Abstract
Objective
Identify patient characteristics and polysomnogram (PSG) parameters associated with postoperative respiratory complications after adenotonsillectomy (AT) among children with high‐risk obstructive sleep apnea (OSA).
Study Design
Case series with chart review.
Setting
Tertiary care children's hospital.
Methods
Pediatric patients (<18 years) with high‐risk OSA (any 1 of: apnea‐hypopnea index [AHI] >30, O2 nadir <80% and peak CO2 >60 mm Hg) on overnight PSG from 2019 to 2021 were included. Primary outcomes were major respiratory intervention during the postoperative admission, prolonged hospitalization, and intensive care unit (ICU) stay.
Results
A total of 307 patients met inclusion criteria. Median age was 6.5 years and 63% were male. Twenty‐five (8.1%) required major respiratory intervention and 29 (9.7%) required ICU admission after AT. Major interventions and ICU admissions were significantly associated with neuromuscular disease (P < .01), higher obstructive apnea‐hypopnea index (oAHI), higher CO2 peak, and lower O2 nadir. Prolonged admission had similar findings except oAHI was not significantly associated. Younger children were significant more likely to require ICU admission or prolonged admission.
Conclusion
Increased oAHI and worsening O2 and CO2 parameters on preoperative PSG were associated with postoperative respiratory complications in children with high‐risk OSA. Children with neuromuscular disease and age 0 to 2 had higher risk of ICU stay and prolonged hospitalization. Clinicians should recognize the importance of parameters beyond oAHI when anticipating postoperative monitoring.
Keywords: high‐risk sleep apnea, obstructive sleep apnea, postoperative complications, profound sleep apnea, very severe sleep apnea
Obstructive sleep apnea (OSA) refers to intermittent nocturnal airway obstruction that affects approximately 1% to 4% of the pediatric population. 1 Definitive diagnosis requires an overnight polysomnogram (PSG) where the most commonly used metric to categorize severity, the apnea‐hypopnea index (AHI), averages the number of mixed, obstructive, and central apneas/hypopneas per hour during sleep. 2 Additional parameters such as hypoxemia, hypercarbia, and heart rate are among several additional factors that are collected. The first‐line treatment for OSA in the majority of children is adenotonsillectomy (AT).
The American Academy of Otolaryngology–Head and Neck Surgery (AAO‐HNS) Foundation identified several research needs in the updated Clinical Practice Guideline for tonsillectomy in children. 3 This included defining PSG parameters that predispose children to having respiratory complications and determining if the risk of postoperative complications can be stratified to the patient's disease severity as defined by PSG. To date, there is evidence that suggests patients with severe OSA and significant hypoxemia and hypercarbia are at higher risk for postoperative complications. 4 , 5
At our institution, a certain population of children is marked as “high‐risk” after their PSG. The primary objective of this study was to determine if there were specific patient factors or PSG parameters in this high‐risk OSA population that were associated with higher risks of postoperative complications such as: major respiratory interventions, prolonged admissions, and intensive care unit (ICU) admissions. We hypothesize that worse O2 and CO2 parameters would have a significant effect on these complications.
Materials and Methods
The study was approved by the University of Texas Southwestern Medical Center Institutional Review Board. Study ID was STU‐042014‐001. Study data was collected and managed using REDCAP electronic data capture tools hosted at the University of Texas Southwestern Medical Center. 6
Data Collection
We retrospectively reviewed all PSGs from January 1, 2019, to December 31, 2021 that were labeled as high‐risk. Patients were identified as high‐risk by the pediatric sleep lab at the time of their PSG. Parameters that were used included: AHI >30, O2 nadir <80%, max CO2 >60 mm Hg, emergent split to positive airway pressure (PAP), arrhythmias, and seizures. Any 1 of these findings would lead to the study being labeled as high‐risk. AHI included both obstructive and central apneas. Due to their disease severity, these patients' sleep study interpretations were expedited, and the pediatric sleep physicians would aim to read their PSGs within 24 hours of completion of the study. Referral to Otolaryngology clinic for surgical evaluation was also expedited.
For this study, we were primarily interested in OSA so we excluded PSGs that were flagged for arrhythmias and seizures. Other exclusion criteria were age >18, history of prior AT and patients who did not undergo AT. We then parsed the electronic medical record for these patients.
We collected demographic information including gender (male or female), age, race (white/Caucasian, black/African‐American, Asian, other), ethnicity (Hispanic or not Hispanic), body mass index (BMI), BMI z score, and BMI as a percentage of the 95th percentile based on sex and age (%BMIp95). %BMIp95 data was not available for patients who were less than 2 years of age or had BMI <95% percentile. We also identified comorbidities including: Trisomy 21, cardiac comorbidities, asthma, craniofacial abnormalities, mucopolysaccharidoses, sickle cell anemia, neuromuscular conditions, and achondroplasia. Our practice consists of 11 surgeons and all but 1 perform total tonsillectomies. The only provider who performs partial tonsillectomies was only present for the latter part of the study period and due to this small subgroup we did not perform a separate subgroup analysis.
Initial PSG reports were identified and the following variables were collected: sleep efficiency, obstructive apnea‐hypopnea index (oAHI), central AHI, O2 nadir, total sleep time (TST) O2 less than 90%, 88%, and 80%, peak CO2, average CO2, percentage of TST with CO2 >50 mm Hg. For the sake of this paper, we will discuss the O2 nadir and TST O2 >90%, 88%, and 80% as O2 parameters. We will discuss peak CO2, average CO2, and percentage of TST CO2 >50 mm Hg as CO2 parameters. We then looked at surgical and perioperative data including: age at surgery, postoperative ICU admission, length of stay (LOS), need for major interventions (noninvasive positive pressure ventilation, intubation). We defined a prolonged admission as admission 48 hours or longer.
Statistical Analysis
Descriptive analysis was performed on demographic data. For our continuous variables, we used means and standard deviations and categorical variables were described using counts with percentage. For certain variables such as age and BMI z score, we used median and interquartile range as there was a large range. Univariate analysis was performed using Pearson's χ 2 testing for categorical data and Student's t testing for continuous variables. A 1‐way analysis of variance was used for comparison of multiple continuous variables. We performed a logistical regression with major interventions and ICU admission with O2 nadir and peak CO2.
This study adhered to Strengthening the Reporting of Observational Studies in Epidemiology guidelines for observational studies. 7 All analyses were performed using Stata Statistical Software, version 16. Significance was set at P < .05.
Results
The Children's Health Sleep Disorders Center is a large tertiary care referral center. The sleep lab at the time of this study was comprised of 20 pediatric beds across 2 sites. Between January of 2019 and December of 2022 a total of 15,943 studies were completed. We identified 1420 PSGs which were labeled as high‐risk. We reviewed these charts and identified 307 patients who met inclusion criteria. The mean age at the time of surgery was 6.5 years. The age breakdown was: ages 0 to 2 (33, 11%), ages 2 to 5 (132, 43%), ages 5 to 12 (95, 31%), and ages 12 to 18 (47, 15%). There were 194 (63%) males overall. The racial breakdown was: 59 white/Caucasian (19%), 113 black/African‐American (37%), 122 Hispanic (40%), and 7 other (4%). There were no age‐related differences in the incidence of Trisomy 21, cardiac disease, asthma, craniofacial disorders, or sickle cell disease. There was a significantly higher incidence of neuromuscular disease in the 0 to 2 year age group (21%, P = .02). The BMI z score was significantly higher in the 5 to 12 and 12 to 18 year age groups (2.4 and 2.5, P < .001). The median %BMIp95 was not significantly different between age groups. Preoperative PSG parameters were also compared. There were significant differences in oAHI and O2 nadir. The oAHI increased with each consecutive age group (P = .003) and the O2 nadir also increased with each consecutive age group (P = .001). Full demographic and preoperative PSG information can be found in Table 1.
Table 1.
Demographics and Comorbidities of Children With High Risk OSA by Age Group
| Age 0‐2, y | Age 2‐5, y | Age 5‐12, y | Age 12‐18, y | P value | |
|---|---|---|---|---|---|
| Total, N (%) | 33 (11) | 132 (43) | 95 (31) | 47 (15) | ‐ |
| Males, N (%) | 18 (55) | 79 (60) | 61 (64) | 36 (76) | .15 |
| Race | |||||
| White/Caucasian | 8 (24) | 28 (21) | 16 (17) | 7 (15) | .009 |
| Black/African‐American | 19 (58) | 53 (40) | 23 (24) | 18 (38) | |
| Hispanic | 6 (18) | 45 (34) | 52 (55) | 19 (40) | |
| Other | 0 | 6 (4.5) | 4 (4.2) | 3 (6.4) | |
| Trisomy 21 | 1 (3.0) | 9 (6.8) | 4 (4.2) | 2 (4.3) | .73 |
| Cardiac disease | 4 (12) | 9 (6.8) | 6 (6.3) | 1 (2.1) | .36 |
| Asthma | 3 (9.1) | 27 (20) | 11 (12) | 10 (21) | .16 |
| Craniofacial | 3 (9.1) | 6 (4.6) | 2 (2.1) | 0 | .13 |
| MPS | 0 | 0 | 0 | 0 | ‐ |
| Sickle cell disease | 1 (3.0) | 1 (0.8) | 1 (1.1) | 0 | .58 |
| Neuromuscular disease | 7 (21) | 9 (6.8) | 5 (5.3) | 2 (4.3) | .02 |
| Achondroplasia | 1 (3.0) | 1 (1.5) | 0 | 0 | .36 |
| BMI z score, mean (SD) | 0.9 (1.8) | 1.1 (2.2) | 2.4 (1.2) | 2.5 (0.8) | <.001 |
| BMI% 95th, mean (SD) | ‐ | 133 (25) | 134 (23) | 134 (24) | .92 |
| Sleep efficiency | 76.7 (15.3) | 83.4 (43.7) | 80.1 (12.7) | 76.8 (12.5) | .50 |
| oAHI | 28.1 (26.7) | 33.6 (25.0) | 36.0 (22.3) | 47.4 (28.8) | .003 |
| CAI | 1.6 (2.6) | 0.8 (1.0) | 0.6 (1.1) | 0.9 (2.1) | .01 |
| O2 nadir | 71.7 (8.2) | 71.7 (9.3) | 75.3 (9.9) | 77.2 (9.3) | .001 |
| TST O2 <90% | 21.4 (30.8) | 23.8 (30.5) | 20.7 (27.4) | 20.0 (31.6) | .83 |
| TST O2 <88% | 9.7 (14.3) | 13.1 (18.1) | 10.6 (14.7) | 10.8 (18.4) | .58 |
| TST O2 <80% | 2.6 (5.1) | 4.5 (9.1) | 3.4 (7.4) | 2.7 (7.6) | .44 |
| Peak CO2 | 52.3 (5.7) | 53.2 (7.6) | 53.0 (4.9) | 52.4 (4.5) | .80 |
| % TST CO2 >50 mm Hg | 6.8 (12.0) | 10.9 (19.4) | 9.0 (17.1) | 9.2 (18.9) | .64 |
Abbreviations: BMI, body mass index; CAI, central apnea index; MPS, mucopolysaccharidosis; oAHI, obstructive apnea‐hypopnea index; OSA, obstructive sleep apnea; SD, standard deviation; TST, total sleep time; %BMIp95, BMI as a percentage of the 95th percentile based on sex and age.
We looked for associations between patient demographics or preoperative PSG findings and need for major postoperative interventions. A total of 25 (8.1%) children required major intervention and we found no significant difference based on race. However, children who required a major intervention had significantly higher BMI z score (3.1 vs 1.7, P < .001) and %BMIp95 (148 vs 132, P = .01). Children with cardiac disease (20%, P < .01), craniofacial disease (12%, P = .02), and neuromuscular disease (24%, P = .004) were found to require significantly more major interventions. Children who needed major interventions had significantly higher oAHI (50.1 vs 34.6, P = .003), lower O2 nadir (64.7% vs 74.4%, P < .001), and TST O2 >90%, 88%, and 80% (P < .001). These children also had significantly higher peak CO2 (56.5 vs 52.6, P = .003) and percentage of TST with CO2 >50 mm Hg (25.4% vs 8.2%, P < .001). Full analysis of major interventions can be found in Table 2.
Table 2.
Association Between Patient Demographics and Preoperative PSG Findings With Postoperative Major Interventions
| Major intervention (N = 25, 8.1%) | No intervention (N = 282, 92%) | P value | |
|---|---|---|---|
| Males | 17 (68) | 177 (63) | .60 |
| Race | |||
| White/Caucasian | 3 (12) | 56 (20) | .41 |
| Black/African‐American | 13 (52) | 100 (35) | |
| Hispanic | 8 (32) | 114 (40) | |
| Other | 1 (4.0) | 12 (4.3) | |
| Trisomy 21 | 0 | 16 (5.7) | .22 |
| Cardiac disease | 5 (20) | 15 (5.3) | .004 |
| Asthma | 3 (12) | 48 (17) | .52 |
| Craniofacial | 3 (12) | 8 (2.8) | .02 |
| MPS | 0 | 0 | ‐ |
| Sickle cell disease | 0 | 3 (1.0) | .60 |
| Neuromuscular disease | 6 (24) | 17 (6.0) | .001 |
| Achondroplasia | 1 (4.0) | 2 (0.7) | .11 |
| BMI z score, mean (SD) | 3.1 (2.4) | 1.7 (1.7) | <.001 |
| BMI% 95th, mean (SD) | 148 (24) | 132 (23) | .01 |
| Sleep efficiency | 79.8 (13.9) | 80.7 (31.4) | .88 |
| oAHI | 50.1 (36.9) | 34.6 (23.9) | .003 |
| CAI | 0.4 (0.1) | 0.9 (1.5) | .13 |
| O2 nadir | 64.7 (12.7) | 74.4 (8.9) | <.001 |
| TST O2 <90% | 60.4 (53.9) | 18.6 (23.8) | <.001 |
| TST O2 <88% | 34.2 (32.5) | 9.6 (12.9) | <.001 |
| TST O2 <80% | 13.6 (13.4) | 2.8 (6.7) | <.001 |
| Peak CO2 | 56.5 (5.0) | 52.6 (6.2) | .003 |
| % TST CO2 >50 mm Hg | 25.4 (31.7) | 8.2 (15.5) | <.001 |
Abbreviations: BMI, body mass index; CAI, central apnea index; MPS, mucopolysaccharidosis; oAHI, obstructive apnea‐hypopnea index; PSG, polysomnogram; SD, standard deviation; TST, total sleep time; %BMIp95, BMI as a percentage of the 95th percentile based on sex and age.
We looked for associations between patient demographics and preoperative PSG findings with admissions greater than 48 hours and need for ICU admission following AT. There were 49 (16%) patients who required prolonged admission. There were no differences in demographics. We found that children with worse O2 and CO2 parameters preoperatively were significantly more likely to have a prolonged admission. This was seen in O2 nadir (67.8% vs 74.7%, P < .001) and TST <90%, 88%, and 80% (P < .001). Children with prolonged admissions had significantly higher peak CO2 (54.9 vs 52.6 mm Hg, P = .02) and TST with CO2 >50 mm Hg (17.3% vs 8.2%, P = .001). There was no significant association with oAHI (40.0 vs 35.1, P = .21). Of these 49 patients who required a prolonged admission, only 6 were for nonrespiratory reasons such as pain control and poor oral intake. Full analysis of prolonged admissions can be found in Table 3.
Table 3.
Association Between Patient Demographics and Preoperative PSG Findings With Prolonged Postoperative Admission (>48 hours)
| Admit >48 h (N = 49, 16%) | Admit <48 h (N = 258, 84%) | P value | |
|---|---|---|---|
| Males | 27 (55) | 167 (65) | .20 |
| Race | |||
| White/Caucasian | 6 (12) | 53 (21) | .08 |
| Black/African‐American | 26 (53) | 87 (34) | |
| Hispanic | 15 (31) | 107 (41) | |
| Other | 2 (4.1) | 11 (4.3) | |
| Trisomy 21 | 4 (8.2) | 12 (4.7) | .31 |
| Cardiac disease | 6 (12) | 14 (5.4) | .08 |
| Asthma | 10 (20) | 41 (16) | .44 |
| Craniofacial | 4 (8.2) | 7 (2.7) | .06 |
| MPS | 0 | 0 | ‐ |
| Sickle cell disease | 1 (2.0) | 2 (0.8) | .41 |
| Neuromuscular disease | 7 (14) | 16 (6.2) | .05 |
| Achondroplasia | 3 (6.1) | 0 | <.001 |
| BMI z score, mean (SD) | 1.9 (2.4) | 1.8 (1.7) | .64 |
| BMI% 95th, mean (SD) | 141 (29) | 133 (23) | .17 |
| Sleep efficiency | 78.3 (13.1) | 81.1 (32.6) | .56 |
| oAHI | 40.0 (33.6) | 35.1 (23.6) | .21 |
| CAI | 0.6 (1.0) | 0.9 (1.6) | .30 |
| O2 nadir | 67.8 (10.0) | 74.7 (9.1) | <.001 |
| TST O2 <90% | 43.0 (44.9) | 18.0 (23.9) | <.001 |
| TST O2 <88% | 23.5 (25.9) | 9.3 (13.3) | <.001 |
| TST O2 <80% | 8.4 (11.5) | 2.8 (6.9) | <.001 |
| Peak CO2, mm Hg (SD) | 54.9 (6.3) | 52.6 (6.2) | .02 |
| % TST CO2 >50 mm Hg | 17.3 (25.9) | 8.2 (15.6) | .001 |
Abbreviations: BMI, body mass index; CAI, central apnea index; MPS, mucopolysaccharidosis; oAHI, obstructive apnea‐hypopnea index; PSG, polysomnogram; SD, standard deviation; TST, total sleep time; %BMIp95, BMI as a percentage of the 95th percentile based on sex and age.
A total of 29 (9.4%) children required postoperative ICU admission. Of these 29, 10 (34.5%) were planned prior to admission. Of these 10, 5 required a major intervention. ICU admissions was significantly higher in children with craniofacial disorders (14%, P = .002) and children with neuromuscular disease (21%, P = .005). Children with a higher BMI z score were significantly more likely to require ICU admission (2.6 vs 1.7, P = .03). Children with higher oAHI were significantly more likely to require ICU admission (48.8 vs 34.5, P = .004). Worse O2 and CO2 parameters on preoperative PSG were also found to be significantly associated with ICU admission. This included O2 nadir (64.8% vs 74.6%, P < .001), TST <90%, 88%, 80% (P < .001), peak CO2 (56.8 vs 52.5, P < .001), and TST with CO2 > 50 mm Hg (22.3% vs 8.3%, P < .001). Full analysis of ICU admissions can be found in Table 4.
Table 4.
Association Between Patient Demographics and Preoperative PSG Findings With Postoperative ICU Admission
| ICU (N = 29, 9.4%) | No ICU (N = 278, 91%) | P value | |
|---|---|---|---|
| Males | 20 (69) | 174 (63) | .50 |
| Race | |||
| White/Caucasian | 6 (21) | 53 (19) | .14 |
| Black/African‐American | 15 (52) | 98 (35) | |
| Hispanic | 6 (21) | 116 (42) | |
| Other | 2 (6.9) | 11 (4.0) | |
| Trisomy 21 | 1 (3.5) | 15 (5.4) | .65 |
| Cardiac disease | 4 (14) | 16 (5.8) | .10 |
| Asthma | 3 (10) | 48 (17) | .34 |
| Craniofacial | 4 (14) | 7 (2.5) | .002 |
| MPS | 0 | 0 | ‐ |
| Sickle cell disease | 0 | 3 (1.1) | .57 |
| Neuromuscular disease | 6 (21) | 17 (6.1) | .005 |
| Achondroplasia | 3 (10) | 0 | <.001 |
| BMI z score, mean (SD) | 2.6 (2.4) | 1.7 (1.8) | .03 |
| BMI% 95th, mean (SD) | 140 (30) | 133 (23) | .31 |
| Sleep efficiency | 78.8 (13.7) | 80.8 (31.6) | .73 |
| oAHI | 48.8 (37.2) | 34.5 (23.6) | .004 |
| CAI | 0.6 (0.9) | 0.9 (1.5) | .39 |
| O2 nadir | 64.8 (10.3) | 74.6 (9.0) | <.001 |
| TST O2 <90% | 51.5 (48.2) | 18.9 (25.3) | <.001 |
| TST O2 <88% | 28.8 (28.5) | 9.8 (13.9) | <.001 |
| TST O2 <80% | 11.3 (12.6) | 2.9 (7.0) | <.001 |
| Peak CO2 | 56.8 (5.0) | 52.5 (6.2) | <.001 |
| % TST CO2 >50 mm Hg | 22.3 (30.0) | 8.3 (15.7) | <.001 |
Abbreviations: BMI, body mass index; CAI, central apnea index; ICU, intensive care unit; MPS, mucopolysaccharidosis; oAHI, obstructive apnea‐hypopnea index; PSG, polysomnogram; SD, standard deviation; TST, total sleep time; %BMIp95, BMI as a percentage of the 95th percentile based on sex and age.
We stratified children into age groups as described above to look at postoperative outcomes. Children age 0 to 2 had significantly higher rate of ICU stays (22%) when compared to other age groups (P = .02). They also had a significant higher rate of prolonged admission over 48 hours (36.0%, P < .001). The full results of this analysis can be found in Table 5.
Table 5.
Postoperative Outcomes in High‐Risk OSA Patients Following AT
| Age 0‐2, y | Age 2‐5, y | Age 5‐12, y | Age 12‐18, y | P value | |
|---|---|---|---|---|---|
| Total hospital length of stay, d (SD) | 1.7 (1.5) | 1.7 (2.1) | 1.2 (0.9) | 1.1 (0.4) | .03 |
| ICU admission, N (%) | 7 (22) | 15 (12) | 6 (6.5) | 1 (2.1) | .02 |
| Length of ICU stay, d (SD) | 0.6 (1.6) | 0.4 (1.6) | 0.2 (0.9) | 0.0 (0.2) | .25 |
| Major intervention, N (%) | 4 (12) | 11 (8.3) | 8 (8.4) | 2 (4.3) | .65 |
| +RVP during admission, N (%) | 4 (12) | 8 (6.2) | 2 (2.1) | 0 | .04 |
| Admission >48 h, N (%) | 12 (36) | 27 (20) | 9 (9.5) | 1 (2.1) | <.001 |
Abbreviations: AT, adenotonsillectomy; ICU, intensive care unit; OSA, obstructive sleep apnea; RVP, respiratory viral panel.
Logistical regression was performed looking at need for major intervention and ICU admission with preoperative O2 nadir and preoperative peak CO2. This provides a visual that shows the predicted probability of major intervention or ICU admission with 95% confidence interval based on logistical regression modeling. These tables can be found in Figure 1.
Figure 1.
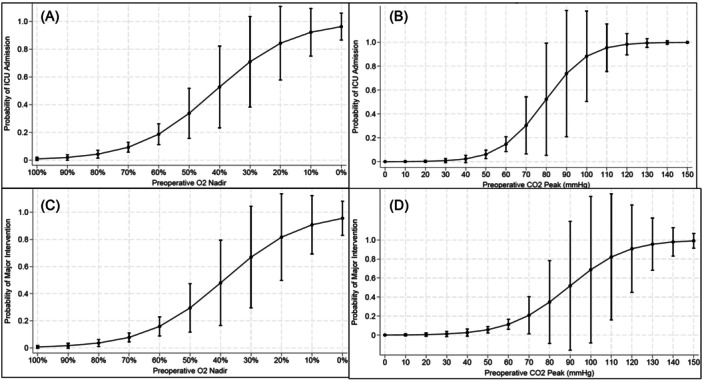
Logistical regression models for major interventions and ICU admission based on preoperative O2 nadir and peak CO2 with 95% confidence intervals. (A) Logistical regression model for probability of ICU admission based on preoperative O2 nadir. (B) Logistical regression model for probability of ICU admission based on preoperative peak CO2. (C) Logistical regression model for probability of major intervention based on preoperative O2 nadir. (D) Logistical regression model for probability of major intervention based preoperative peak CO2. ICU, intensive care unit.
Discussion
In this single‐institution study of patients with high‐risk OSA, our goal was to identify specific patient characteristics and PSG parameters that are associated with higher risks of postoperative complications. The impetus for this study was based on the published AAO‐HNS guidelines that identified gaps in the current literature. The guidelines recommended identification of PSG findings that predispose to respiratory complications and determine if the risk of complications could be stratified by disease severity. We found that higher AHI, O2 parameters, and CO2 parameters had the highest association with postoperative complications. Children who were obese or had neuromuscular or cardiac disease were significantly more likely to require major intervention. Younger children were more likely to require postoperative ICU admission and had longer LOS.
Reported rates of respiratory complication following AT have a wide range due to various definitions of complications. 8 In our study, we found that 8.1% of our patients required major intervention. This rate is higher than that of most studies, reflecting the increased severity of OSA in our population. Saur and Brietzke performed a systematic review that showed only a 5.8% rate of major respiratory interventions. 5 This study included all patients regardless of OSA severity. Using the KIDS database, Kou et al reported a 3.6% rate of major interventions. 9 This study included all children admitted after AT so the lower rate likely reflects the inclusion of patients with less severe disease. Kirkham et al found a rate of serious respiratory events (which included use of PAP or intubation) of 1.7% in their study of all patients who underwent AT for tonsillectomy and adenoidectomy. 8
In our population, 9.7% of the patients required postoperative ICU admission. This is higher than other studies, which again reflects the increased severity of OSA in our population. Lim et al looked at 887 children with severe OSA and reported a 3.2% rate of ICU admission. 4 We found that ICU admission was associated with neuromuscular disease, higher AHI, and worse O2 and CO2 parameters. Than et al studied 278 children with severe OSA and also found that peak CO2 > 60 mm Hg and neuromuscular disease were significantly associated with ICU admission. 10 Vandjelovic et al studied 94 patients who were admitted to the ICU after AT. They found that children with neuromuscular disease, who were on PAP at home, and had O2 nadir 80% were significantly related with requiring ICU. 11 These findings are similar to those found in our study. In addition, they found that 31% of the ICU admissions were unplanned, which is very similar to our rate of 34.5%.
Identification of clinical and PSG risk factors for postoperative respiratory complications has been identified as a research need by the AAO‐HNS. Our study identified neuromuscular disease and worsened O2 and CO2 parameters on preoperative PSG as significant risk factors for postoperative interventions, prolonged admissions, and ICU admissions. We did not find race to be a risk factor for postoperative interventions in our cohort. Previous studies by Lim and colleagues had found African American race to be a risk factor for respiratory complications after AT. 4 , 9
Children with high‐risk OSA represent a subset of children with severe OSA that are at higher risk for postoperative respiratory interventions and ICU admissions. This is important for providers to recognize as these patients may require closer postoperative monitoring and a lower threshold for ICU admission. Figure 1 provides a visual guide for providers to see the predicted probability of patients requiring major intervention or ICU admission based on their preoperative O2 nadir and peak CO2. The confidence intervals get wider as the O2 nadir decreases and the peak CO2 increases due to low sample sizes in those ranges.
There are limitations to this study. Due to the retrospective nature, there are inherent limitations with biases and potential errors in the medical record. There are multiple factors that affect the generalizability of this study. Primarily, this was a single‐center experience, so the demographics and racial distributions may not reflect that of other centers. There are also differences in practice patterns. One example of variability in practice patterns nationally and within our own institution is the use of preoperative PSGs. Many providers do not routinely order PSGs for patients due to obesity. However, the most recently published Clinical Practice Guideline for Tonsillectomy does recommend a PSG for patients with obesity so we felt this was an important factor to account for.
Variability in practices and subsequent small sample size of studies has continued to leave knowledge gaps in the postoperative care and determinants of success when treating children with high‐risk or profound OSA. To our knowledge, this is one of the largest studies of children with high‐risk or profound OSA. However, the results should be interpreted with caution. Overall, respiratory complications and ICU admissions are still relatively rare so our sample size may be insufficient to fully examine these patients. This study provides valuable information that will help with patient risk stratification and counseling for caregivers. Identifying patients who are at higher risk of postoperative complications can help anticipate and decrease these complications and their sequelae.
Future studies should include multiple centers and further efforts should be taken to explore risk factors for postoperative complications so this population can be closely monitored. There are multiple terms used for this select group of patients within the “severe OSA” definition. Some of these terms include: profound, very severe, and high‐risk OSA. Future studies and guidelines should aim to establish a set of diagnostic criteria and nomenclature for these patients. In addition, we believe that it is important for future studies to further study the effects of hypoxemia and hypercapnia on patient outcomes.
Conclusion
Increased oAHI and worsening O2 and CO2 parameters on preoperative PSG were associated with postoperative respiratory complications in children with high‐risk OSA. This population of high‐risk OSA has higher rates of respiratory complications when compared to other children with severe OSA. Children with neuromuscular disease and age 0 to 2 had higher risk of ICU stay and prolonged hospitalization. Clinicians should recognize the importance of parameters beyond oAHI when anticipating postoperative monitoring.
Author Contributions
Yann‐Fuu Kou, design, data collection, analysis, manuscript preparation, editing; Jonathan R. Korpon, design, data collection, analysis, manuscript preparation, presentation, editing; Helene Dabbous, design, data collection, analysis, manuscript preparation, editing; Romaine F. Johnson, design, data collection, analysis, manuscript preparation, editing; Ron B. Mitchell, design, data collection, analysis, manuscript preparation, editing; Anna Wani, design, data collection, analysis, manuscript preparation, editing; Stephen R. Chorney, design, data collection, analysis, manuscript preparation, editing.
Disclosures
Competing interests
None.
Funding source
None.
This article was presented at the AAO‐HNSF 2023 Annual Meeting & OTO Experience; September 30 to October 4, 2023; Nashville, Tennessee.
References
- 1. Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576.e84. [DOI] [PubMed] [Google Scholar]
- 2. Bhattacharjee R, Kheirandish‐Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182:676‐683. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell RB, Archer SM, Ishman SL, et al. Clinical Practice Guideline: Tonsillectomy in Children (Update). Otolaryngol Head Neck Surg. 2019;160(2):187‐205. [DOI] [PubMed] [Google Scholar]
- 4. Lim J, Garigipati P, Liu K, Johnson RF, Liu C. Risk factors for post‐tonsillectomy respiratory events in children with severe obstructive sleep apnea. Laryngoscope. 2023;133(5):1251‐1256. [DOI] [PubMed] [Google Scholar]
- 5. Saur JS, Brietzke SE. Polysomnography results versus clinical factors to predict post‐operative respiratory complications following pediatric adenotonsillectomy. Int J Pediatr Otorhinolaryngol. 2017;98:136‐142. [DOI] [PubMed] [Google Scholar]
- 6. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495‐1499. [DOI] [PubMed] [Google Scholar]
- 8. Kirkham EM, Puglia MP, Haydar B, et al. Preoperative predictors of severe respiratory events after tonsillectomy: consideration for pediatric intensive care admission. Otolaryngol Head Neck Surg. 2023;168(6):1535‐1544. [DOI] [PubMed] [Google Scholar]
- 9. Kou YF, Sakai M, Shah GB, Mitchell RB, Johnson RF. Postoperative respiratory complications and racial disparities following inpatient pediatric tonsillectomy: a cross‐sectional study. Laryngoscope. 2019;129(4):995‐1000. [DOI] [PubMed] [Google Scholar]
- 10. Than K, Mun‐Price C, Klein MJ, Ross PA, Gomez G, Nagoshi M. PICU admission and complications following adenotonsillectomies in pediatric patients: a retrospective cohort study. Int J Pediatr Otorhinolaryngol. 2022;158:111166. 10.1016/j.ijporl.2022.111166 [DOI] [PubMed] [Google Scholar]
- 11. Vandjelovic ND, Briddell JW, Crippen MM, Schmidt RJ. Evaluating pediatric intensive care unit utilization after tonsillectomy. Int J Pediatr Otorhinolaryngol. 2020;128:109693. 10.1016/j.ijporl.2019.109693 [DOI] [PubMed] [Google Scholar]


