version of the Medical Research Council dyspnea scale, and Leicester Cough Questionnaire. AE and admissions due to severe AE and/or CAP were prospectively monitored for 12 months after enrollment. AE was defined as an acute worsening of respiratory symptoms requiring additional treatments, such as bronchodilators, antibiotics, and systemic corticosteroids, for ≥ 3 consecutive days. Additionally, a history of AE, CAP, and hospitalization within two years prior to enrollment were also evaluated12.
SEs-IgE measurements
We measured serum levels of SEs-IgE (SEA-IgE and SEB-IgE, respectively) in titers (ImmunoCAP®; Phadia K.K., Tokyo, Japan) using the collected samples. The detection limit for each specific IgE titer was 0.10 UA/mL.
Sputum culture
Sputum samples were cultured to increase colonization by pathogenic microorganisms if it could be collected from patients. Bacterial culture was performed according to the national guideline issued by the Japan Registered Clinical Laboratories Association (uploaded in Japanese: https://www.jrcla.or.jp/).
Statistical analysis
Statistical analysis was performed using JMP 14.3 software (SAS Institute Japan, Tokyo, Japan). Continuous variables were presented as median (interquartile range), and categorical variables as numbers (%). Although total IgE levels were not measured at enrollment in four patients, values were obtained from stored samples. Two C-reactive protein values were missing, but were not measured and treated as blanks because they did not affect the main results of this study. COPD patients were divided into SEs sensitization (+) [SE (+)] and SEs sensitization (−) [SE (−)] groups. Comparisons between the two groups were made using the Wilcoxon rank-sum test, Chi-squared test, and Fisher’s exact test, as appropriate. Total IgE levels were compared among SE (+), SE (−), and healthy individuals using the Steel–Dwass test.
Correlations between IgE levels (total IgE and SEs-IgE) and C-CS (C2 and C5) were evaluated using Pearson’s correlation coefficient. Levels of serum total IgE and SEs-IgE were log-transformed when assessing such correlations.
To adjust for the influence of confounders on the association of SEs sensitization with total IgE levels, declined C-CS, and AE, multiple logistic regression analyses were performed. Confounders identified were incorporated into each analysis. Current smoking was considered a confounder for all outcomes. Additionally, other potential confounders included: (1) total IgE levels: age, asthma, and rhinitis; (2) declined C-CS: cerebrovascular disease, male gender, and use of antidepressant drugs; (3) AE during the follow-up period: history of AE within the past two years, not taking inhaled corticosteroids, eosinophils ≥ 300/µL, and severe airflow limitation (postbronchodilator FEV1/FVC). We considered that SE-IgE was associated with them if the p value was ≤ 0.05 in the multiple logistic regression analyses.
Patient’s characteristics
Table 1 presents the characteristics of the patients. The cohort included patients with severe COPD, with 29 (39.7%) having severe AE and/or community-acquired pneumonia that necessitated hospitalization within the two years preceding enrollment, and nine (13.4%) receiving home oxygen therapy. According to the GOLD 2018 guidelines, twenty (29.4%) patients were classified in Group D. Additionally, ten (14.7%) had comorbid asthma. Sputum samples were collected from 36 (52.9%) patients. S. Aureus was found in seven patients. When compared to the healthy individuals (Table S1), ages were older, the proportion of males were higher, and lung function were lower in patients with COPD (p < 0.0001 for all). Meanwhile, body mass index and levels of total IgE and FeNO were similar between the two groups.
Table 1.
Patient’s characteristics.
| All patients (n = 68) | SE (−) (n = 52) | SE (+) (n = 16) | p values | |
|---|---|---|---|---|
| Age | 75 (70, 75) | 74 (70, 77) | 75 (70, 80) | 0.36 |
| Body Mass Index, kg/m2 | 21.8 (19.1, 24.0) | 21.9 (19.5, 24.0) | 21.3 (18.8, 24.2) | 0.57 |
| Sex, male | 58 (85.3) | 43 (82.7) | 15 (93.8) | 0.43 |
| Smoking, ex/ current | 54 (79.4)/14 (20.6) | 44 (84.6)/ 8(15.4) | 10 (62.5)/ 6(37.5) | 0.078 |
| Pack-years | 51.5 (36, 73.5) | 52.5 (37.5, 73.5) | 46.5 (24.6, 83.3) | 0.66 |
| GOLD classification, A/ B/ C/ D |
19 (27.9)/ 23 (33.8)/ 6 (8.8)/ 20 (29.4) |
14 (27)/17 (33)/ 6 (12)/15 (29) |
5 (31)/6 (38)/ 0 (0)/5 (31) |
0.33 |
| COPD stage 1/2/3/4 |
14 (20.6)/38 (55.9)/ 12 (17.6)/4 (5.9) |
12 (23)/29 (56)/ 8 (15)/3 (6) |
2 (13)/9 (56)/ 4 (25)/1 (6) |
0.72 |
| Sputum, + | 36 (52.9) | 26 (50.0) | 10 (62.5) | 0.41 |
| SA colonization, +* | 7 (10.3) | 3 (11.5) | 4 (40.0) | 0.076 |
| LAMA, + | 48 (70.6) | 35 (67.3) | 13 (81.3) | 0.36 |
| LABA, + | 57 (83.8) | 42 (80.8) | 15 (93.8) | 0.44 |
| ICS, + | 21 (30.9) | 14 (26.9) | 7 (43.8) | 0.23 |
| Home oxygen use, + | 9 (13.2) | 6 (11.5) | 3 (18.8) | 0.43 |
| Antidepressant drugs, + | 3 (4.4) | 2 (3.9) | 1 (6.3) | 0.56 |
| ACE inhibitors, + | 1 (1.5) | 1 (1.9) | 0 (0) | > 0.99 |
| Pneumococcal vaccination, + | 34 (50) | 27 (51.9) | 7 (43.8) | 0.78 |
| Asthma, + | 10 (14.7) | 10 (19.2) | 0 (0) | 0.10 |
| Cerebrovascular disease, + | 7 (10.3) | 4 (7.7) | 3 (18.8) | 0.34 |
| Pneumonia < 2 year, + | 20 (29.4) | 16 (30.8) | 4 (25.0) | 0.76 |
| A history of pneumonia ≥ 2, + | 5 (7.4) | 3 (5.8) | 2 (12.5) | 0.58 |
| AE < 2 year, + | 19 (27.9) | 15 (28.8) | 4 (25.0) | > 0.99 |
| A history of AE ≥ 2, + | 8 (11.8) | 7 (13.5) | 1 (6.3 | 0.67 |
| Hospitalization < 2 year, + | 27 (39.7) | 21 (40.4) | 6 (37.5) | > 0.99 |
| A history of hospitalization ≥ 2, + | 10 (14.7) | 7 (13.5) | 3 (18.8) | 0.69 |
| mMRC scale, Grade | 1 (1, 3) | 1 (1, 3) | 1.5 (1, 3) | 0.52 |
| mMRC ≥ Grade 2, + | 33 (48.5) | 25 (48.1) | 8 (50) | > 0.99 |
| CAT, point | 13 (6, 18) | 14 (6, 19) | 12 (6, 18) | 0.88 |
| CAT ≥ 10, + | 39 (57.4) | 22 (42.3) | 7 (43.8) | > 0.99 |
| LCQ, point | 19.1 (17.3, 20.2) | 19.1 (16.9, 20.2) | 19.3 (17.4, 20.3) | 0.67 |
*n = 36 (SE [−]: n = 26/ SE [+]: n = 10)
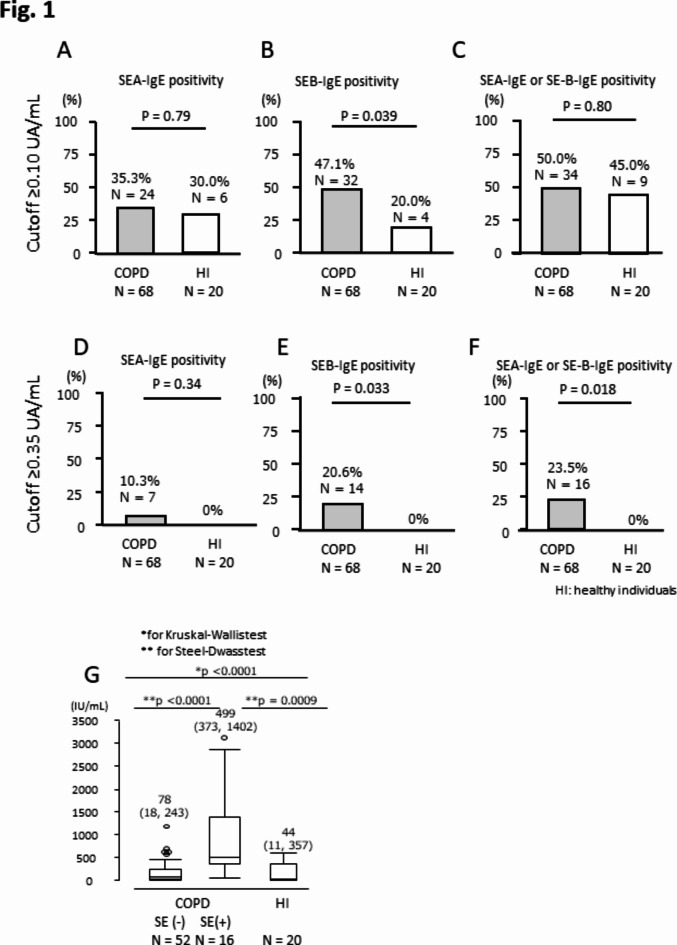
In the previous study, we considered that levels of SEs-IgE ≥ 0.10 UA/mL were clinically meaningful in patients with CRS, particularly when they had comorbid asthma10. However, the rate of individuals whose values of SEs-IgE were ≥ 0.10 UA/mL was similar between patients with COPD and healthy individuals (Fig. 1 A and C). In contrast, no healthy individuals showed levels of SEs-IgE ≥ 0.35 UA/mL (Fig. 1D and F). Sensitization to SEs (n = 16 [23.5%]), especially SEB (n = 14 [20.6%]), was more frequent in patients with COPD than in healthy individuals (0%) when the cut-off value was set at 0.35 UA/mL (p = 0.033 for SEB and p = 0.018 for SEs, respectively. Figure 1E and F).
Fig. 1.
An impact of SEs sensitization on COPD and IgE levels. The SEs-IgE cut-off values were set at ≥ 0.10 UA/mL (A–C) and ≥ 0.35 UA/mL(D–F). Comparison of total IgE levels among SE(+), SE(–), and HI(G).
Stratifying patients based on the presence or absence of SEs sensitization revealed no differences in terms of disease severity, COPD-related symptoms, cough-specific QoL, history of AE, pneumonia, hospitalization within the past two years, or comorbid asthma. However, S. aureus colonization tended to be more prevalent in the SE (+) group compared to the SE (−) group (Table 1).
Results
Biomarkers and physiological functions
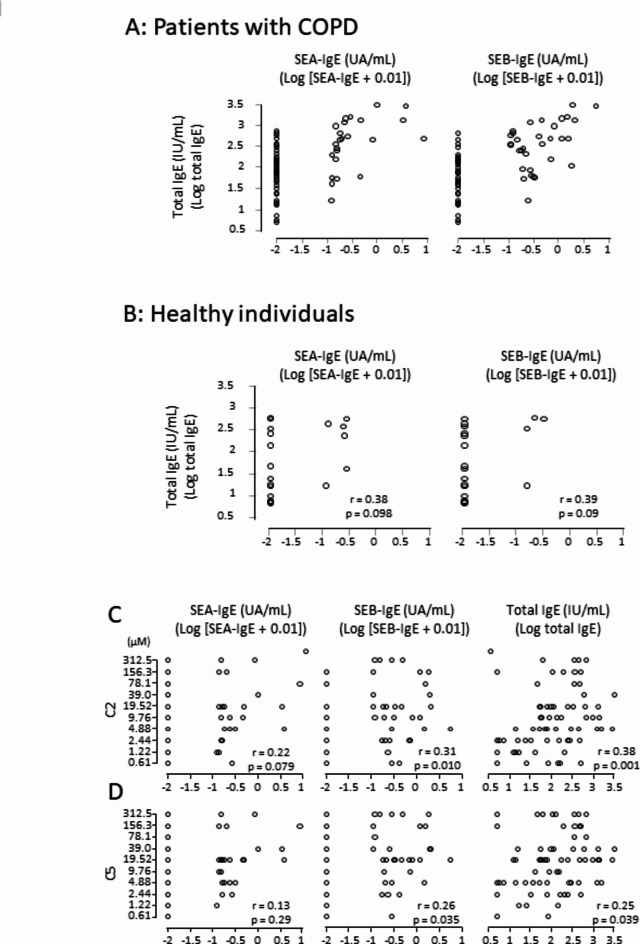
Serum total IgE levels were significantly higher in the SE (+) group compared to both the SE (−) group and healthy individuals (Fig. 1G). In patients with COPD, a significant, but moderate correlation was observed between serum IgE levels and SEs-IgE levels (Fig. 2A), a correlation not detected in healthy individuals (Fig. 2B). Blood eosinophil counts and FeNO, both biomarkers of Type-2 inflammation, showed no significant differences between the SE (+) and the SE (−) groups. Notably, concentrations of both C2 and C5 were higher in the SE (+) group than in the SE (−) group, indicating a more prominent decline in C-CS levels in the SE (+) group (Table 2). Higher levels of serum total IgE and SEB-IgE were significantly correlated with higher values of C2 and C5, suggesting an association between increased levels of serum total IgE, SEs sensitization, and a decline in C-CS (Fig. 2C and D).
Fig. 2.
Correlations between SEs-IgE and total IgE/C-CS. Patients with COPD (A), healthy individuals (B), C2 (C) and C5 (D).
Table 2.
Biomarkers (except for serum total IgE) and physiological functions when stratified according to SE sensitization.
| All patients (n = 68) | SE (-) (n = 52) | SE (+) (n = 16) | p values | |
|---|---|---|---|---|
| Neutrophils, /µL | 3709 (2921, 4768) | 3746 (2967, 4672) | 3450 (2489, 5871) | 0.83 |
| Eosinophils, /µL | 196 (119, 259) | 196 (116, 252) | 195 (134, 330) | 0.72 |
| Eosinophils ≥ 300 /µL, + | 12 (17.6) | 8 (15.4) | 4 (25) | 0.46 |
| Albumin, g/dL | 4.1 (3.8, 4.3) | 4.1 (3.9, 4.3) | 4.0 (3.6, 4.1) | 0.061 |
| Lactate dehydrogenase, IU/L | 192 (170, 212) | 194 (175, 213) | 184 (158, 205) | 0.27 |
| Hemoglobin A1c, % | 5.8 (5.6, 6.2) | 5.8 (5.6, 6.2) | 6.8 (5.4, 6.4) | 0.70 |
| Brain natriuretic peptide, pg/mL | 34 (18, 67) | 42 (20, 73) | 28 (13, 58) | 0.46 |
| C-reactive protein, mg/L* | 0.12 (0.07, 0.29) | 0.12 (0.07, 0.28) | 0.19 (0.06, 0.52) | 0.54 |
| FeNO, ppb | 23.2 (14.4, 31.2) | 21.7 (13.3, 31.2) | 26.0 (16.2, 32.7) | 0.47 |
| C2, Number of doubling concentrations† | 4 (3, 5) | 4 (3, 6) | 5.5 (4, 8.75) | 0.036 |
| C5, Number of doubling concentrations† | 6 (4, 7) | 5 (4, 7) | 6 (6, 9) | 0.038 |
| Ejection Fraction, % | 64 (61, 70) | 66 (61, 71) | 62 (61, 66) | 0.20 |
| Postbronchodilator FEV1, mL | 1535 (1153, 1905) | 1560 (1285, 1956) | 1280 (943, 1803) | 0.22 |
| Postbronchodilator FEV1 predicted, % | 63.8 (50.4, 76.2) | 64.7 (51.7, 76.2) | 54.5 (43.7, 75.4) | 0.14 |
| Postbronchodilator FEV1/FVC, % | 51.2 (41.0, 60.2) | 53.7 (43.4, 60.5) | 46.5 (32.7, 55.7) | 0.073 |
| Reversibility, % | 3.2 (0, 9.2) | 2.9 (0, 8.5) | 4.2 (− 4.0, 14.3) | > 0.99 |
*n = 66(SE [−]: n = 50/ SE [+]: n = 16), †The number of doubling concentrations of capsaicin ranges from 1 (0.61 µM) to 10(312.5 µM), with a higher number indicating decreased cough sensitivity to inhaled capsaicin. It reflects base 2 logarithmic values of C2 and C5, respectively.
AE, hospitalization, and pneumonia during one-year follow-up period
During the 12-month follow-up period, 15 patients experienced AE, with eight of them requiring hospitalizations due to severe AE and/or CAP. A higher proportion of individuals in the SE (+) group experienced AE compared to those in the SE (−) group (Fig. S2A). However, SEs sensitization did not have an impact on hospitalization or pneumonia (Figures S2B, C).
The influence of total IgE levels, C-CS, and AE in stratification with SEA/SEB
When analyses of the influence of total IgE levels, C-CS and AE were performed for the stratification with SEA and that with SEB, the trends observed were consistent with the combined analysis. However, some of the variables (the proportion of AE, and C5 for both, and C2 for SEB) lacked statistical significance (p < 0.05) likely because of the limited sample size (SEA-IgE positive: n = 7, SEB-IgE positive n = 14).
Role in SE sensitization on elevated total IgE levels, declined C-CS, and AE-COPD
To determine the association of SEs sensitization with elevated total IgE levels, declined C-CS, and AE, we performed multivariate analyses adjusting for confounders. The results of the multivariate analysis after adjusting confounders suggested that SEs sensitization contributed to elevated levels of serum total IgE, decline in C-CS, and increased frequency of AE during the follow-up period in patients with COPD (Tables 3A–3D).
Table 3.
An association of SE sensitization with elevation of total IgE (A), decline in capsaicin cough reflex sensitivity (C-CS) (B, C), and acute exacerbation (AE) during follow-up period (D).
| (A) Elevation of total IgE* | ||||
|---|---|---|---|---|
| Estimates | 95% C. I. | Standardized β | p value | |
| SE sensitization, + | 0.50 | 0.30, 0.69 | 0.57 | < 0.0001 |
| Smoking, current smoking | −0.02 | −0.23, 0.18 | −0.03 | 0.80 |
| Age | 0.002 | −0.02, 0.02 | 0.02 | 0.82 |
| Asthma, + | 0.10 | −0.12, 0.33 | 0.10 | 0.36 |
| Rhinitis, + | −0.15 | −0.34, 0.04 | −0.17 | 0.11 |
| (B) Decline in C-CS (C2†) | ||||
| Estimates | 95% C. I. | Standardized β | p value | |
| SE sensitization, + | 0.85 | 0.07, 1.62 | 0.28 | 0.033 |
| Smoking, current smoking | −0.17 | −0.98, 0.65 | −0.05 | 0.68 |
| Cerebrovascular disease, + | −0.06 | −1.11, 0.99 | −0.01 | 0.91 |
| Sex, males | 0.07 | −0.86, 0.99 | 0.02 | 0.88 |
| Antidepressant drugs, + | −1.00 | −2.55, 0.55 | −0.16 | 0.20 |
| (C) Decline in C-CS (C5†) | ||||
| Estimates | 95% C. I. | Standardized β | p value | |
| SE sensitization, + | 0.80 | 0.06, 1.53 | 0.27 | 0.035 |
| Smoking, current smoking | −0.23 | −0.55, 1.01 | −0.07 | 0.56 |
| Cerebrovascular disease, + | 0.28 | −0.72, 1.28 | 0.07 | 0.58 |
| Sex, males | 0.09 | −0.96, 0.80 | −0.02 | 0.85 |
| Antidepressant drugs, + | −1.31 | −2.78, − 0.17 | −0.22 | 0.083 |
| (D) AE during follow-up period | ||||
| Estimates | 95% C. I. | Standardized β | p value | |
| SE sensitization, + | 13.8 | 2.43, 25.2 | 0.28 | 0.018 |
| Smoking, current smoking | −9.33 | −21.5, 2.83 | −0.18 | 0.13 |
| A history of AE < 2 year, + | 11.3 | 0.38, 22.1 | 0.24 | 0.043 |
| ICS, - | 0.48 | −10.4, 11.3 | 0.01 | 0.93 |
| Eosinophil ≥ 300 /µl, + | 4.79 | −7.28, 16.9 | 0.09 | 0.43 |
| Post bronchodilator FEV1/FVC | −0.80 | −1.57, − 0.04 | −0.24 | 0.039 |
*Total IgE was log-transformed because it was not normally distributed. †The number of doubling concentrations of capsaicin ranges from 1 (0.61 µM) to 10(312.5 µM), with a higher number indicating decreased cough sensitivity to inhaled capsaicin. It reflects base 2 logarithmic values of C2 and C5, respectively.
During the follow-up, we further evaluated whether C-CS was associated with AE through SE sensitization. However, we found no evidence supporting this hypothesis (data not shown).
Discussion
This posthoc study is the first to reveal that SEs sensitization at a threshold of ≥ 0.35 UA/mL, particularly SEB sensitization, is more prevalent in patients with COPD than in healthy subjects. Importantly, we found that SEs sensitization was associated with a lower C-CS. Although S. aureus colonization is potentially more relevant to clinical outcomes, SE sensitization may influence the decline in C-CS in patients with COPD by inducing IgE production. A recent study by Karakioulaki et al.11 reported that SEs sensitization did not impact eosinophil and FeNO levels under stable patient conditions, it led to elevated levels of serum total IgE. Although the results were inconclusive because of the small sample size, SE sensitization may worsen the clinical condition of patients with COPD.
In our previous study, we observed that SE-IgE levels of ≥ 0.10 UA/mL influenced clinical phenotype of patients with CRS10. Even at low sensitization levels, SEs induced increased expression of Type-2 inflammation systemically and locally in these patients10. Moreover, other studies have demonstrated that SE-IgE levels of ≥ 0.10 UA/mL are associated with the development and exacerbation of asthma, in terms of severity, use of oral corticosteroids, hospitalization, and lower lung function7,17,18. The current study revealed a difference in SEs sensitization, especially SEB sensitization, between patients with COPD and healthy controls when the cut-off value was set at 0.35 UA/mL. Meanwhile, Karakioulaki et al. did not observe a difference in SEs-IgE positivity between COPD patients and healthy individuals11. This discrepancy may be explained by the characteristics of healthy individuals. Most of the healthy individuals in our cohort comprised never smokers (n = 18), and Karakioulaki’s cohort included current smokers. The GA2LEN study clearly demonstrated that heavy smoking was associated with sensitization to SEs18. Thus, different rate of smokers may lead to a conflict outcome between ours and Karakioulaki’s. Age may also influence the rate of SE sensitization between patients with COPD and healthy individuals. However, the relationship between age and SE sensitization in the adult population remains controversial. A recent study showed that older age was a risk factor for SE sensitization in the general population19, whereas age had no effect in patients with asthma18. These findings suggest that the minimum levels of serum SEs-IgE necessary to trigger a response might vary across different diseases.
Elevated levels of total IgE have been correlated with an increased risk of dyspnea, AE, and a decline in lung function in patients with COPD20,21. Prevalence of SEs sensitization is higher in patients with COPD during stable and exacerbated conditions, compared to nonsmoking and smoking healthy individuals22. Although we did not observe a difference in the levels of total IgE between patients with and without COPD, we observed a moderate correlation in patients with COPD between the levels of total IgE and SEs-IgE. However, this correlation was absent in either nonsmoking or smoking healthy individuals22. Thus, SEs sensitization may stimulate the production of IgE during a chronic inflammatory response by acting as superantigens.
C-CS recognized as a biomarker of airway neuronal dysfunction in asthma, and heightened C-CS was associated with the pathophysiology of nonatopic asthma, poorly controlled asthma, asthma severity, and asthma exacerbation13,16. Lower levels of total IgE have contributed to heightened C-CS13. In this study, we observed a similar trend in the influence of total IgE levels on C-CS in patients with COPD, as higher levels of total IgE correlated with a decline in C-CS (Fig. 2B). These suggest that the nonallergic response is associated with airway neuronal dysfunction. In contrast, direct challenge with house dust mites into the airways could increase the cough response through airway hyperresponsiveness23. Thus, allergens may alter airway nerve fiber hyper- and hyporesponsiveness. SE may cause food poisoning by inducing the release of neuropeptides from sensory neurons24. SE-IgE may induce airway nerve hyporesponsiveness to capsaicin by triggering neuropeptides such as substance P and CGRP. However, the mechanism by which SE-IgE decreases C-CS remains unknown. Further studies are warranted to elucidate this.
Our previous work demonstrated that declined C-CS was a predictor of AE and/or community-acquired pneumonia requiring hospitalization in patients with COPD12. Aligning with these data, previous literature reported an association between heightened C-CS and AE-COPD25,26. The cough reflex is a natural and inevitable function to protect the lower airways from external particles, microorganisms, and aspirates. Viral infections, a well-known cause of AE-COPD, can trigger heightened C-CS27, while declined C-CS causes recurrent pneumonia28. Therefore, we speculated that both heightened and declined C-CS may contribute to the worsening of COPD. However, no association was observed between SE sensitization and hospitalization in this study. The association of SE sensitization with C-CS may be small in terms of future hospitalization or AE. Although the difference in C5 between the SE (+) and SE (−) groups was small, there is a difference of two doubling doses of C2 between the two groups. The European Respiratory Society guidelines for the management of cough published in 2007 clearly stated that not only C5 but also C2 should be recorded when performing the cough challenge test29. The C2 value may be preferred over the C5 value when patients reach C5 near the maximum inhaled doses of capsaicin. In our cohort, only five patients had heightened C-CS [C5 ≤ 2.44 µM (the third lowest concentration among the 10 doubling concentrations)]. When most of the population has normal cough reflex sensitivity, the difference in C2 values between the two groups may be more crucial than that in C5 values. SE sensitization contributed to the decline in C5 as well as C2 values after adjusting for confounders. Thus, we believe that SE sensitization contributed to the decline in C-CS in patients with COPD.
This study has some limitations. First, we did not measure aeroallergens, such as those from house dust mites, molds, dogs, or cats. Sensitization to rhinitis-related allergens was associated with SEs-IgE positivity in patients with COPD11. Increased exposure to sensitized aeroallergens has been associated with a higher risk of adverse clinical outcomes, including perceived wheezing, cough, dyspnea, emergency department visits, and the use of antibiotics, in patients with COPD30. A recent report identified higher levels of allergen-specific IgE, measured using the S1 screening panel, as a risk factor for a decline in lung function21. While sensitization to SEs facilitates the production of IgE in patients with COPD22, other allergens may also contribute to exacerbation of the condition. Second, it remains unclear whether SEs can enhance Type-2-dependent inflammatory responses and the decline of C-CS during COPD exacerbations. Unfortunately, we could not assess this hypothesis as we did not collect samples from patients with exacerbated COPD during the study. Third, due to the small sample size in our study, we were unable to evaluate the association between colonization of S. aureus and sensitization to SEs due to the small study sample size. S. aureus colonization, but not SE sensitization, is associated with the decline in C-CS because of less effective airway defenses. Therefore, we cannot exclude the possibility that colonization of S. aureus may play a more important role than sensitization. Meanwhile, measuring serum SE-IgE levels is easier than collecting sputum samples because some patients have difficulty expectorating sputum. Therefore, SE sensitization may be a marker for declined C-CS in patients with COPD. Last, SE sensitization is associated with the pathophysiology of CRS. CRS may confound the high IgE levels in patients with COPD. However, we did not evaluate comorbid CRS using sinus CT. Meanwhile, the relationship between allergy and pathophysiology of CRS remains inconclusive31. Thus, CRS may not have a substantial effect on high IgE levels in COPD.
Although further studies are needed to address these limitations, we propose that SEs sensitization may lead to declined C-CS, resulting in future AE in patients with COPD. In conclusion, sensitization to SEs could be a therapeutic target for COPD, as targeting IgE levels might be effective in improving respiratory symptoms and preventing AE in patients with COPD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Yoshihiro Kanemitsu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. Kensuke Fukumitsu: Data curation, Funding acquisition, Resources. Ryota Kurokawa: Data curation, Methodology, Project administration, Resources. Taisuke Akamatsu: Data curation, Methodology, Resources. Satoshi Fukuda: Data curation, Resources. Yutaka Ito: Data curation, Resources. Yuki Amakusa: Data curation, Resources. Tatsuro Suzuki: Data curation, Resources. Keima Ito: Data curation, Resources. Yuta Mori: Data curation, Resources. Takehiro Uemura: Data curation, Resources. Hirotsugu Ohkubo: Data curation, Resources. Tomoko Tajiri: Data curation, Resources. Toshihiro Shirai: Data curation, Resources, Supervision. Akio Niimi: Resources, Supervision, Writing – review & editing.
Funding
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government [grant number 20K17220].
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
There is no conflict of interest in the submitted work. The authors have competing interests outside the submitted work. Y.K. received research grants from MSD life foundation and personal fees from GSK, Novartis Pharma, AstraZeneca, Sanofi, and Kyorin. K.F. received research grants from Novartis Pharma and GSK. S.F. received personal fees from AstraZeneca and Eli Lilly. H.O. received a research grant from Boehringer Ingelheim. A.N. reports personal fees from Astellas, AstraZeneca, Kyorin, GSK, MSD, Shionogi, Bayer, Sanofi, Taiho, and Boehringer Ingelheim, and research grants from Astellas, Kyorin, Boehringer Ingelheim, Novartis, MSD, Daiichi Sankyo, Taiho, Teijin, Ono, Takeda, and Sanofi Pharmaceutical. R.K., T. A., Y.I., Y. A., T. S., K.I., Y. M., T. U., T.T., or T.S. did not receive any grants or personal fees.
Ethics approval and consent to participate
This study was approved by the ethics committee of Nagoya City University (60-18-0012) and registered in the UMIN Clinical Trials Registry (Registry ID UMIN000032497). Written informed consent was obtained from all participants. All methods were performed in accordance with the Declaration of Helsinki.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-81297-y.
References
- 1.Collaborators, G. D. I. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet396, 1204–1222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brightling, C. & Greening, N. Airway inflammation in COPD: progress to precision medicine. Eur. Respir J. 54. (2019). [DOI] [PubMed]
- 3.Flora, M. et al. Staphylococcus Aureus in chronic airway diseases: an overview. Respir Med.155, 66–71 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Sakr, A., Brégeon, F., Mège, J. L., Rolain, J. M. & Blin, O. Nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections. Front. Microbiol.9, 2419 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sørensen, M. et al. Staphylococcus aureus enterotoxin sensitization is associated with allergic poly-sensitization and allergic multimorbidity in adolescents. Allergy72, 1548–1555 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Bachert, C. et al. Staphylococcus aureus and its IgE-inducing enterotoxins in asthma: current knowledge. Eur. Respir J. 55. (2020). [DOI] [PubMed]
- 7.Bachert, C. et al. Specific IgE against Staphylococcus aureus enterotoxins: an independent risk factor for asthma. J. Allergy Clin. Immunol.130, 376–381e378 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Tomassen, P. et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J. Allergy Clin. Immunol.137, 1449–1456e1444 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Song, W. J. et al. Staphylococcal enterotoxin IgE sensitization in late-onset severe eosinophilic asthma in the elderly. Clin. Exp. Allergy. 46, 411–421 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Kanemitsu, Y. et al. Moulds and Staphylococcus aureus enterotoxins are relevant allergens to affect type 2 inflammation and clinical outcomes in chronic rhinosinusitis patients. ERJ Open. Res.6, 00265–2020 (2020). [DOI] [PMC free article] [PubMed]
- 11.Karakioulaki, M. et al. Staphylococcus aureus enterotoxin A- and B-specific IgE in chronic obstructive pulmonary disease. Respir Res.24, 225 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanemitsu, Y. et al. Decreased capsaicin cough reflex sensitivity predicts hospitalisation due to COPD. BMJ Open. Respir Res.10, e001283 (2023). [DOI] [PMC free article] [PubMed]
- 13.Satia, I. et al. Capsaicin-evoked cough responses in asthmatic patients: evidence for airway neuronal dysfunction. J. Allergy Clin. Immunol.139, 771–779e710 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Yanagisawa, S. & Ichinose, M. Definition and diagnosis of asthma-COPD overlap (ACO). Allergol. Int.67, 172–178 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Kanemitsu, Y. et al. A novel pathophysiologic link between upper and lower airways in patients with chronic rhinosinusitis: Association of sputum periostin levels with upper airway inflammation and olfactory function. World Allergy Organ. J.13, 100094 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanemitsu, Y. et al. Increased capsaicin sensitivity in patients with severe asthma is Associated with worse clinical outcome. Am. J. Respir Crit. Care Med.201, 1068–1077 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Sintobin, I. et al. Sensitisation to staphylococcal enterotoxins and asthma severity: a longitudinal study in the EGEA cohort. Eur. Respir J. 54, 1900198 (2019). [DOI] [PubMed]
- 18.Tomassen, P. et al. Staphylococcus aureus enterotoxin-specific IgE is associated with asthma in the general population: a GA(2)LEN study. Allergy68, 1289–1297 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Park, H. K. et al. Differences between Staphylococcus aureus nasal carriage and IgE-sensitization to Staphylococcus aureus enterotoxin on risk factors and effects in adult population. Allergy Asthma Clin. Immunol.18, 6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin, J., Liu, X. & Sun, Y. The prevalence of increased serum IgE and aspergillus sensitization in patients with COPD and their association with symptoms and lung function. Respir Res.15, 130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lommatzsch, M. et al. Group cs: IgE is associated with exacerbations and lung function decline in COPD. Respir Res.23, 1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohde, G. et al. Increased IgE-antibodies to Staphylococcus aureus enterotoxins in patients with COPD. Respir Med.98, 858–864 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Satia, I. et al. Allergen challenge increases capsaicin-evoked cough responses in patients with allergic asthma. J. Allergy Clin. Immunol.144, 788–795e781 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Popoff, M. R. & Poulain, B. Bacterial toxins and the nervous system: neurotoxins and multipotential toxins interacting with neuronal cells. Toxins (Basel). 2, 683–737 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terada, K. et al. Cough-reflex sensitivity to inhaled capsaicin in COPD associated with increased exacerbation frequency. Respirology14, 1151–1155 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Cho, P. S. P. et al. The relationship between Cough Reflex sensitivity and exacerbation frequency in Chronic Obstructive Pulmonary Disease. Lung198, 617–628 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dicpinigaitis, P. V., Spinner, L., Santhyadka, G. & Negassa, A. Effect of tiotropium on cough reflex sensitivity in acute viral cough. Lung186, 369–374 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Niimi, A. et al. Impaired cough reflex in patients with recurrent pneumonia. Thorax58, 152–153 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morice, A. H. et al. ERS guidelines on the assessment of cough. Eur. Respir J.29, 1256–1276 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Jamieson, D. B. et al. Effects of allergic phenotype on respiratory symptoms and exacerbations in patients with chronic obstructive pulmonary disease. Am. J. Respir Crit. Care Med.188, 187–192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcus, S., Roland, L. T., DelGaudio, J. M. & Wise, S. K. The relationship between allergy and chronic rhinosinusitis. Laryngoscope Investig Otolaryngol.4, 13–17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.