Abstract
Objective
Mandibular plate reconstruction (MPR) is often indicated after tumor ablation, osteoradionecrosis excision, and traumatic bone loss to restore oral functionality and facial cosmetics. There are limited analyses identifying risk factors that lead to plate infection (PIn), exposure, and removal (“plate complications”).
Study Design
Retrospective cohort study.
Setting
Academic tertiary medical center.
Methods
Patients who underwent MPR from 2013 to 2022 were identified. Risk factors for plate complications were analyzed based on demographic, clinical, intraoperative, and postoperative factors. Multivariable analysis was conducted with logistic regression. Survival analysis was conducted with a Cox model.
Results
Of the 188 patients analyzed, 48 (25.5%) had a plate complication [infection: 22 (11.7%); exposure: 23 (12.2%); removal: 35 (18.6%)]. Multivariate analysis revealed predictive associations between at least 1 plate complication and the following variables: smoking status, soft tissue defect size, number of plates, average screw length, and various postoperative complications. Other associations approached the threshold for significance. Prior and adjuvant radiation therapy, type of free flap, stock versus custom plates, and perioperative antibiotic prophylaxis regimens were not associated with plate complications. No plate complication was independently associated with lower overall survival. PIn (hazard ratio, HR: 7.99, confidence interval, CI [4.11, 15.54]) and exposure (HR: 3.56, CI [1.79, 7.08]) were independently associated with higher rates of plate removal.
Conclusion
Plate complications are relatively common after MPR. Smoking history, specific disease characteristics, hardware used during surgery, and postoperative complications may help identify higher‐risk patients, but additional larger‐scale studies are needed to validate our findings and resolve discrepancies in the current literature.
Keywords: mandibular reconstruction, microvascular reconstruction, plate complications, plate exposure, plate infection, plate removal
Mandibular plate reconstruction (MPR) is frequently indicated after tumor ablation, osteoradionecrosis (ORN), and traumatic bone loss to reestablish the continuity of the mandible and preserve oral intake, a patent airway, and articulate speech. 1 To reduce the number of revisions and improve outcomes, modern MPR approaches are often performed immediately following extirpative surgery. The gold standard donor flap is the fibula free flap (FFF) given its appropriate soft tissue, bone stock, and low morbidity. 2 Osteocutaneous radial forearm (RFFF) and scapular tip free flaps are additional options for bony reconstruction. 3 , 4 Anterolateral thigh, soft‐tissue‐only RFFF, and the latissimus dorsi free flaps are alternatives if bony reconstruction is deferred. 5 These reconstructions are often stabilized by stock or patient‐specific titanium plates, given the strong torsional and bending forces the mandible must withstand. 6 , 7 Properly approximating plates with the defect is critical to avoid altering facial structure and impairing functionality. 8 Thus, surgeons must balance esthetics and performance with medical treatment of the defect. 9 , 10
While some studies claim success rates for MPR up to 86% to 100%, significant perioperative and postoperative morbidities can occur. 11 , 12 , 13 , 14 , 15 , 16 , 17 Major problems include plate infection (PIn), plate exposure (PEx), and pain, all of which can reduce quality of life and can necessitate plate removal (PRem) or revision surgeries. PIn and PEx specifically are among the most common indications for PRem. 18 , 19 Despite these known adverse outcomes, there has been limited research regarding their incidence and risk factors. Two meta‐analyses investigating MPR secondary to ORN calculated the rates of postoperative complications such as free flap failure, fistula formation, PEx, and flap infection. 14 , 15 A recent retrospective cohort study of 91 patients found that preoperative radiotherapy and secondary mandibular reconstruction were predictors of hardware failure. 20 Another study of 28 oromandibular cancer cases determined that adjuvant radiation therapy was correlated with PIn and PEx. 21 Nevertheless, many demographic, clinical, intraoperative, and postoperative variables remain to be analyzed.
Hence, our study aims to elucidate the rates of PIn, PEx, and PRem (which henceforth may collectively be referred to as “plate complications”) in patients who have undergone MPR, identify associated risk factors, and investigate their effect on survival. Existing literature is limited in this regard, especially within the same large and diverse patient cohort. 14 , 22 , 23 , 24 , 25 , 26 Our study seeks to address this gap and add to the growing research exploring the efficacy and outcomes of modern MPR.
Methods and Materials
Study Design, Setting, and Sample
This was a retrospective cohort study involving patients who underwent MPR with transosteal bone plate implantation at a single tertiary academic institution from May 2013 to May 2022. Clinical notes and operative documents from the medical records of included patients were reviewed, and data was managed with Research Electronic Data Capture software. 27 Inclusion criteria were age ≥ 18 years, single‐stage MPR with vascularized free flap, and follow‐up of ≥12 months. Patients were excluded if any other part of their head or neck was simultaneously reconstructed or if reconstruction occurred without transosteal bone plating. This study was approved by the University of Pennsylvania Institutional Review Board (project number 826710) and adheres to the “Strengthening the Reporting of Observational Studies in Epidemiology” framework. 28
Study Outcomes
The primary outcomes of interest were the rates of and risk factors associated with PRem (explantation of transosteal bone plate(s) any time after MPR), PIn (surgical site or hardware infection more than 30 days after MPR), and PEx (visible extrusion of transosteal bone plate(s) any time after MPR). PIn and PEx were clinical diagnoses based on the presence of erythema, swelling, and/or drainage and visible exposure at the surgical site, respectively. Secondary outcomes of interest included 5‐year survival rates and median time to plate complications.
Study Variables
Study variables included demographic information, medical history, diagnostic workup, surgical management, and adjuvant therapy. Patient demographics included age at MPR, sex, race, body mass index, and smoking status. Medical history included American Society of Anesthesiologist physical status, immunosuppression, Charlson Comorbidity Index (CCI) score and its component comorbidities, hypothyroidism, and nutrition status. Diagnostic workup included prior history of radiation to or cancer of the head and neck; indication for MPR; histology, primary site, and T stage of malignancy; and histology of benign tumor. Surgical management included length of inpatient stay, type of free flap, size of soft tissue defect, number of osteotomies, number and average size of plates and screws, type of plate, and various postoperative complications, including surgical site infection (wound infection within thirty days of reconstruction), wound dehiscence, hardware complications (loose screws or plate fracture), free flap compromise, and thrombotic events. Composition and duration of perioperative antibiotic prophylaxis, along with history of penicillin allergy, were also documented. Finally, survival status was recorded, as were instances and dates of plate complications.
Statistical Methods
Wilcoxon rank‐sum test, Pearson's χ 2 test, and Fisher's exact test were used to conduct univariate analyses. Significance was set at P ≤ .05, P values were 2‐sided, and 95% confidence intervals (CIs) were constructed where appropriate. Significant variables in the univariate analysis that were nonconfounding, as determined by variance inflation factor (VIF) analysis, were incorporated into a multivariable logistic regression model to determine predictive associations. Unadjusted Kaplan‐Meier estimates were used for univariate comparison of overall survival and plate complications at 5 years, and a Cox proportional hazard model was used for multivariate analysis of these outcomes. Statistical analyses were performed using R version 4.3.1 (R Project for Statistical Computing) via RStudio version 2023.06.0 (RStudio Inc).
Results
Demographic and Clinical Characteristics
The final study population consisted of 188 patients. Of these individuals, 48 (25.5%) had a plate complication; 22 (11.7%) had PIn, 23 (12.2%) had PEx, and 35 (18.6%) had PRem. The mean age of patients in the sample was 61.0 years old (SD: 14.8 years). Most patients were male (66.5%), white (80.9%), and current or former smokers (61.2%). The median CCI was 5.0, and many patients had prior radiation to (34.0%) or cancer of (39.9%) the head and neck. The most common indication for reconstruction was malignancy (67.6%). Additional information about the patient cohort can be found in Supplemental Table S1, available online.
Intraoperative and Postoperative Characteristics
The typical reconstruction utilized a FFF (71.3%) with a single stock plate that was ≥1.5 mm thick. Almost half (47.9%) of reconstructions were associated with surgical complications, with thrombotic events (18.1%), surgical site infection (16.0%), and wound dehiscence (14.9%) the most common. A minority of patients received adjuvant radiation (22.3%) or chemoradiation (13.3%). Additional characteristics can be found in Supplemental Table S2, available online.
Most (80.1%) patients received a prophylactic perioperative antibiotic regimen of ampicillin‐sulbactam, with a median duration of 3 days. Few (12.3%) patients had a penicillin allergy. Additional data regarding antibiotic regimens can be found in Tables 1 and 2.
Table 1.
Characteristics of Perioperative Antibiotic Prophylaxis
| Any plate complication | Plate removal | Plate infection | Plate exposure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Overall, N = 146a | No, N = 110a | Yes, N = 36a | P valueb | No, N = 121a | Yes, N = 25a | P valueb | No, N = 131a | Yes, N = 15a | P valueb | No, N = 129a | Yes, N = 17a | P valueb |
| Antibiotic regimen | .55 | .78 | .34 | .23 | |||||||||
| Ampicillin‐sulbactam | 117 (80.1) | 90 (81.8) | 27 (75.0) | 97 (80.2) | 19 (76.0) | 105 (80.2) | 12 (80.0) | 105 (81.4) | 12 (70.6) | ||||
| Cephalosporin+metronidazole | 17 (11.6) | 11 (10.0) | 6 (16.7) | 14 (11.6) | 4 (16.0) | 14 (10.7) | 3 (20.0) | 13 (10.1) | 4 (23.5) | ||||
| Other | 12 (8.2) | 9 (8.2) | 3 (8.3) | 10 (8.3) | 2 (8.0) | 12 (9.2) | 0 (0) | 11 (8.5) | 1 (5.9) | ||||
| Average duration, d | 4.6 (2.5) | 4.6 (2.6) | 4.3 (2.0) | .66 | 4.6 (2.5) | 4.3 (2.1) | .68 | 4.7 (2.5) | 3.6 (1.3) | .17 | 4.6 (2.5) | 4.2 (2.1) | .55 |
| Median (IQR) | 3 (3, 7) | 3 (3, 7) | 3 (3, 5.5) | 3 (3, 7) | 3 (3, 6) | 3 (3, 7) | 3 (3, 3) | 3 (3, 7) | 3 (3, 5) | ||||
| Mode | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||||
| PCN allergy | .39 | .52 | .69 | .44 | |||||||||
| No | 128 (87.7) | 98 (89.1) | 30 (83.3) | 106 (87.6) | 21 (84.0) | 114 (87.0) | 14 (93.3) | 114 (88.4) | 14 (82.4) | ||||
| Yes | 18 (12.3) | 12 (10.9) | 6 (16.7) | 15 (12.4) | 4 (16.0) | 17 (13.0) | 1 (6.7) | 15 (11.6) | 3 (17.6) | ||||
Abbreviations: IQR, interquartile range; PCN, penicillin.
Mean (SD); n (%).
Wilcoxon rank sum test; Pearson's χ 2 test; Fisher's exact test.
Table 2.
Additional Information Regarding Perioperative Prophylactic Antibiotic Regimens, Broken Down by Specific Antibiotics Used, Frequency, and Median and Mean Duration of Regimen
| Antibiotic regimen | Specific antibiotics used | Count (%) | Median (IQR) length | Mean (SD) length |
|---|---|---|---|---|
| Ampicillin‐sulbactam | Ampicillin‐sulbactam 3 g every 6 h | 91 (62.3) | 3 (3, 6) | 4.41 (2.33) |
| Ampicillin‐sulbactam 1.5 g every 6 h | 26 (17.8) | 5.5 (3, 7) | 5.77 (3.10) | |
| Cephalosporin with metronidazole | Ceftriaxone 1 g daily with metronidazole 500 mg every 12 h | 12 (8.2) | 3 (3, 3) | 3.33 (1.11) |
| Cefazolin 1 g every 8 h with metronidazole 500 mg every 12 h | 5 (3.4) | 3 (3, 6) | 4.20 (1.60) | |
| Other | Levofloxacin 750 mg daily with metronidazole 500 mg every 12 h | 6 (4.1) | 3 (3, 4) | 4.00 (1.83) |
| Clindamycin 600 mg every 8 h | 3 (2.1) | 7 (‐) | 6.33 (0.94) | |
| Individualized antibiotic regimens, per Infectious Disease | 3 (2.1) | 3 (‐) | 3.67 (1.70) |
Abbreviation: IQR, interquartile range.
The 5‐year overall survival rate for the entire cohort was 75.5% (CI [66.9, 82.2]). Median time to PRem, PIn, and PEx was 0.84 years (interquartile range, IQR [0.44, 1.21]), 0.46 years (IQR [0.32, 1.69]), and 0.58 years (IQR [0.14, 1.50]), respectively.
Predictive Factors of Plate Complications
After univariate analysis, smoking status (P = .021), primary site of malignancy (P = .008), soft tissue defect size (P = .035), plate thickness (P = .016), number of screws (P = .032), average screw length (P = .025), surgical site infection (p = 0.047), wound dehiscence (P = .001), hardware complications (P = .033), and free flap compromise (P = .023) were significantly associated with plate complications. Stratifying by type of plate complication revealed other associations. Primary site of malignancy (P = .005), soft tissue defect size (P = .008), number of plates (P = .032), average screw length (P < .001), surgical site infection (P = .006), wound dehiscence (P = .046), and hardware complications (P = .034) were associated with PRem. PIn was significantly associated with diabetes mellitus (P = .042), primary site of malignancy (P = .017), soft tissue defect size (P = .011), plate thickness (P = .025), wound dehiscence (P= .007), and free flap compromise (P = .050). Smoking status (P = .023), history of myocardial infarction (P = .027) or rheumatologic disease (P = .028), primary site of malignancy (P = .003), number (P = .027) and thickness (P = .044), and wound dehiscence (P = .009) were significantly associated with PEx. PIn (P < .001) and PEx (P < .001) were significantly associated with PRem. Of note, hypothyroidism, nutrition status, prior or adjuvant radiation, number of osteotomies, type of plate, duration and composition of perioperative antibiotic prophylaxis, and penicillin allergy were not associated with any plate complications. Other associations approached significance; a summary of univariate analyses conducted can be found in Supplemental Tables S1 and S2.
Multivariable regression models were then created using these covariates to identify factors independently associated with plate complications. Patients with a smoking history (odds ratio, OR: 2.70 [1.18, 6.67]), soft tissue defect ≤ 45 cm2 (OR: 2.70 [1.23, 6.25]), wound dehiscence (OR: 2.68 [1.00, 7.13]), and hardware complications (OR: 5.40 [1.37, 22.1] were more likely to have plate complications. Individuals with soft tissue defect ≤ 45 cm2 (OR: 3.70 [1.54, 9.09]), average screw length < 9 mm (OR: 3.57 [1.30, 10.0]), surgical site infection (OR: 4.05 [1.50, 11.0]), wound dehiscence (OR: 3.53 [1.25, 9.83]), and hardware complications (OR: 4.36 [1.00, 18.8]) were more likely to have PRem. Patients with soft tissue defect ≤ 45 cm2 (OR: 4.76 [1.54, 16.7]) and wound dehiscence (OR: 3.99 [1.19, 12.9]) were more likely to have PIn. Those with prior MI (OR: 3.72 [1.06, 12.2]) and >2 plates (OR: 3.87 [1.09, 12.9]) were more likely to have PEx. Other ORs approached the threshold for significance; a summary of multivariate analyses can be found in Table 3.
Table 3.
Results of Multivariable Logistic Regression
| Any plate complication | Plate removal | Plate infection | Plate exposure | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | OR [95% CI] | P value | OR [95% CI] | P value | OR [95% CI] | P value | OR [95% CI] | P value |
| Smoking Status | ||||||||
| Never‐smoker | 1 [Reference] | ‐ | ‐ | ‐ | ‐ | ‐ | 1 [Reference] | ‐ |
| Ever‐smoker | 2.70 [1.18, 6.67] | .023 | ‐ | ‐ | ‐ | ‐ | 2.63 [0.87, 10.0] | .11 |
| History of MI | ||||||||
| No | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 1 [Reference] | ‐ |
| Yes | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 3.72 [1.06, 12.2] | .032 |
| History of rheumatic disorder | ||||||||
| No | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 1 [Reference] | ‐ |
| Yes | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 2.28 [0.80, 6.25] | .11 |
| History of DM | ||||||||
| No | ‐ | ‐ | ‐ | ‐ | 1 [Reference] | ‐ | ‐ | ‐ |
| Yes | ‐ | ‐ | ‐ | ‐ | 2.84 [0.83, 9.85] | .081 | ‐ | ‐ |
| Soft tissue defect size, cm2 | ||||||||
| <45 | 1 [Reference] | ‐ | 1 [Reference] | ‐ | 1 [Reference] | ‐ | ‐ | ‐ |
| ≥45 | 0.37 [0.16, 0.81] | .015 | 0.27 [0.11, 0.65] | .004 | 0.21 [0.06, 0.65] | .011 | ‐ | ‐ |
| Number of plates | ||||||||
| ≤2 | 1 [Reference] | ‐ | ‐ | ‐ | 1 [Reference] | ‐ | ||
| >2 | 1.08 [0.29, 3.71] | >.99 | ‐ | ‐ | 3.87 [1.09, 12.9] | .029 | ||
| Plate thickness, mm | ||||||||
| <1.5 | 1 [Reference] | ‐ | ‐ | ‐ | 1 [Reference] | ‐ | 1 [Reference] | ‐ |
| ≥1.5 | 0.49 [0.23, 1.06] | .072 | ‐ | ‐ | 0.41 [0.15, 1.09] | .078 | 0.48 [0.18, 1.25] | .14 |
| Number of screws | ||||||||
| ≤12 | 1 [Reference] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| >12 | 1.86 [0.85, 4.13] | .12 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Average screw length, mm | ||||||||
| <9 | 1 [Reference] | ‐ | 1 [Reference] | ‐ | ‐ | ‐ | ‐ | ‐ |
| ≥9 | 0.043 [0.18, 1.08] | .068 | 0.28 [0.10, 0.77] | .013 | ‐ | ‐ | ‐ | ‐ |
| Surgical site infection | ||||||||
| No | 1 [Reference] | ‐ | 1 [Reference] | ‐ | ‐ | ‐ | ‐ | ‐ |
| Yes | 1.95 [0.70, 5.29] | .20 | 4.05 [1.50, 11.0] | .005 | ‐ | ‐ | ‐ | ‐ |
| Wound dehiscence | ||||||||
| No | 1 [Reference] | ‐ | 1 [Reference] | ‐ | 1 [Reference] | ‐ | 1 [Reference] | ‐ |
| Yes | 2.68 [1.00, 7.13] | .048 | 3.53 [1.25, 9.83] | .015 | 3.99 [1.19, 12.9] | .021 | 2.12 [0.69, 6.15] | History of DM.20 |
| Hardware complications | ||||||||
| No | 1 [Reference] | ‐ | 1 [Reference] | ‐ | ‐ | ‐ | ‐ | ‐ |
| Yes | 5.40 [1.37, 22.1] | .015 | 4.36 [1.00, 18.8] | .046 | ‐ | ‐ | ‐ | ‐ |
| Free flap compromise | ||||||||
| No | 1 [Reference] | ‐ | ‐ | 1 [Reference] | ‐ | ‐ | ‐ | |
| Yes | 4.29 [0.93, 19.8] | .059 | ‐ | 4.01 [0.59, 25.1] | .14 | ‐ | ‐ | |
Statistically significant differences are indicated in bold.
Only factors that were incorporated into each model are displayed; all other significant univariate variables were excluded given concerns for confounding or overfitting.
Abbreviations: CI, confidence interval; DM, diabetes mellitus; MI, myocardial infarction; OR, odds ratio.
Rates of Overall Survival and PRem
On univariate Kaplan‐Meier analysis, 5‐year overall survival was not significantly different between patients with and without PRem (87.3% [69.0, 95.1] vs 72.5% [62.3, 80.4]; P = .14), PIn (95.5% [71.9, 99.4] vs 72.3% [63.2, 80.2]; P = 0.058), PEx (64.4% [31.5, 84.5] vs 77.1% [68.1, 83.8]; P = .53), and plate complications (79.5% [70.5, 89.8] vs 74.1% [63.7, 81.9]; P = .41) (Figure 1). These associations remained insignificant for PRem (hazard ratio, HR: 0.46 [0.16, 1.31]), PIn (HR: 0.18 [0.02, 1.33]), PEx (HR: 1.33 [0.55, 3.18]), and plate complications (HR: 0.72 [0.33, 1.57]) when controlling for significant demographic, clinical, and operative traits.
Figure 1.
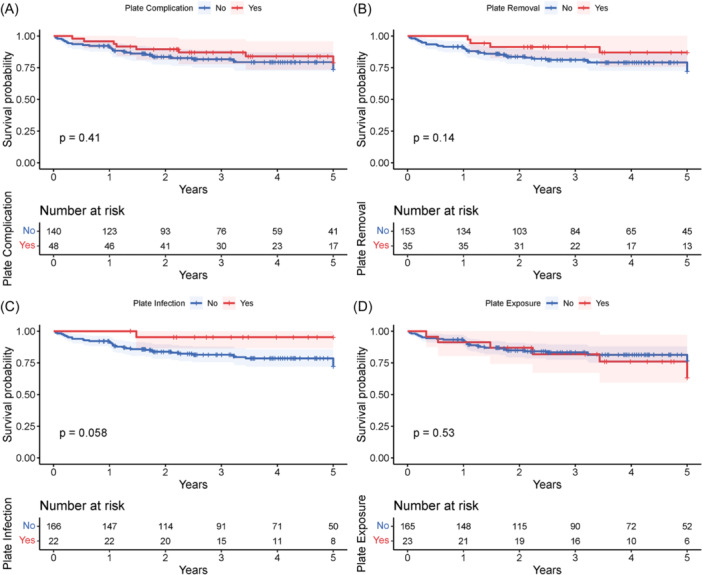
Unadjusted Kaplan‐Meier curve comparing 5‐year overall survival between patients who did and did not have (A) plate complications in general, (B) PRem, (C) PIn, and (D) PEx. No plate complication was associated with decreased survival. PEx, plate exposure; PIn, plate infection; PRem, plate removal.
Rates of PRem were significantly different among patients with and without PIn (72.9% [46.1, 93.6] vs 16.2% [10.6, 24.3]; P < .001) and PEx (84.4% [63.5, 96.8] vs 13.5% [8.5, 20.9]; P < .001) (Figure 2). PIn (HR: 7.99 [4.11, 15.54]) and PEx (HR: 3.56 [1.79, 7.08]) were associated with higher rates of PRem on multivariable Cox models, too.
Figure 2.
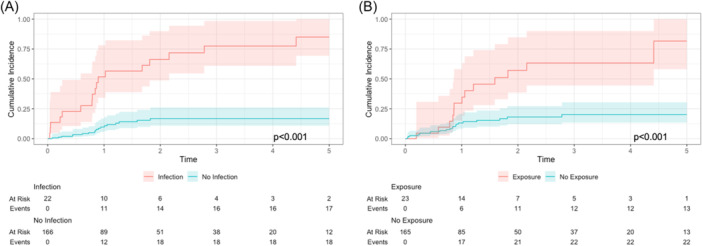
Unadjusted Kaplan‐Meier curve comparing 5‐year incidence rates of PRem between patients who did and did not have (A) PIn and (B) PEx. Rates of PRem were significantly different in both cases. PEx, plate exposure; PIn, plate infection; PRem, plate removal.
Discussion
MPR is performed in many contexts to achieve functional and esthetic recovery and improve postoperative quality of life, but adverse outcomes do occur. Risk factors leading to such outcomes are not entirely understood as previous studies analyzed plate complications in isolation or with smaller cohorts. In this single‐institution sample of 188 patients, plate complications were relatively common (25.5%); 35 (18.6%) patients had PRem, 22 (11.7%) had PIn, and 23 (12.2%) had PEx, correlating with reported values. 19 , 29 , 30 , 31 Smoking status, soft tissue defect size, number and thickness of plates, number and average length of screws, surgical site infection, wound dehiscence, and hardware complications were associated with at least 1 plate complication on multivariate analysis, with several other variables approaching significance (Table 3). No plate complication was associated with decreased overall survival (Figure 1).
Some risk factors we identified are original contributions to the literature. For instance, shorter screws were associated with increased rates of PRem and approached significance for plate complications. It is presumed that shorter screws allow for less secure attachment to the reconstructed mandible; patients requiring shorter screws may also have small and/or atrophic mandibles, making them inherently more prone to complications. Soft tissue defects ≥ 45 cm2 were correlated with decreased rates of plate complications, PRem, and PIn, perhaps because scar tissue formed by larger defects may protect hardware and anatomic structures from external factors while better stabilizing implanted plates. We do not advocate deliberately enlarging the surgical site in all patients, but further study is merited to see if this would be beneficial in specific scenarios, given its consistency across multiple plate complications. Implanting more than 2 plates was associated with greater rates of PEx, although this may be a function of probability. Finally, various surgical complications (surgical site infection, wound dehiscence, hardware complications, and free flap compromise) were associated with or approached significance for multiple plate complications, likely due to interruptions in the normal wound healing process. Other covariates—tumor histology, type of free flap, plate thickness, and number of screws—also approached significance for multiple plate complications on univariate and multivariate analysis, thus warranting further analysis in larger studies.
Other risk factors match those already recognized in the literature. For instance, PRem, PIn, and PEx have been linked to smoking history, site of defect, and surgical site infection. 26 , 32 , 33 , 34 , 35 Of note, primary tumor site was not included in our multivariate analysis due to its distribution and possible confounding per VIF analysis. One study showed that PEx was associated with free flap compromise, while another connected PIn with diabetes mellitus. 30 , 36 These variables approached significance on our univariate and multivariate analysis, respectively. Finally, PIn and PEx were the 2 variables most strongly associated with PRem, validating our statistical analyses and findings. 18 , 19
Additional covariates were insignificant on univariate analysis. Stock plates performed similarly to patient‐specific plates, although the latter approached significance in decreasing rates of PEx. This suggests that without specific indications, such as location of injury or previous hardware failure, stock plates are a cost‐effective implant for successful MPR. The reduction of mean plate‐to‐bone gap by custom plates may explain their apparent effect on PEx. 37 Perioperative antibiotic regimen also was not significantly associated with plate complications (Table 1). Our institution has a standard regimen of ampicillin‐sulbactam for all bony reconstruction patients, even for those with ORN, with levofloxacin/metronidazole as an alternative for penicillin‐allergic patients (Table 2). This protocol is only altered in consultation with our Infectious Disease colleagues based on drug allergies, underlying immunocompromise, and concern for infection at the time of surgery. Our outcomes provide evidence that if coverage is adequate, different prophylactic regimens and penicillin‐allergic patients are not associated with increased plate complications. Of note, all patients between July 2019 and February 2020 had a perioperative regimen of cephalosporin/metronidazole due to an institutional antibiotic shortage, explaining why it is the second‐most common regimen documented. Finally, aside from a history of myocardial infarction, rheumatologic disease, or diabetes mellitus, no comorbidity approached significance. This suggests that pre‐existing conditions should largely not be considered contraindications to MPR. Additionally, it implies that aside from specific aspects of medical history, hardware used intraoperatively and postoperative complications—and perhaps radiation therapy, as discussed by other papers—are primarily why plate complications occur.
Multiple risk factors previously associated with plate complications were insignificant in our analysis. The most prominent were preoperative and adjuvant radiation therapy. Although one study also found that radiation therapy did not affect the rate of PEx, 35 others associated it with increased incidence of all plate complications. 19 , 38 , 39 , 40 As plate complications are multifactorial, our findings may reflect the size and diversity of our cohort, which could have introduced other factors that mitigated the effect of radiation; improved surgical techniques over the course of the study period; or our institution's protocols to identify and monitor patients undergoing radiation, thereby optimizing the population while intervening at the earliest signs of complications. The discrepancy between the rates of patients with advanced‐stage malignancy (73.6%) and adjuvant therapy (35.6%) may stem from patient concerns about quality of life, physician refusal given patient health status or comorbidities, prior radiation to the same area or ineffective chemotherapy, or advanced disease progression. One study positively correlated osteotomies with PEx, 30 but it only examined PEx that occurred within a year of MPR. Others associated certain free flaps with PRem, but our analysis did not show any increased plate complications with osteocutaneous versus soft tissue reconstruction. 19 , 41
Examined together, the results of this investigation suggest some guidelines for MPR that may lead to decreased morbidity. First, minimizing the number of plates and screws while ensuring adequate thickness and length, respectively, seems to be beneficial. Shorter monocortical screws have historically been used to secure the osteocutaneous flap to prevent the theoretical disruption of the periosteal blood supply on the nonplating surface, but the success of longer bicortical screws suggests that this fear may be unfounded. 42 , 43 , 44 Taking precautions to reduce the rate of surgical complications—adequate antibiotics to curtail surgical site infections, properly suturing fascia and overlying skin to prevent wound dehiscence, and ensuring viable anastomoses to lessen rates of free flap compromise—may also improve patient outcomes. Unless custom plates are specifically indicated, stock plates appear to be as efficacious and more cost‐effective. Finally, our data clearly supports PIn and PEx generally being indications for PRem. However, not all patients with PIn and PEx had PRem and vice versa. More investigation must be done to demarcate when PRem, PIn, and/or PEx occur without the other(s), but the existence of these cases emphasize that these plate complications can be considered independent phenomena to some extent and that PRem should ultimately be a collaborative discussion between the patient and physician based on chronic pain, functional limitations, esthetic concerns, and medical stability for additional surgery.
Our study should be interpreted in the context of certain limitations. Given the relatively low frequency of plate complications and that 12.8% of our cohort was lost to follow‐up, it is possible that our multivariable models may be skewed due to overfitting. Moreover, we did not account for surgeon experience or variations in hardware when analyzing the data. Indeed, previous studies have shown conflicting results regarding the impact of the type and model of implants on plate complications. 45 , 46 , 47 , 48 Additionally, perioperative antibiotic data was not available for the 42 (22.3%) patients prior to November 2016, but study authors who operated during this time agree that our regimens were unchanged. Our study also did not account for the location of the mandibular defect in our analysis, which may be pertinent as anterior and lateral defects carry distinctive load‐bearing issues and reconstructive challenges. 49 Finally, as most of our patients had squamous cell carcinoma, our findings may not be as generalizable for ORN, benign tumors, facial trauma, and other indications of MPR.
Conclusion
PRem, PIn, and PEx are relatively common after MPR but are not associated with decreased overall survival. History of smoking, soft tissue defect size, characteristics of hardware used, and surgical complications may be used to identify patients at risk of a plate complication. Additional larger‐scale studies must be conducted to fully elucidate and validate our findings of the risk factors associated with PRem, PIn, and PEx and resolve discrepancies in the current literature.
Author Contributions
Keshav V. Shah, design, conduct, analysis, writing, editing, and presentation of research; Saawan D. Patel, analysis, writing, and editing; Karthik Rajasekaran, editing; Steven B. Cannady, editing; Ara A. Chalian, editing; Robert M. Brody, design and editing.
Disclosures
Competing interests
None.
Funding source
No internal or external funding was received for this research.
Supporting information
Supporting information.
Supporting information.
This article was presented at the AAO‐HNSF 2023 Annual Meeting & OTO Experience; September 30 to October 4, 2023; Nashville, Tennessee.
References
- 1. Kumar BP, Venkatesh V, Kumar KAJ, Yadav BY, Mohan SR. Mandibular reconstruction: overview. J Maxillofac Oral Surg. 2016;15(4):425‐441. 10.1007/s12663-015-0766-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84(1):71‐79. [PubMed] [Google Scholar]
- 3. Arganbright JM, Tsue TT, Girod DA, et al. Outcomes of the osteocutaneous radial forearm free flap for mandibular reconstruction. JAMA Otolaryngol Head Neck Surg. 2013;139(2):168‐172. 10.1001/jamaoto.2013.1615 [DOI] [PubMed] [Google Scholar]
- 4. Blumberg JM, Walker P, Johnson S, et al. Mandibular reconstruction with the scapula tip free flap. Head Neck. 2019;41(7):2353‐2358. 10.1002/hed.25702 [DOI] [PubMed] [Google Scholar]
- 5. Brown JS, Lowe D, Kanatas A, Schache A. Mandibular reconstruction with vascularised bone flaps: a systematic review over 25 years. Br J Oral Maxillofac Surg. 2017;55(2):113‐126. 10.1016/j.bjoms.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 6. Zeller AN, Neuhaus MT, Weissbach LVM, et al. Patient‐specific mandibular reconstruction plates increase accuracy and long‐term stability in immediate alloplastic reconstruction of segmental mandibular defects. J Maxillofac Oral Surg. 2020;19(4):609‐615. 10.1007/s12663-019-01323-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong RCWT H, Kin L, Merkx MAW. Biomechanics of mandibular reconstruction: a review. Int J Oral Maxillofac Surg. 2010;39(4):7. 10.1016/j.ijom.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 8. Klotch D. Application of reconstruction plates to the mandible. Oper Tech Otolaryngol Head Neck Surg. 1995;6(2):89‐96. 10.1016/S1043-1810(05)80017-X [DOI] [Google Scholar]
- 9. Haller JR, Sullivan MJ. Contemporary techniques of mandibular reconstruction. Am J Otolaryngol. 1995;16(1):19‐23. 10.1016/0196-0709(95)90004-7 [DOI] [PubMed] [Google Scholar]
- 10. Seol GJ, Jeon EG, Lee JS, et al. Reconstruction plates used in the surgery for mandibular discontinuity defect. J Korean Assoc Oral Maxillofac Surg. 2014;40(6):266‐271. 10.5125/jkaoms.2014.40.6.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ang E, Black C, Irish J, et al. Reconstructive options in the treatment of osteoradionecrosis of the craniomaxillofacial skeleton. Br J Plast Surg. 2003;56(2):92‐99. 10.1016/s0007-1226(03)00085-7 [DOI] [PubMed] [Google Scholar]
- 12. Buchbinder D, Hilaire St, H . The use of free tissue transfer in advanced osteoradionecrosis of the mandible. J Oral Maxillofac Surg. 2006;64(6):961‐964. 10.1016/j.joms.2006.02.013 [DOI] [PubMed] [Google Scholar]
- 13. Celik N, Wei F, Chen H, et al. Osteoradionecrosis of the mandible after oromandibular cancer surgery. Plast Reconstr Surg. 2002;109(6):1875‐1881. 10.1097/00006534-200205000-00014 [DOI] [PubMed] [Google Scholar]
- 14. Lee M, Chin RY, Eslick GD, Sritharan N, Paramaesvaran S. Outcomes of microvascular free flap reconstruction for mandibular osteoradionecrosis: a systematic review. J Craniomaxillofac Surg. 2015;43(10):2026‐2033. 10.1016/j.jcms.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 15. Markiewicz MR, Bell RB, Bui TG, et al. Survival of microvascular free flaps in mandibular reconstruction: a systematic review and meta‐analysis. Microsurgery. 2015;35(7):576‐587. 10.1002/micr.22471 [DOI] [PubMed] [Google Scholar]
- 16. Store G, Boysen M, Skjelbred P. Mandibular osteoradionecrosis: reconstructive surgery. Clin Otolaryngol Allied Sci. 2002;27(3):197‐203. 10.1046/j.1365-2273.2002.00564.x [DOI] [PubMed] [Google Scholar]
- 17. Werle AH, Tsue TT, Toby EB, Girod DA. Osteocutaneous radial forearm free flap: its use without significant donor site morbidity. Otolaryngol Head Neck Surg. 2000;123(6):711‐717. 10.1067/mhn.2000.110865 [DOI] [PubMed] [Google Scholar]
- 18. Kreutzer K, Steffen C, Nahles S, et al. Removal of patient‐specific reconstruction plates after mandible reconstruction with a fibula free flap: is the plate the problem. Int J Oral Maxillofac Surg. 2022;51(2):182‐190. 10.1016/j.ijom.2021.04.003 [DOI] [PubMed] [Google Scholar]
- 19. Wood CB, Shinn JR, Amin SN, Rohde SL, Sinard RJ. Risk of plate removal in free flap reconstruction of the mandible. Oral Oncol. 2018;83:91‐95. 10.1016/j.oraloncology.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 20. de Vicente JC, Rodríguez‐Santamarta T, de Villalaín L, Ruiz‐Ranz M, Rodríguez‐Torres N, Cobo JL. Risk factors associated with fixation‐related complications in microsurgical free flap reconstruction of the mandible. Microsurgery. 2023;43(1):27‐38. 10.1002/micr.30888 [DOI] [PubMed] [Google Scholar]
- 21. Sakakibara A, Hashikawa K, Yokoo S, Sakakibara S, Komori T, Tahara S. Risk factors and surgical refinements of postresective mandibular reconstruction: a retrospective study. Plast Surg Int. 2014;2014:893746. 10.1155/2014/893746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abdulrazaq SS, Riyadh S. The fate of the mandibular reconstruction plate. J Craniofac Surg. 2019;30(2):e97‐e101. 10.1097/SCS.0000000000004920 [DOI] [PubMed] [Google Scholar]
- 23. Boyd TG, Huber KM, Verbist DE, Bumpous JM, Wilhelmi BJ. Case report removal of exposed titanium reconstruction plate after mandibular reconstruction with a free fibula osteocutaneous flap with large surgical pin cutters: a case report and literature review. Eplasty. 2012;12:42. [PMC free article] [PubMed] [Google Scholar]
- 24. Ritschl LM, Mücke T, Hart D, et al. Retrospective analysis of complications in 190 mandibular resections and simultaneous reconstructions with free fibula flap, iliac crest flap or reconstruction plate: a comparative single centre study. Clin Oral Investig. 2021;25(5):2905‐2914. 10.1007/s00784-020-03607-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shibahara T, Noma H, Furuya Y, Takaki R. Fracture of mandibular reconstruction plates used after tumor resection. J Oral Maxillofac Surg. 2002;60(2):182‐185. 10.1053/joms.2002.29817 [DOI] [PubMed] [Google Scholar]
- 26. Maurer P, Eckert AW, Kriwalsky MS, Schubert J. Scope and limitations of methods of mandibular reconstruction: a long‐term follow‐up. Br J Oral Maxillofac Surg. 2010;48(2):100‐104. 10.1016/j.bjoms.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 27. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377‐381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500‐1524. 10.1016/j.ijsu.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 29. Rymer BC. Quantification of the bimodal plate‐specific complication profile associated with reconstruction of segmental mandibular defects with reconstruction plate and soft tissue flap: evidence from systematic review. J Craniofac Surg. 2022;33(7):2072‐2075. 10.1097/SCS.0000000000008578 [DOI] [PubMed] [Google Scholar]
- 30. West JD, Tang L, Julian A, Das S, Chambers T, Kokot NC. Risk factors for plate extrusion after mandibular reconstruction with vascularized free flap. J Oral Maxillofac Surg. 2021;79(8):1760‐1768. 10.1016/j.joms.2021.02.009 [DOI] [PubMed] [Google Scholar]
- 31. Capucha T, Shilo D, Abdalla‐Aslan R, et al. Is open reduction internal fixation using titanium plates in the mandible as successful as we think. J Craniofac Surg. 2022;33(4):1032‐1036. 10.1097/SCS.0000000000008258 [DOI] [PubMed] [Google Scholar]
- 32. Asero R. Food additives intolerance: a possible cause of perennial rhinitis. J Allergy Clin Immunol. 2002;110(6):937‐938. 10.1067/mai.2002.130054 [DOI] [PubMed] [Google Scholar]
- 33. Yao CM, Ziai H, Tsang G, et al. Surgical site infections following oral cavity cancer resection and reconstruction is a risk factor for plate exposure. J Otolaryngol Head Neck Surg. 2017;46(1):30. 10.1186/s40463-017-0206-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Rijt EEM, Noorlag R, Koole R, Abbink JH, Rosenberg AJWP. Predictive factors for premature loss of Martin 2.7 mandibular reconstruction plates. Br J Oral Maxillofac Surg. 2015;53(2):121‐125. 10.1016/j.bjoms.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 35. Nicholson RE, Schuller DE, Forrest LA, Mountain RE, Ali T, Young D. Factors involved in long‐ and short‐term mandibular plate exposure. Arch Otolaryngol Head Neck Surg. 1997;123(2):217‐222. 10.1001/archotol.1997.01900020107016 [DOI] [PubMed] [Google Scholar]
- 36. Liu SP, Cai ZG, Zhang J, Zhang JG, Zhang Y. Plate related complication after mandibular reconstruction. Zhonghua Kou Qiang Yi Xue Za Zhi. 2013;48(10):586‐590. [PubMed] [Google Scholar]
- 37. Davies JC, Chan HHL, Yao CMKL, et al. Association of plate contouring with hardware complications following mandibular reconstruction. Laryngoscope. 2022;132(1):61‐66. 10.1002/lary.29706 [DOI] [PubMed] [Google Scholar]
- 38. Fanzio PM, Chang KP, Chen HH, et al. Plate exposure after anterolateral thigh free‐flap reconstruction in head and neck cancer patients with composite mandibular defects. Ann Surg Oncol. 2015;22(9):3055‐3060. 10.1245/s10434-014-4322-1 [DOI] [PubMed] [Google Scholar]
- 39. Coletti DP, Ord R, Liu X. Mandibular reconstruction and second generation locking reconstruction plates: outcome of 110 patients. Int J Oral Maxillofac Surg. 2009;38(9):960‐963. 10.1016/j.ijom.2009.03.721 [DOI] [PubMed] [Google Scholar]
- 40. Dean A, Alamillos F, Heredero S, Redondo‐Camacho A, Guler I, Sanjuan A. Fibula free flap in maxillomandibular reconstruction. J Craniomaxillofac Surg. 2020;48(10):994‐1003. 10.1016/j.jcms.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 41. Knitschke M, Sonnabend S, Bäcker C, et al. Partial and total flap failure after fibula free flap in head and neck reconstructive surgery: retrospective analysis of 180 flaps over 19 years. Cancers. 2021;13(4):865. 10.3390/cancers13040865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manson PN. Facial Bone Healing and Bone Grafts. Clin Plast Surg. 1994;21(3):331‐348. [PubMed] [Google Scholar]
- 43. Kabasawa Y, Sato M, Kikuchi T, et al. Analysis and comparison of clinical results of bilateral sagittal split ramus osteotomy performed with the use of monocortical locking plate fixation or bicortical screw fixation. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(5):e333‐e341. 10.1016/j.oooo.2012.02.025 [DOI] [PubMed] [Google Scholar]
- 44. Fujioka M, Fujii T, Hirano A. Comparative study of mandibular stability after sagittal split osteotomies: biocortical versus monocortical osteosynthesis. Cleft Palate Craniofac J. 2000;37(6):551‐555. 10.1597/1545-1569_2000_037_0551_csomsa_2.0.co_2 [DOI] [PubMed] [Google Scholar]
- 45. Zhang ZL, Wang S, Sun CF, Xu ZF. Miniplates Versus Reconstruction Plates in Vascularized Osteocutaneous Flap Reconstruction of the Mandible. J Craniofac Surg. 2019;30(2):e119‐e125. 10.1097/SCS.0000000000005020 [DOI] [PubMed] [Google Scholar]
- 46. Zavattero E, Fasolis M, Garzino‐Demo P, Berrone S, Ramieri GA. Evaluation of plate‐related complications and efficacy in fibula free flap mandibular reconstruction. J Craniofac Surg. 2014;25(2):397‐399. 10.1097/SCS.0000000000000656 [DOI] [PubMed] [Google Scholar]
- 47. Robey AB, Spann ML, McAuliff TM, Meza JL, Hollins RR, Johnson PJ. Comparison of miniplates and reconstruction plates in fibular flap reconstruction of the mandible. Plast Reconstr Surg. 2008;122(6):1733‐1738. 10.1097/PRS.0b013e31818a9ac5 [DOI] [PubMed] [Google Scholar]
- 48. Chen Y, Wu J, Gokavarapu S, Shen Q, Ji T. Radiotherapy and smoking history are significant independent predictors for osteosynthesis‐associated late complications in vascular free fibula reconstruction of mandible. J Craniofac Surg. 2017;28(6):1508‐1513. 10.1097/SCS.0000000000003704 [DOI] [PubMed] [Google Scholar]
- 49. Kakarala K, Shnayder Y, Tsue TT, Girod DA. Mandibular reconstruction. Oral Oncol. 2018;77:111‐117. 10.1016/j.oraloncology.2017.12.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.


