Abstract
1. In order adequately to control eye movements, oculomotoneurones have to be supplied with both an eye-velocity signal and an eye-position signal. However, all the command signals of the oculomotor system are velocity signals. Nowadays, there is general agreement about the existence of a brainstem network that would convert velocity command-signals into an eye-position signal. This circuit, because of its function, is called the oculomotor neural integrator. The most obvious symptom of its eventual failure is a gaze-holding deficit: in this case, saccades are followed by a centripetal post-saccadic drift. Although the oculomotor neural integrator is central in oculomotor theory, its precise location is still a matter for debate. 2. Previously, microinjections of kainic acid (KA) into the region of the nucleus prepositus hypoglossi (NPH) and of the medial vestibular nucleus (MVN) were found to induce a horizontal gaze-holding failure both in the cat and in the monkey. However, the relatively large volumes (1-3 microliters) and concentrations (2-4 micrograms microliters-1) used in these injections made it difficult to know if the observed deficit was due to a disturbance of the NPH or of the nearby MVN. These considerations led us to inject very small amounts of kainic acid (50 nl, 0.1 microgram microliter-1) either into the rostral part of the MVN or into different sites along the NPH of the cat. 3. The search coil technique was used to record (1) spontaneous eye movements (2) the vestibulo-ocular reflex (VOR) induced by a constant-velocity rotation (50 deg s-1 for 40 s) and the optokinetic nystagmus (OKN) elicited by rotating an optokinetic drum at 30 deg s-1 for 40 s. 4. In each injection experiment, the location of the abducens nucleus of the alert cat was mapped out by recording the antidromic field potentials evoked by the stimulation of the abducens nerve. Two micropipettes were then glued together in such a way that when the tip of the recording micropipette was in the centre of the abducens nucleus the tip of the injection micropipette was in a target area. The twin pipettes were then lowered in the brainstem until the recording micropipette reached the centre of the abducens nucleus. Kainic acid was then injected into the brainstem of the alert cat through the injection micropipette by an air pressure system. 5. Carried out according to such a protocol, KA injections into the NPH or the rostral part of the MVN consistently led to specific eye-movement changes.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


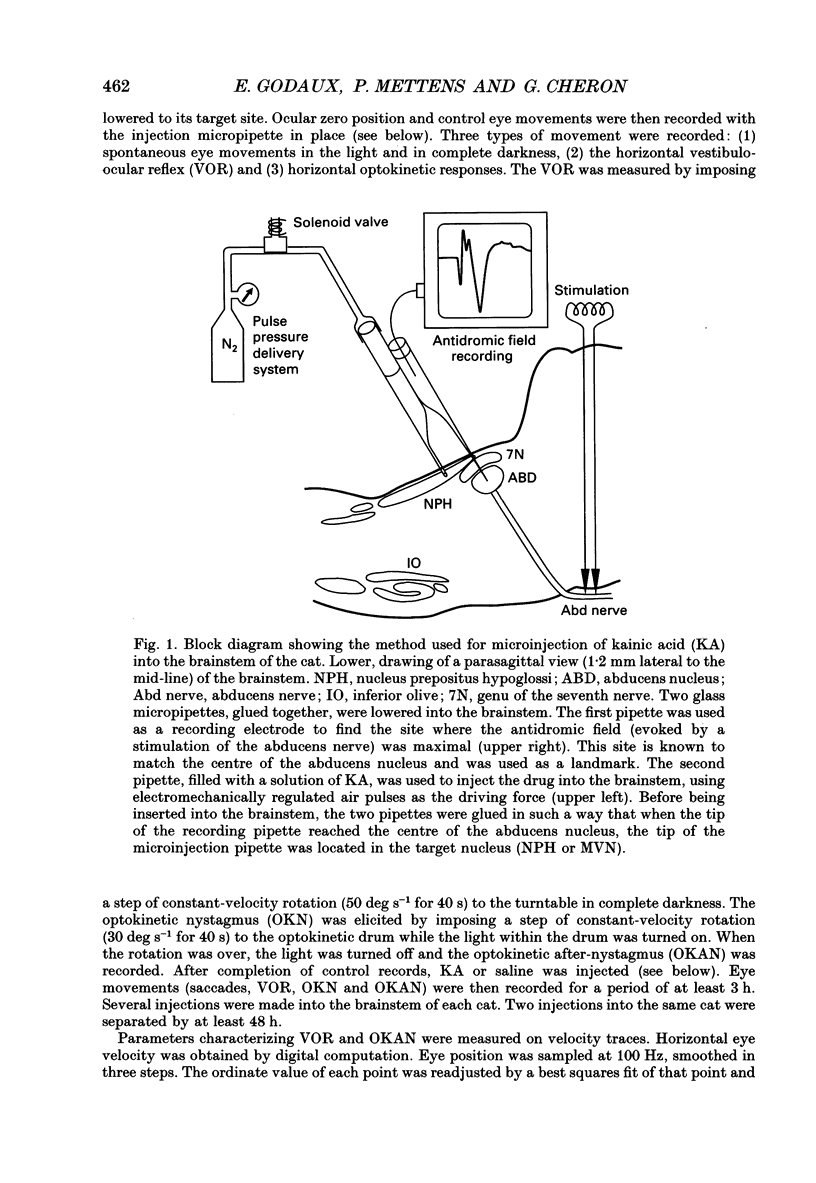




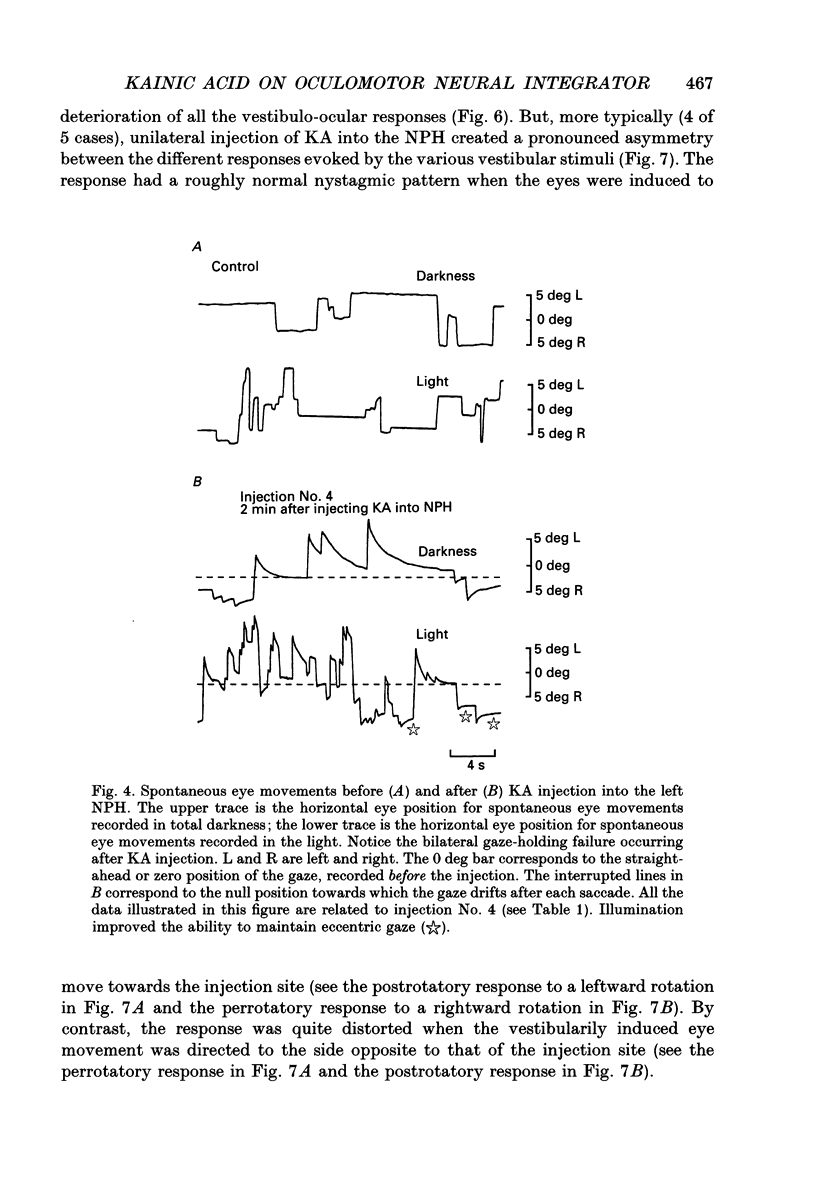
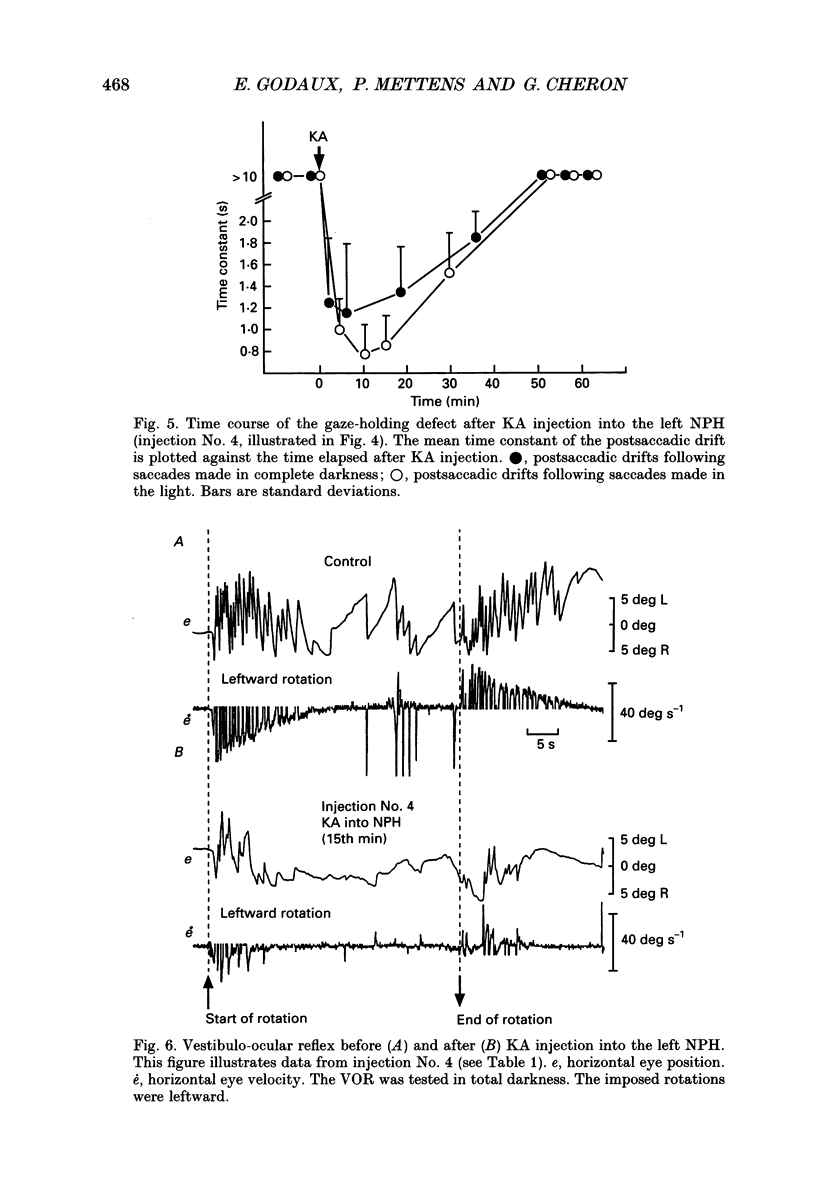

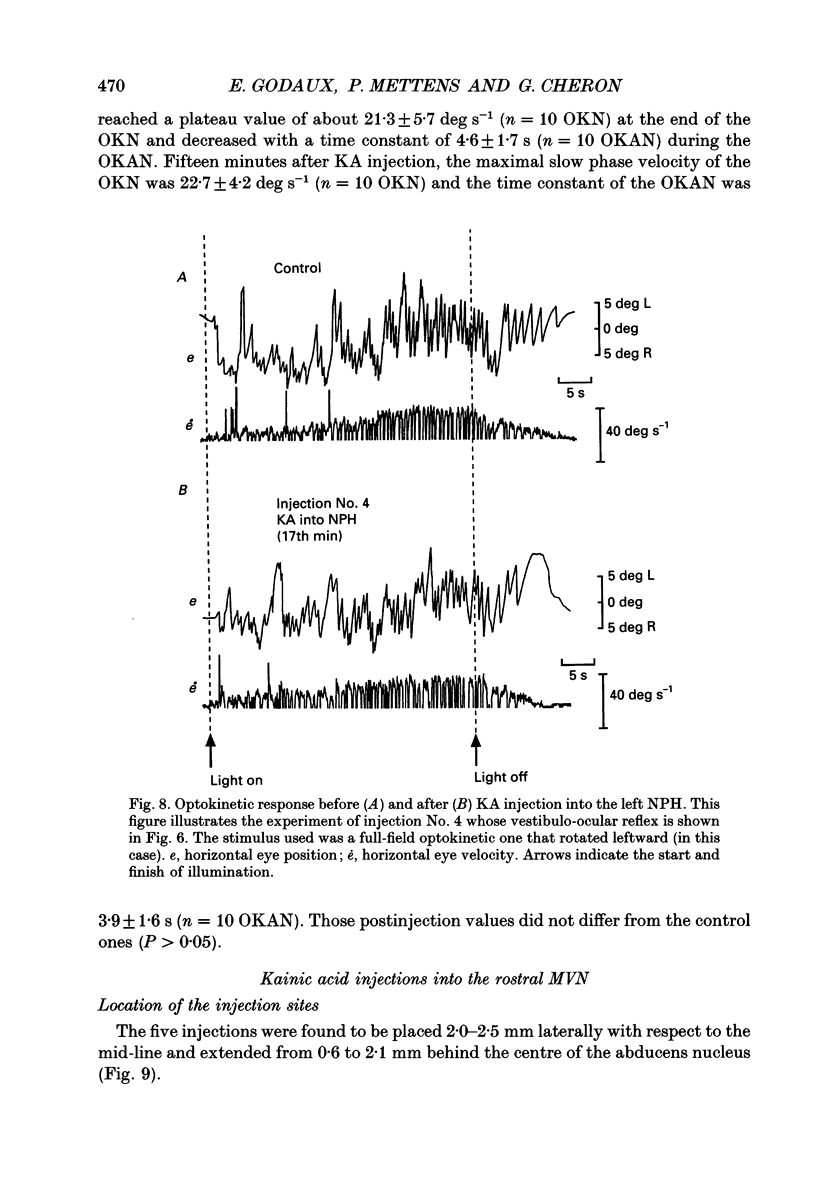








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaral D. G., Price J. L. An air pressure system for the injection of tracer substances into the brain. J Neurosci Methods. 1983 Sep;9(1):35–43. doi: 10.1016/0165-0270(83)90107-3. [DOI] [PubMed] [Google Scholar]
- Baker R. G., Mano N., Shimazu H. Postsynaptic potentials in abducens motoneurons induced by vestibular stimulation. Brain Res. 1969 Oct;15(2):577–580. doi: 10.1016/0006-8993(69)90189-9. [DOI] [PubMed] [Google Scholar]
- Baker R., Evinger C., McCrea R. A. Some thoughts about the three neurons in the vestibular ocular reflex. Ann N Y Acad Sci. 1981;374:171–188. doi: 10.1111/j.1749-6632.1981.tb30869.x. [DOI] [PubMed] [Google Scholar]
- Baker R., Gresty M., Berthoz A. Neuronal activity in the prepositus hypoglossi nucleus correlated with vertical and horizontal eye movement in the cat. Brain Res. 1976 Jan 16;101(2):366–371. doi: 10.1016/0006-8993(76)90278-x. [DOI] [PubMed] [Google Scholar]
- Berthoz A., Droulez J., Vidal P. P., Yoshida K. Neural correlates of horizontal vestibulo-ocular reflex cancellation during rapid eye movements in the cat. J Physiol. 1989 Dec;419:717–751. doi: 10.1113/jphysiol.1989.sp017895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon S. C., Robinson D. A. Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. J Neurophysiol. 1987 May;57(5):1383–1409. doi: 10.1152/jn.1987.57.5.1383. [DOI] [PubMed] [Google Scholar]
- Cheron G., Gillis P., Godaux E. Lesions in the cat prepositus complex: effects on the optokinetic system. J Physiol. 1986 Mar;372:95–111. doi: 10.1113/jphysiol.1986.sp015999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G., Godaux E. Disabling of the oculomotor neural integrator by kainic acid injections in the prepositus-vestibular complex of the cat. J Physiol. 1987 Dec;394:267–290. doi: 10.1113/jphysiol.1987.sp016870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G., Godaux E., Laune J. M., Vanderkelen B. Lesions in the cat prepositus complex: effects on the vestibulo-ocular reflex and saccades. J Physiol. 1986 Mar;372:75–94. doi: 10.1113/jphysiol.1986.sp015998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G., Mettens P., Godaux E. Gaze holding defect induced by injections of ketamine in the cat brainstem. Neuroreport. 1992 Jan;3(1):97–100. doi: 10.1097/00001756-199201000-00026. [DOI] [PubMed] [Google Scholar]
- Collewijn H. Direction-selective units in the rabbit's nucleus of the optic tract. Brain Res. 1975 Dec 26;100(3):489–508. doi: 10.1016/0006-8993(75)90154-7. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Crawford J. D., Cadera W., Vilis T. Generation of torsional and vertical eye position signals by the interstitial nucleus of Cajal. Science. 1991 Jun 14;252(5012):1551–1553. doi: 10.1126/science.2047862. [DOI] [PubMed] [Google Scholar]
- Delgado-Garcia J. M., del Pozo F., Baker R. Behavior of neurons in the abducens nucleus of the alert cat--I. Motoneurons. Neuroscience. 1986 Apr;17(4):929–952. doi: 10.1016/0306-4522(86)90072-2. [DOI] [PubMed] [Google Scholar]
- Escudero M., Delgado-García J. M. Behavior of reticular, vestibular and prepositus neurons terminating in the abducens nucleus of the alert cat. Exp Brain Res. 1988;71(1):218–222. doi: 10.1007/BF00247538. [DOI] [PubMed] [Google Scholar]
- Escudero M., de la Cruz R. R., Delgado-García J. M. A physiological study of vestibular and prepositus hypoglossi neurones projecting to the abducens nucleus in the alert cat. J Physiol. 1992 Dec;458:539–560. doi: 10.1113/jphysiol.1992.sp019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C., Goldberg J. M. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971 Jul;34(4):661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- Fuchs A. F., Robinson D. A. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966 May;21(3):1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Godaux E., Vanderkelen B. Vestibulo-ocular reflex, optokinetic response and their interactions in the cerebellectomized cat. J Physiol. 1984 Jan;346:155–170. doi: 10.1113/jphysiol.1984.sp015013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. M., Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol. 1971 Jul;34(4):635–660. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- Henn V., Straumann D., Hess B. J., Hepp K., Vilis T., Reisine H. Generation of vertical and torsional rapid eye movement in the rostral mesencephalon. Experimental data and clinical implications. Acta Otolaryngol Suppl. 1991;481:191–193. doi: 10.3109/00016489109131378. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P., Schoppmann A. Retinal input to direction selective cells in the nucleus tractus opticus of the cat. Brain Res. 1975 Dec 5;99(2):359–366. doi: 10.1016/0006-8993(75)90037-2. [DOI] [PubMed] [Google Scholar]
- Judge S. J., Richmond B. J., Chu F. C. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20(6):535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kaneko C. R. Effects of ibotenic acid lesions of nucleus prepositus hypoglossi on optokinetic and vestibular eye movements in the alert, trained monkey. Ann N Y Acad Sci. 1992 May 22;656:408–427. doi: 10.1111/j.1749-6632.1992.tb25225.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J., Darlot C., Berthoz A., Baker R. Neuronal activity in prepositus nucleus correlated with eye movement in the alert cat. J Neurophysiol. 1982 Feb;47(2):329–352. doi: 10.1152/jn.1982.47.2.329. [DOI] [PubMed] [Google Scholar]
- McCrea R. A., Yoshida K., Berthoz A., Baker R. Eye movement related activity and morphology of second order vestibular neurons terminating in the cat abducens nucleus. Exp Brain Res. 1980;40(4):468–473. doi: 10.1007/BF00236156. [DOI] [PubMed] [Google Scholar]
- McFarland J. L., Fuchs A. F. Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movement in behaving macaques. J Neurophysiol. 1992 Jul;68(1):319–332. doi: 10.1152/jn.1992.68.1.319. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. The distribution of [3H]kainic acid binding sites in rat CNS as determined by autoradiography. Brain Res. 1982 Dec 2;252(1):91–100. doi: 10.1016/0006-8993(82)90981-7. [DOI] [PubMed] [Google Scholar]
- Robinson D. A. Eye movement control in primates. The oculomotor system contains specialized subsystems for acquiring and tracking visual targets. Science. 1968 Sep 20;161(3847):1219–1224. doi: 10.1126/science.161.3847.1219. [DOI] [PubMed] [Google Scholar]
- Robinson D. A. The effect of cerebellectomy on the cat's bestibulo-ocular integrator. Brain Res. 1974 May 17;71(2-3):195–207. doi: 10.1016/0006-8993(74)90961-5. [DOI] [PubMed] [Google Scholar]
- Robinson J. H., Deadwyler S. A. Kainic acid produces depolarization of CA3 pyramidal cells in the vitro hippocampal slice. Brain Res. 1981 Sep 21;221(1):117–127. doi: 10.1016/0006-8993(81)91067-2. [DOI] [PubMed] [Google Scholar]
- Shimazu H., Precht W. Inhibition of central vestibular neurons from the contralateral labyrinth and its mediating pathway. J Neurophysiol. 1966 May;29(3):467–492. doi: 10.1152/jn.1966.29.3.467. [DOI] [PubMed] [Google Scholar]
- Skavenski A. A., Robinson D. A. Role of abducens neurons in vestibuloocular reflex. J Neurophysiol. 1973 Jul;36(4):724–738. doi: 10.1152/jn.1973.36.4.724. [DOI] [PubMed] [Google Scholar]
- Van Gisbergen J. A., Robinson D. A., Gielen S. A quantitative analysis of generation of saccadic eye movements by burst neurons. J Neurophysiol. 1981 Mar;45(3):417–442. doi: 10.1152/jn.1981.45.3.417. [DOI] [PubMed] [Google Scholar]
- Wroblewski J. T., Danysz W. Modulation of glutamate receptors: molecular mechanisms and functional implications. Annu Rev Pharmacol Toxicol. 1989;29:441–474. doi: 10.1146/annurev.pa.29.040189.002301. [DOI] [PubMed] [Google Scholar]
- Zee D. S., Yamazaki A., Butler P. H., Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981 Oct;46(4):878–899. doi: 10.1152/jn.1981.46.4.878. [DOI] [PubMed] [Google Scholar]




